

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4690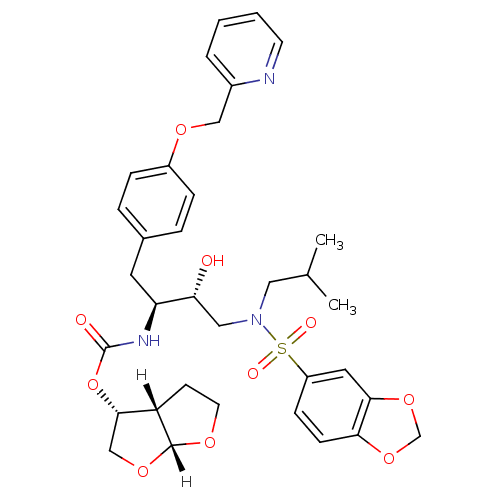 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW0385 from HIV1 protease | Bioorg Med Chem Lett 16: 1788-94 (2006) Article DOI: 10.1016/j.bmcl.2006.01.035 BindingDB Entry DOI: 10.7270/Q2WS8V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4685 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW0385 from HIV1 protease | Bioorg Med Chem Lett 16: 1788-94 (2006) Article DOI: 10.1016/j.bmcl.2006.01.035 BindingDB Entry DOI: 10.7270/Q2WS8V1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042365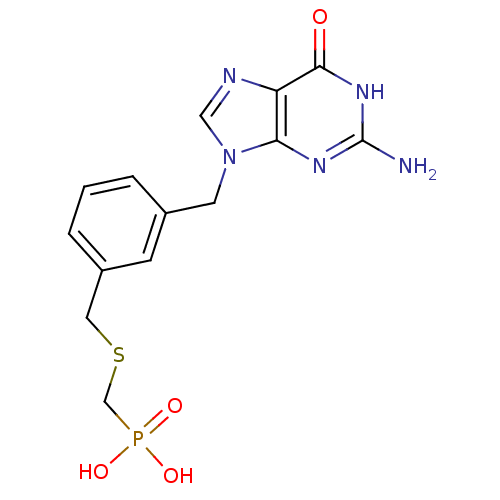 (CHEMBL117417 | [3-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033660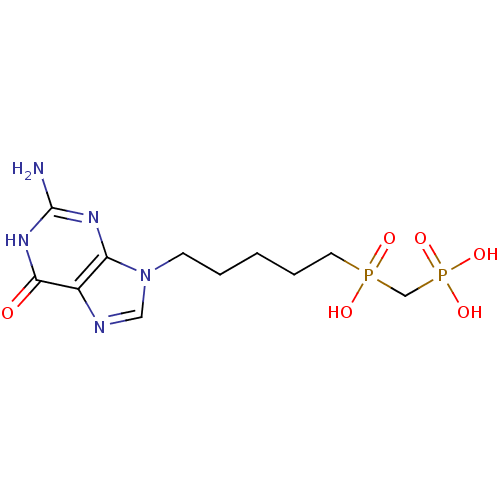 (CHEMBL269745 