 Found 444 hits with Last Name = 'mclaughlin' and Initial = 'jp'
Found 444 hits with Last Name = 'mclaughlin' and Initial = 'jp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(CALF) | BDBM85393
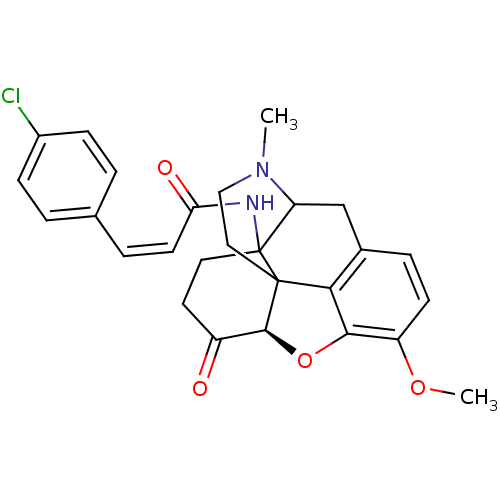
(14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27ClN2O4/c1-30-14-13-26-23-17-6-9-20(33-2)24(23)34-25(26)19(31)11-12-27(26,21(30)15-17)29-22(32)10-5-16-3-7-18(28)8-4-16/h3-10,21,25H,11-15H2,1-2H3,(H,29,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50001022
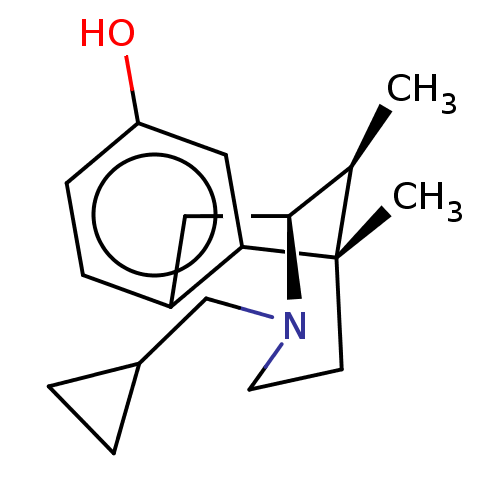
((2S,6S,11S)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC1CC1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3/t12-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 374-80 (2002)
Article DOI: 10.1124/jpet.302.1.374
BindingDB Entry DOI: 10.7270/Q2959G3P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50001022

((2S,6S,11S)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC1CC1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3/t12-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 374-80 (2002)
Article DOI: 10.1124/jpet.302.1.374
BindingDB Entry DOI: 10.7270/Q2959G3P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50097579
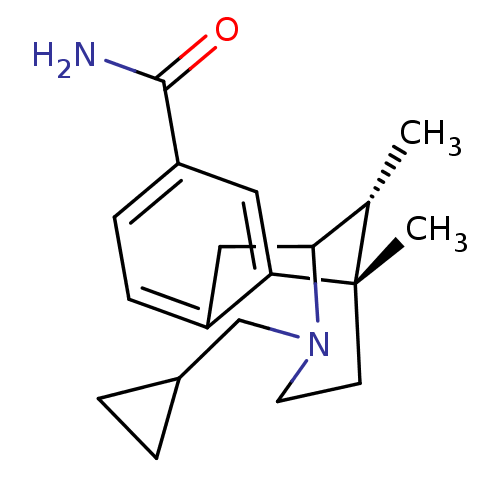
((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...)Show SMILES C[C@H]1C2Cc3ccc(cc3[C@@]1(C)CCN2CC1CC1)C(N)=O |r,TLB:8:9:1:14.12.13,15:14:1:4.9.3| Show InChI InChI=1S/C19H26N2O/c1-12-17-10-14-5-6-15(18(20)22)9-16(14)19(12,2)7-8-21(17)11-13-3-4-13/h5-6,9,12-13,17H,3-4,7-8,10-11H2,1-2H3,(H2,20,22)/t12-,17?,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 374-80 (2002)
Article DOI: 10.1124/jpet.302.1.374
BindingDB Entry DOI: 10.7270/Q2959G3P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50169774
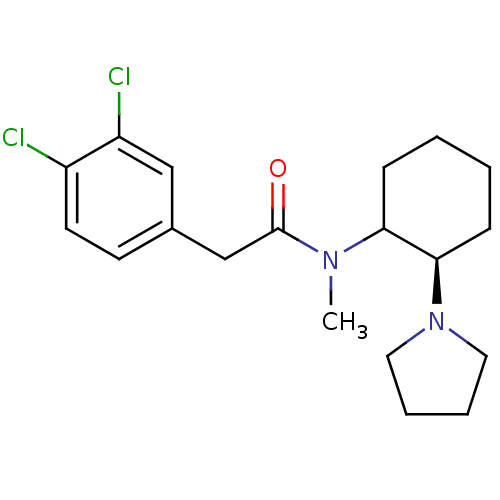
(2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN(C1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17?,18-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 374-80 (2002)
Article DOI: 10.1124/jpet.302.1.374
BindingDB Entry DOI: 10.7270/Q2959G3P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50097579

((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4...)Show SMILES C[C@H]1C2Cc3ccc(cc3[C@@]1(C)CCN2CC1CC1)C(N)=O |r,TLB:8:9:1:14.12.13,15:14:1:4.9.3| Show InChI InChI=1S/C19H26N2O/c1-12-17-10-14-5-6-15(18(20)22)9-16(14)19(12,2)7-8-21(17)11-13-3-4-13/h5-6,9,12-13,17H,3-4,7-8,10-11H2,1-2H3,(H2,20,22)/t12-,17?,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 374-80 (2002)
Article DOI: 10.1124/jpet.302.1.374
BindingDB Entry DOI: 10.7270/Q2959G3P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM85396
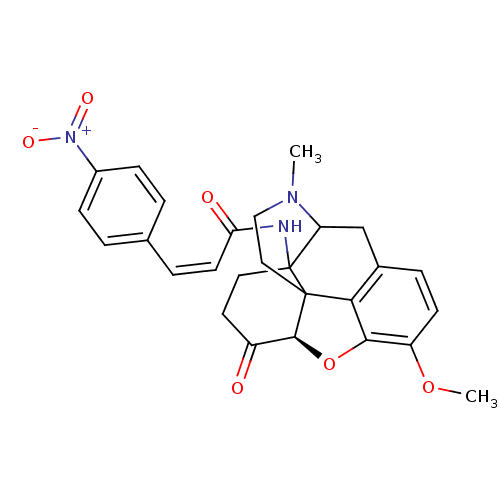
(CACO)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27N3O6/c1-29-14-13-26-23-17-6-9-20(35-2)24(23)36-25(26)19(31)11-12-27(26,21(29)15-17)28-22(32)10-5-16-3-7-18(8-4-16)30(33)34/h3-10,21,25H,11-15H2,1-2H3,(H,28,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50533716
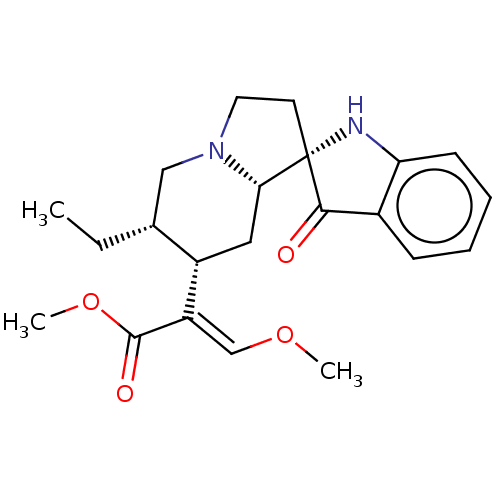
(CHEMBL4453504)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2ccccc2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C22H28N2O4/c1-4-14-12-24-10-9-22(20(25)15-7-5-6-8-18(15)23-22)19(24)11-16(14)17(13-27-2)21(26)28-3/h5-8,13-14,16,19,23H,4,9-12H2,1-3H3/b17-13+/t14-,16+,19+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM50029138
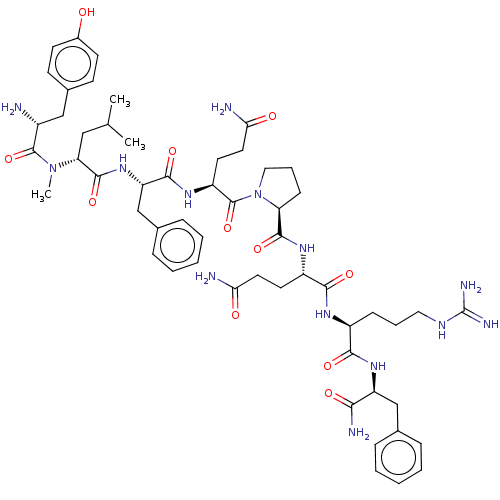
(CHEMBL3360830)Show SMILES CC(C)C[C@@H](N(C)C(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C55H78N14O11/c1-32(2)28-44(68(3)53(79)37(56)29-35-18-20-36(70)21-19-35)52(78)67-42(31-34-14-8-5-9-15-34)50(76)65-40(23-25-46(58)72)54(80)69-27-11-17-43(69)51(77)64-39(22-24-45(57)71)49(75)63-38(16-10-26-62-55(60)61)48(74)66-41(47(59)73)30-33-12-6-4-7-13-33/h4-9,12-15,18-21,32,37-44,70H,10-11,16-17,22-31,56H2,1-3H3,(H2,57,71)(H2,58,72)(H2,59,73)(H,63,75)(H,64,77)(H,65,76)(H,66,74)(H,67,78)(H4,60,61,62)/t37-,38+,39+,40+,41+,42+,43+,44-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]EYF from human NPFF2 receptor expressed in CHO cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071073
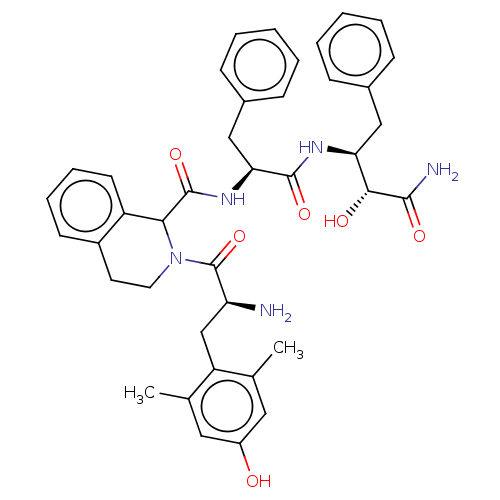
(CHEMBL3409763)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(N)=O |r| Show InChI InChI=1S/C40H45N5O6/c1-24-19-29(46)20-25(2)31(24)23-32(41)40(51)45-18-17-28-15-9-10-16-30(28)35(45)39(50)44-34(22-27-13-7-4-8-14-27)38(49)43-33(36(47)37(42)48)21-26-11-5-3-6-12-26/h3-16,19-20,32-36,46-47H,17-18,21-23,41H2,1-2H3,(H2,42,48)(H,43,49)(H,44,50)/t32-,33-,34-,35?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50533717

(CHEMBL4449284)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(C#N)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H27N3O4/c1-4-14-12-26-9-8-23(19(26)10-16(14)17(13-29-2)22(28)30-3)21(27)20-15(11-24)6-5-7-18(20)25-23/h5-7,13-14,16,19,25H,4,8-10,12H2,1-3H3/b17-13+/t14-,16+,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071072
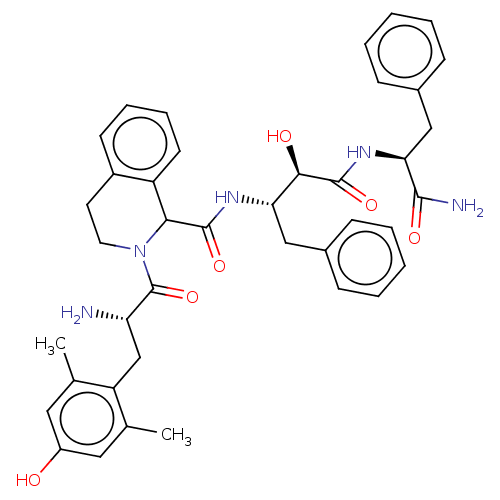
(CHEMBL3409762)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H45N5O6/c1-24-19-29(46)20-25(2)31(24)23-32(41)40(51)45-18-17-28-15-9-10-16-30(28)35(45)38(49)43-33(21-26-11-5-3-6-12-26)36(47)39(50)44-34(37(42)48)22-27-13-7-4-8-14-27/h3-16,19-20,32-36,46-47H,17-18,21-23,41H2,1-2H3,(H2,42,48)(H,43,49)(H,44,50)/t32-,33-,34-,35?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50474150
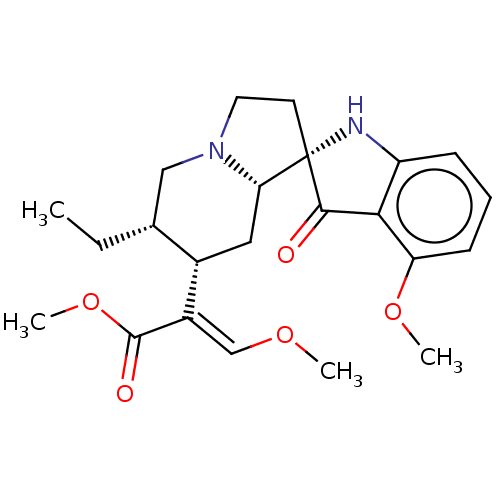
(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Opioid receptor delta 1
(Bos taurus) | BDBM85393

(14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27ClN2O4/c1-30-14-13-26-23-17-6-9-20(33-2)24(23)34-25(26)19(31)11-12-27(26,21(30)15-17)29-22(32)10-5-16-3-7-18(28)8-4-16/h3-10,21,25H,11-15H2,1-2H3,(H,29,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50528969
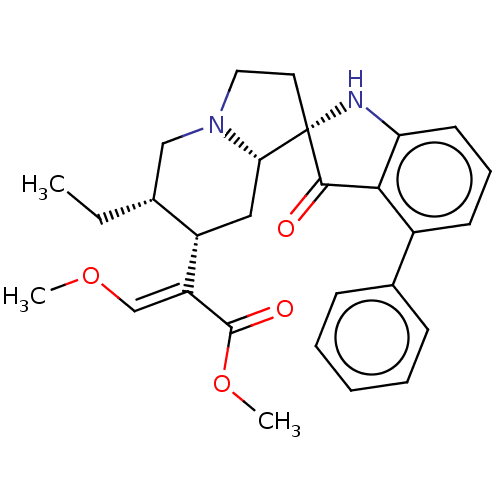
(CHEMBL4458337)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(c2C1=O)-c1ccccc1)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C28H32N2O4/c1-4-18-16-30-14-13-28(24(30)15-21(18)22(17-33-2)27(32)34-3)26(31)25-20(11-8-12-23(25)29-28)19-9-6-5-7-10-19/h5-12,17-18,21,24,29H,4,13-16H2,1-3H3/b22-17+/t18-,21+,24+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071071

(CHEMBL3409761)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(N)=O |r| Show InChI InChI=1S/C42H46N6O6/c1-24-18-29(49)19-25(2)32(24)22-33(43)42(54)48-17-16-27-12-6-7-14-31(27)37(48)41(53)47-36(21-28-23-45-34-15-9-8-13-30(28)34)40(52)46-35(38(50)39(44)51)20-26-10-4-3-5-11-26/h3-15,18-19,23,33,35-38,45,49-50H,16-17,20-22,43H2,1-2H3,(H2,44,51)(H,46,52)(H,47,53)/t33-,35-,36-,37?,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 2
(Homo sapiens (Human)) | BDBM50029138

(CHEMBL3360830)Show SMILES CC(C)C[C@@H](N(C)C(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C55H78N14O11/c1-32(2)28-44(68(3)53(79)37(56)29-35-18-20-36(70)21-19-35)52(78)67-42(31-34-14-8-5-9-15-34)50(76)65-40(23-25-46(58)72)54(80)69-27-11-17-43(69)51(77)64-39(22-24-45(57)71)49(75)63-38(16-10-26-62-55(60)61)48(74)66-41(47(59)73)30-33-12-6-4-7-13-33/h4-9,12-15,18-21,32,37-44,70H,10-11,16-17,22-31,56H2,1-3H3,(H2,57,71)(H2,58,72)(H2,59,73)(H,63,75)(H,64,77)(H,65,76)(H,66,74)(H,67,78)(H4,60,61,62)/t37-,38+,39+,40+,41+,42+,43+,44-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]EYF from human NPFF2 receptor expressed in CHO cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50528969

(CHEMBL4458337)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(c2C1=O)-c1ccccc1)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C28H32N2O4/c1-4-18-16-30-14-13-28(24(30)15-21(18)22(17-33-2)27(32)34-3)26(31)25-20(11-8-12-23(25)29-28)19-9-6-5-7-10-19/h5-12,17-18,21,24,29H,4,13-16H2,1-3H3/b22-17+/t18-,21+,24+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50533719
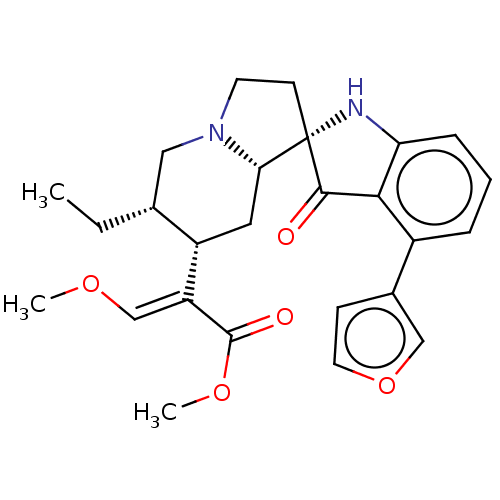
(CHEMBL4441457)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(-c3ccoc3)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C26H30N2O5/c1-4-16-13-28-10-9-26(22(28)12-19(16)20(15-31-2)25(30)32-3)24(29)23-18(17-8-11-33-14-17)6-5-7-21(23)27-26/h5-8,11,14-16,19,22,27H,4,9-10,12-13H2,1-3H3/b20-15+/t16-,19+,22+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071094
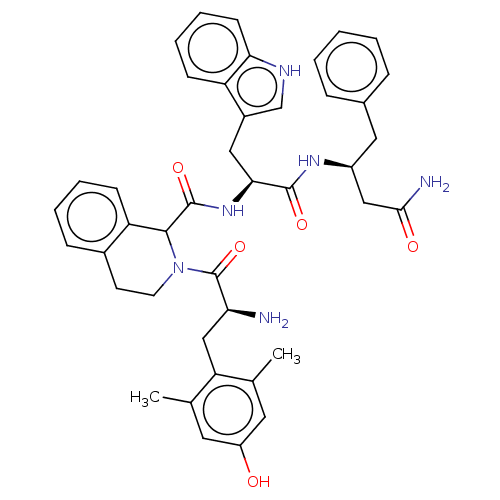
(CHEMBL3409765)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](CC(N)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C42H46N6O5/c1-25-18-31(49)19-26(2)34(25)23-35(43)42(53)48-17-16-28-12-6-7-14-33(28)39(48)41(52)47-37(21-29-24-45-36-15-9-8-13-32(29)36)40(51)46-30(22-38(44)50)20-27-10-4-3-5-11-27/h3-15,18-19,24,30,35,37,39,45,49H,16-17,20-23,43H2,1-2H3,(H2,44,50)(H,46,51)(H,47,52)/t30-,35-,37-,39?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071070
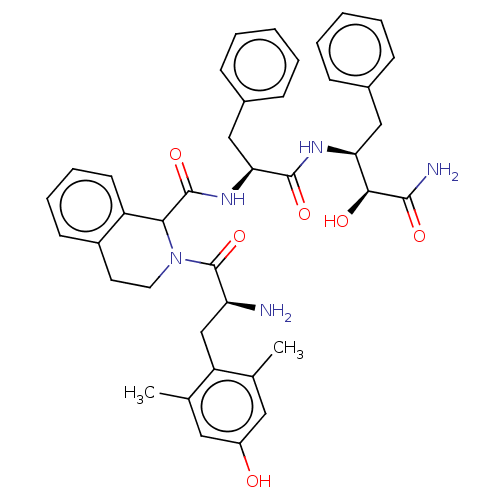
(CHEMBL3409760)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(N)=O |r| Show InChI InChI=1S/C40H45N5O6/c1-24-19-29(46)20-25(2)31(24)23-32(41)40(51)45-18-17-28-15-9-10-16-30(28)35(45)39(50)44-34(22-27-13-7-4-8-14-27)38(49)43-33(36(47)37(42)48)21-26-11-5-3-6-12-26/h3-16,19-20,32-36,46-47H,17-18,21-23,41H2,1-2H3,(H2,42,48)(H,43,49)(H,44,50)/t32-,33-,34-,35?,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071093

(CHEMBL3409764)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CC(N)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C40H45N5O5/c1-25-19-31(46)20-26(2)33(25)24-34(41)40(50)45-18-17-29-15-9-10-16-32(29)37(45)39(49)44-35(22-28-13-7-4-8-14-28)38(48)43-30(23-36(42)47)21-27-11-5-3-6-12-27/h3-16,19-20,30,34-35,37,46H,17-18,21-24,41H2,1-2H3,(H2,42,47)(H,43,48)(H,44,49)/t30-,34-,35-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071095

(CHEMBL3409766)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@H](CC(=O)N[C@@H](Cc1ccccc1)C(N)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C40H45N5O5/c1-25-19-31(46)20-26(2)33(25)24-34(41)40(50)45-18-17-29-15-9-10-16-32(29)37(45)39(49)43-30(21-27-11-5-3-6-12-27)23-36(47)44-35(38(42)48)22-28-13-7-4-8-14-28/h3-16,19-20,30,34-35,37,46H,17-18,21-24,41H2,1-2H3,(H2,42,48)(H,43,49)(H,44,47)/t30-,34-,35-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM85394
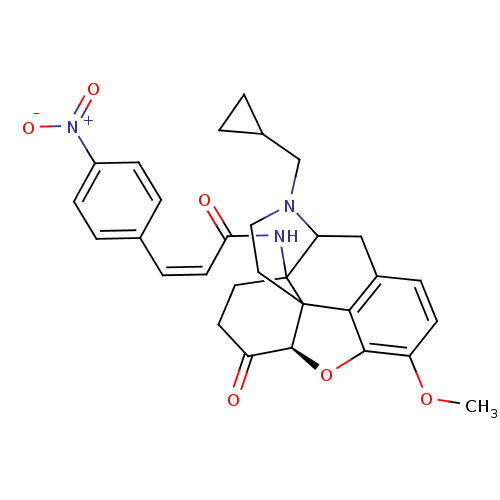
(N-CPM-CACO)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31N3O6/c1-38-23-10-7-20-16-24-30(31-25(35)11-6-18-4-8-21(9-5-18)33(36)37)13-12-22(34)28-29(30,26(20)27(23)39-28)14-15-32(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,31,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(CALF) | BDBM85393

(14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27ClN2O4/c1-30-14-13-26-23-17-6-9-20(33-2)24(23)34-25(26)19(31)11-12-27(26,21(30)15-17)29-22(32)10-5-16-3-7-18(28)8-4-16/h3-10,21,25H,11-15H2,1-2H3,(H,29,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(GUINEA PIG) | BDBM50001022

((2S,6S,11S)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...)Show SMILES C[C@@H]1[C@@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC1CC1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C18H25NO/c1-12-17-9-14-5-6-15(20)10-16(14)18(12,2)7-8-19(17)11-13-3-4-13/h5-6,10,12-13,17,20H,3-4,7-9,11H2,1-2H3/t12-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 374-80 (2002)
Article DOI: 10.1124/jpet.302.1.374
BindingDB Entry DOI: 10.7270/Q2959G3P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM85395
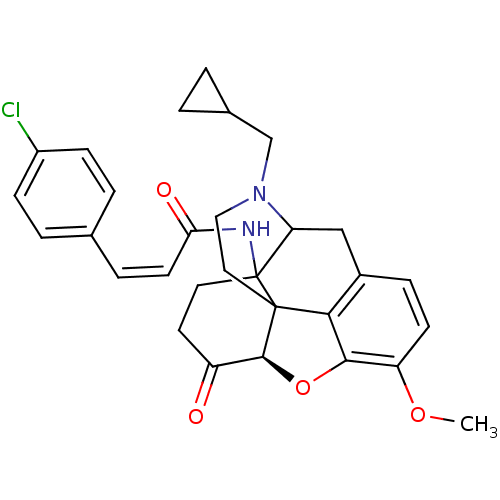
(MC-CAM)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31ClN2O4/c1-36-23-10-7-20-16-24-30(32-25(35)11-6-18-4-8-21(31)9-5-18)13-12-22(34)28-29(30,26(20)27(23)37-28)14-15-33(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,32,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50533718
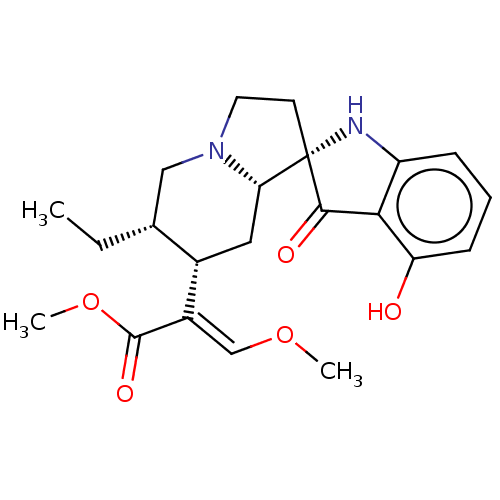
(CHEMBL4530421)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(O)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C22H28N2O5/c1-4-13-11-24-9-8-22(20(26)19-16(23-22)6-5-7-17(19)25)18(24)10-14(13)15(12-28-2)21(27)29-3/h5-7,12-14,18,23,25H,4,8-11H2,1-3H3/b15-12+/t13-,14+,18+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50533719

(CHEMBL4441457)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(-c3ccoc3)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C26H30N2O5/c1-4-16-13-28-10-9-26(22(28)12-19(16)20(15-31-2)25(30)32-3)24(29)23-18(17-8-11-33-14-17)6-5-7-21(23)27-26/h5-8,11,14-16,19,22,27H,4,9-10,12-13H2,1-3H3/b20-15+/t16-,19+,22+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071068
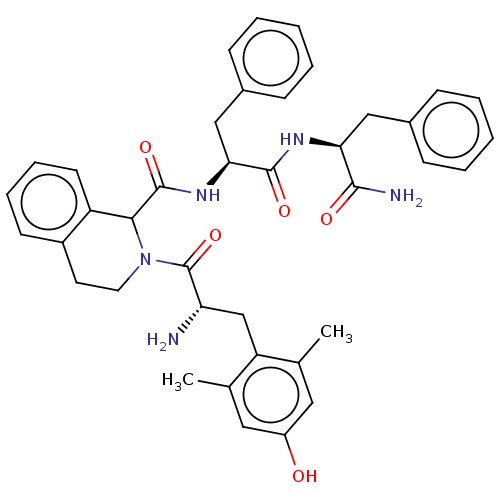
(CHEMBL3409758)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C39H43N5O5/c1-24-19-29(45)20-25(2)31(24)23-32(40)39(49)44-18-17-28-15-9-10-16-30(28)35(44)38(48)43-34(22-27-13-7-4-8-14-27)37(47)42-33(36(41)46)21-26-11-5-3-6-12-26/h3-16,19-20,32-35,45H,17-18,21-23,40H2,1-2H3,(H2,41,46)(H,42,47)(H,43,48)/t32-,33-,34-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071069
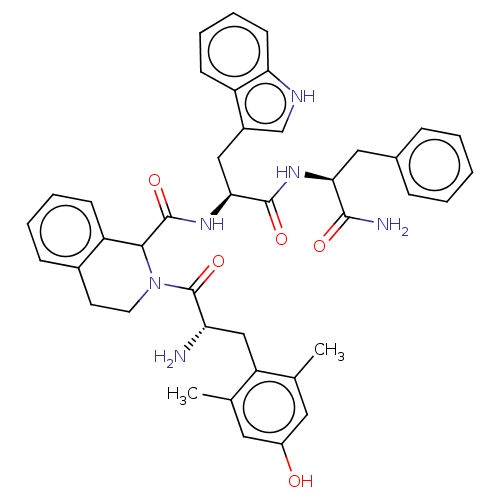
(CHEMBL3409759)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C41H44N6O5/c1-24-18-29(48)19-25(2)32(24)22-33(42)41(52)47-17-16-27-12-6-7-14-31(27)37(47)40(51)46-36(21-28-23-44-34-15-9-8-13-30(28)34)39(50)45-35(38(43)49)20-26-10-4-3-5-11-26/h3-15,18-19,23,33,35-37,44,48H,16-17,20-22,42H2,1-2H3,(H2,43,49)(H,45,50)(H,46,51)/t33-,35-,36-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50533717

(CHEMBL4449284)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(C#N)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C23H27N3O4/c1-4-14-12-26-9-8-23(19(26)10-16(14)17(13-29-2)22(28)30-3)21(27)20-15(11-24)6-5-7-18(20)25-23/h5-7,13-14,16,19,25H,4,8-10,12H2,1-3H3/b17-13+/t14-,16+,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50533720
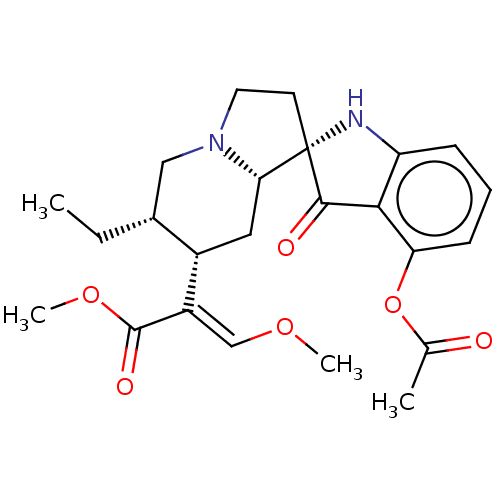
(CHEMBL4557905)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(OC(C)=O)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C24H30N2O6/c1-5-15-12-26-10-9-24(20(26)11-16(15)17(13-30-3)23(29)31-4)22(28)21-18(25-24)7-6-8-19(21)32-14(2)27/h6-8,13,15-16,20,25H,5,9-12H2,1-4H3/b17-13+/t15-,16+,20+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50533716

(CHEMBL4453504)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2ccccc2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C22H28N2O4/c1-4-14-12-24-10-9-22(20(25)15-7-5-6-8-18(15)23-22)19(24)11-16(14)17(13-27-2)21(26)28-3/h5-8,13-14,16,19,23H,4,9-12H2,1-3H3/b17-13+/t14-,16+,19+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071071

(CHEMBL3409761)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(N)=O |r| Show InChI InChI=1S/C42H46N6O6/c1-24-18-29(49)19-25(2)32(24)22-33(43)42(54)48-17-16-27-12-6-7-14-31(27)37(48)41(53)47-36(21-28-23-45-34-15-9-8-13-30(28)34)40(52)46-35(38(50)39(44)51)20-26-10-4-3-5-11-26/h3-15,18-19,23,33,35-38,45,49-50H,16-17,20-22,43H2,1-2H3,(H2,44,51)(H,46,52)(H,47,53)/t33-,35-,36-,37?,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50474150

(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50029183
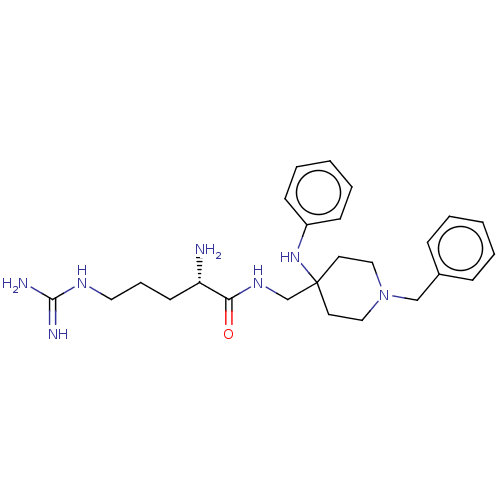
(CHEMBL3360832)Show SMILES N[C@@H](CCCNC(N)=N)C(=O)NCC1(CCN(Cc2ccccc2)CC1)Nc1ccccc1 |r| Show InChI InChI=1S/C25H37N7O/c26-22(12-7-15-29-24(27)28)23(33)30-19-25(31-21-10-5-2-6-11-21)13-16-32(17-14-25)18-20-8-3-1-4-9-20/h1-6,8-11,22,31H,7,12-19,26H2,(H,30,33)(H4,27,28,29)/t22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cells after 60 mins by scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50029188

(CHEMBL2165920)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C45H72N14O10/c1-24(2)21-31(57-40(65)30(23-35(47)61)56-42(67)33-15-10-20-59(33)44(69)36(48)25(3)4)43(68)58-19-9-14-32(58)41(66)54-28(16-17-34(46)60)39(64)53-27(13-8-18-52-45(50)51)38(63)55-29(37(49)62)22-26-11-6-5-7-12-26/h5-7,11-12,24-25,27-33,36H,8-10,13-23,48H2,1-4H3,(H2,46,60)(H2,47,61)(H2,49,62)(H,53,64)(H,54,66)(H,55,63)(H,56,67)(H,57,65)(H4,50,51,52)/t27-,28-,29-,30-,31-,32-,33-,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]NPVF from human NPFF1 receptor expressed in CHO cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50029138

(CHEMBL3360830)Show SMILES CC(C)C[C@@H](N(C)C(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C55H78N14O11/c1-32(2)28-44(68(3)53(79)37(56)29-35-18-20-36(70)21-19-35)52(78)67-42(31-34-14-8-5-9-15-34)50(76)65-40(23-25-46(58)72)54(80)69-27-11-17-43(69)51(77)64-39(22-24-45(57)71)49(75)63-38(16-10-26-62-55(60)61)48(74)66-41(47(59)73)30-33-12-6-4-7-13-33/h4-9,12-15,18-21,32,37-44,70H,10-11,16-17,22-31,56H2,1-3H3,(H2,57,71)(H2,58,72)(H2,59,73)(H,63,75)(H,64,77)(H,65,76)(H,66,74)(H,67,78)(H4,60,61,62)/t37-,38+,39+,40+,41+,42+,43+,44-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]NPVF from human NPFF1 receptor expressed in CHO cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Neuropeptide FF receptor 1
(Homo sapiens (Human)) | BDBM50029138

(CHEMBL3360830)Show SMILES CC(C)C[C@@H](N(C)C(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C55H78N14O11/c1-32(2)28-44(68(3)53(79)37(56)29-35-18-20-36(70)21-19-35)52(78)67-42(31-34-14-8-5-9-15-34)50(76)65-40(23-25-46(58)72)54(80)69-27-11-17-43(69)51(77)64-39(22-24-45(57)71)49(75)63-38(16-10-26-62-55(60)61)48(74)66-41(47(59)73)30-33-12-6-4-7-13-33/h4-9,12-15,18-21,32,37-44,70H,10-11,16-17,22-31,56H2,1-3H3,(H2,57,71)(H2,58,72)(H2,59,73)(H,63,75)(H,64,77)(H,65,76)(H,66,74)(H,67,78)(H4,60,61,62)/t37-,38+,39+,40+,41+,42+,43+,44-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [125I]YVP from human NPFF1 receptor expressed in CHO cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50029183

(CHEMBL3360832)Show SMILES N[C@@H](CCCNC(N)=N)C(=O)NCC1(CCN(Cc2ccccc2)CC1)Nc1ccccc1 |r| Show InChI InChI=1S/C25H37N7O/c26-22(12-7-15-29-24(27)28)23(33)30-19-25(31-21-10-5-2-6-11-21)13-16-32(17-14-25)18-20-8-3-1-4-9-20/h1-6,8-11,22,31H,7,12-19,26H2,(H,30,33)(H4,27,28,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from human KOR expressed in HEK293 cells after 60 mins by scintillation counting analysis |
J Med Chem 57: 8903-27 (2014)
Article DOI: 10.1021/jm500989n
BindingDB Entry DOI: 10.7270/Q2C24Z18 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071068

(CHEMBL3409758)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C39H43N5O5/c1-24-19-29(45)20-25(2)31(24)23-32(40)39(49)44-18-17-28-15-9-10-16-30(28)35(44)38(48)43-34(22-27-13-7-4-8-14-27)37(47)42-33(36(41)46)21-26-11-5-3-6-12-26/h3-16,19-20,32-35,45H,17-18,21-23,40H2,1-2H3,(H2,41,46)(H,42,47)(H,43,48)/t32-,33-,34-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071069

(CHEMBL3409759)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C41H44N6O5/c1-24-18-29(48)19-25(2)32(24)22-33(42)41(52)47-17-16-27-12-6-7-14-31(27)37(47)40(51)46-36(21-28-23-44-34-15-9-8-13-30(28)34)39(50)45-35(38(43)49)20-26-10-4-3-5-11-26/h3-15,18-19,23,33,35-37,44,48H,16-17,20-22,42H2,1-2H3,(H2,43,49)(H,45,50)(H,46,51)/t33-,35-,36-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50580195
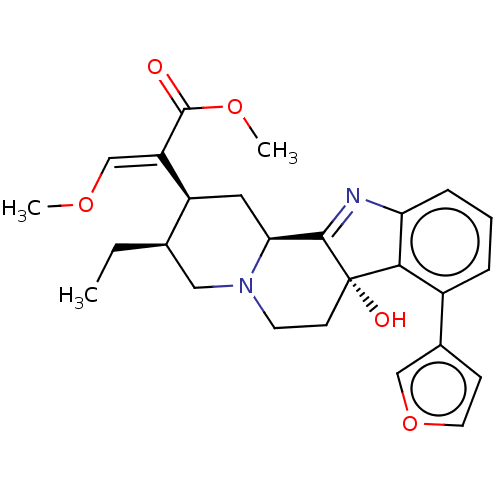
(CHEMBL5071286)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@@]1(O)C2=Nc2cccc(-c3ccoc3)c12)C(=C/OC)\C(=O)OC |r,t:15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor (unknown origin) expressed in CHO cells at 10 uM |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01273
BindingDB Entry DOI: 10.7270/Q25D8WQH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071095

(CHEMBL3409766)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@H](CC(=O)N[C@@H](Cc1ccccc1)C(N)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C40H45N5O5/c1-25-19-31(46)20-26(2)33(25)24-34(41)40(50)45-18-17-29-15-9-10-16-32(29)37(45)39(49)43-30(21-27-11-5-3-6-12-27)23-36(47)44-35(38(42)48)22-28-13-7-4-8-14-28/h3-16,19-20,30,34-35,37,46H,17-18,21-24,41H2,1-2H3,(H2,42,48)(H,43,49)(H,44,47)/t30-,34-,35-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50533718

(CHEMBL4530421)Show SMILES [H][C@@]12C[C@@H]([C@H](CC)CN1CC[C@]21Nc2cccc(O)c2C1=O)C(=C/OC)\C(=O)OC |r| Show InChI InChI=1S/C22H28N2O5/c1-4-13-11-24-9-8-22(20(26)19-16(23-22)6-5-7-17(19)25)18(24)10-14(13)15(12-28-2)21(27)29-3/h5-7,12-14,18,23,25H,4,8-11H2,1-3H3/b15-12+/t13-,14+,18+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins |
J Med Chem 59: 8381-97 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00748
BindingDB Entry DOI: 10.7270/Q27H1P2H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071072

(CHEMBL3409762)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCc2ccccc2C1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H45N5O6/c1-24-19-29(46)20-25(2)31(24)23-32(41)40(51)45-18-17-28-15-9-10-16-30(28)35(45)38(49)43-33(21-26-11-5-3-6-12-26)36(47)39(50)44-34(37(42)48)22-27-13-7-4-8-14-27/h3-16,19-20,32-36,46-47H,17-18,21-23,41H2,1-2H3,(H2,42,48)(H,43,49)(H,44,50)/t32-,33-,34-,35?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50071076
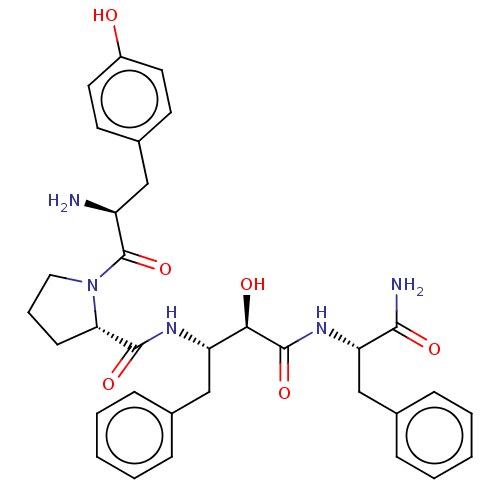
(CHEMBL3409749)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C33H39N5O6/c34-25(18-23-13-15-24(39)16-14-23)33(44)38-17-7-12-28(38)31(42)36-26(19-21-8-3-1-4-9-21)29(40)32(43)37-27(30(35)41)20-22-10-5-2-6-11-22/h1-6,8-11,13-16,25-29,39-40H,7,12,17-20,34H2,(H2,35,41)(H,36,42)(H,37,43)/t25-,26-,27-,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis |
Eur J Med Chem 92: 270-81 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.049
BindingDB Entry DOI: 10.7270/Q22B90RZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data