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033663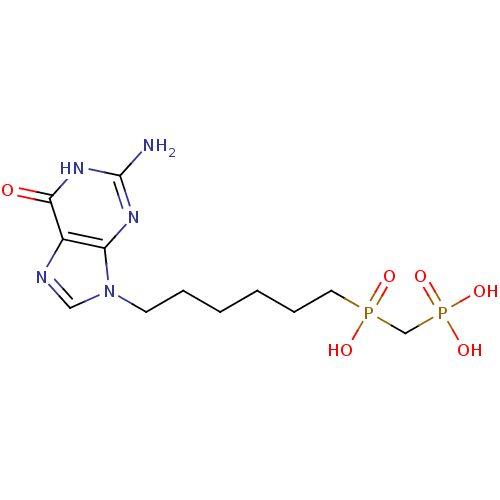 (CHEMBL7319 | {[6-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042370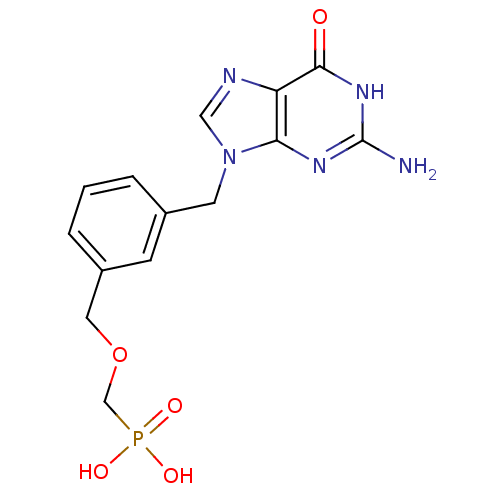 (CHEMBL326595 | [3-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042364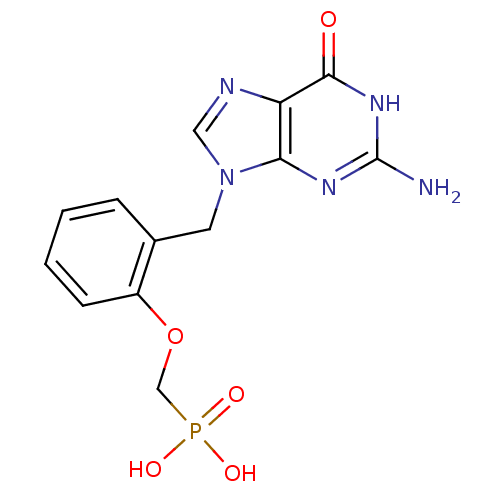 (CHEMBL327010 | [2-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033661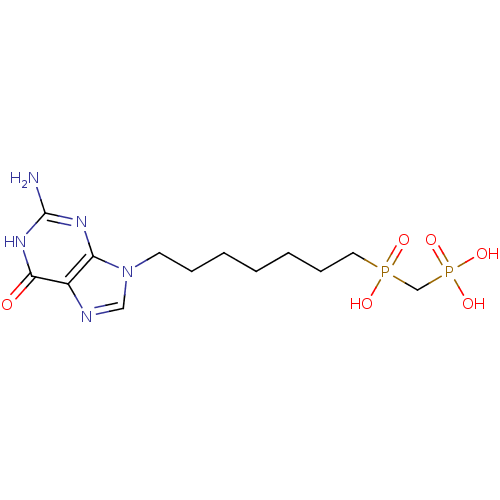 (CHEMBL7247 | {[7-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042372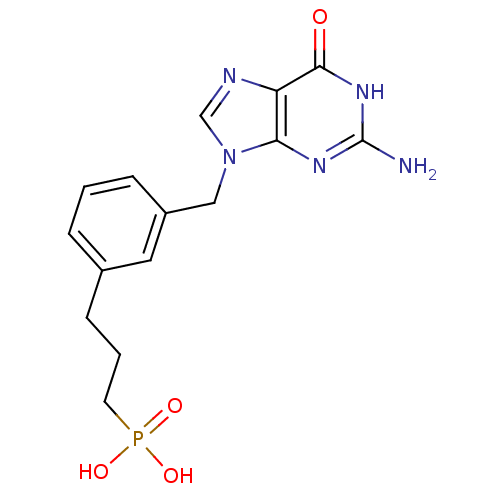 (CHEMBL117127 | {3-[3-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042363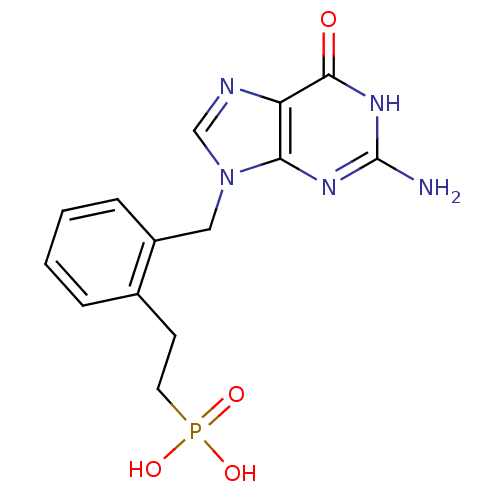 (CHEMBL333946 | {2-[2-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042367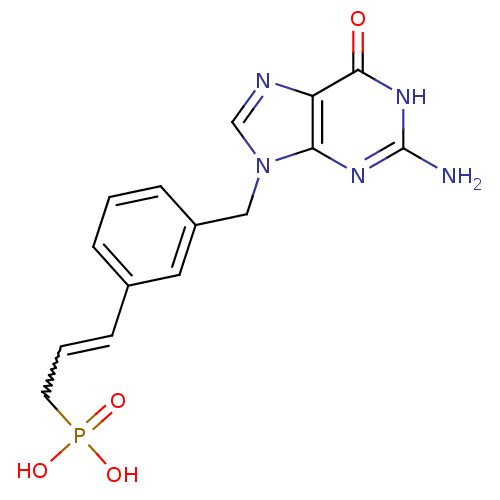 (CHEMBL325716 | {3-[3-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042369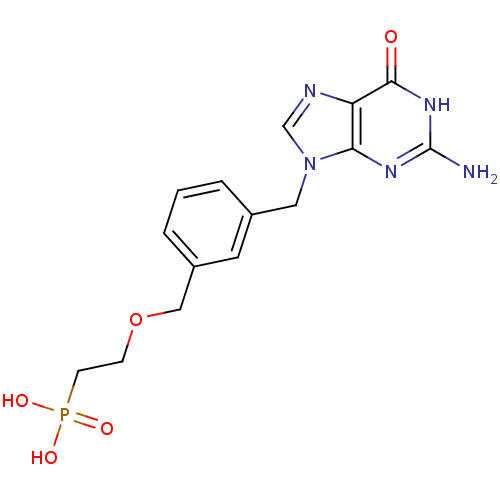 (CHEMBL119243 | {2-[3-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033664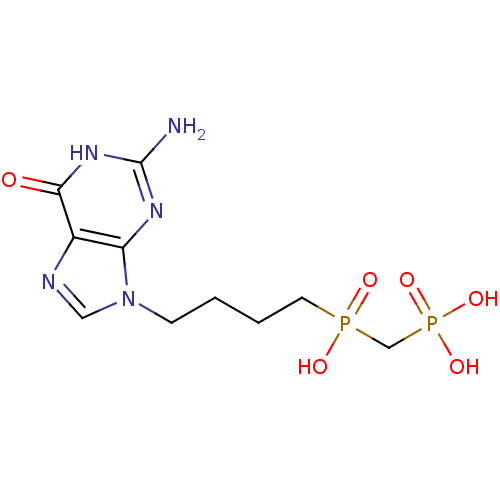 (CHEMBL7068 | {[4-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042362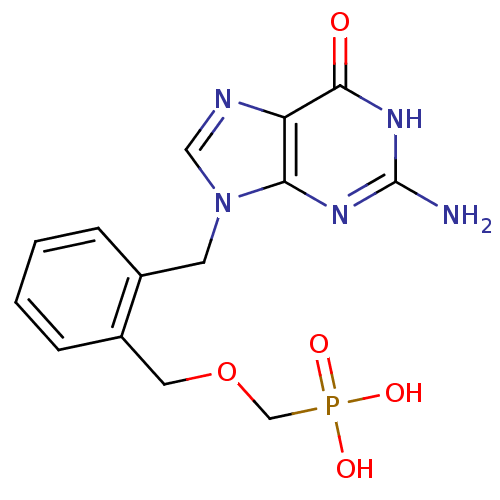 (CHEMBL331133 | [2-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033663 (CHEMBL7319 | {[6-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042366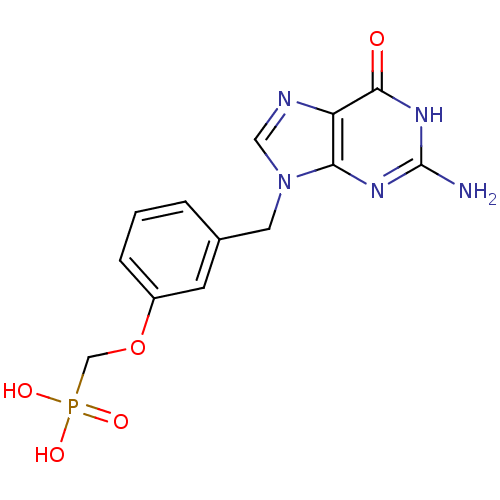 (CHEMBL116580 | [3-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033660 (CHEMBL269745 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042361 (CHEMBL324839 | {3-[2-(2-Amino-6-oxo-1,6-dihydro-pu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033665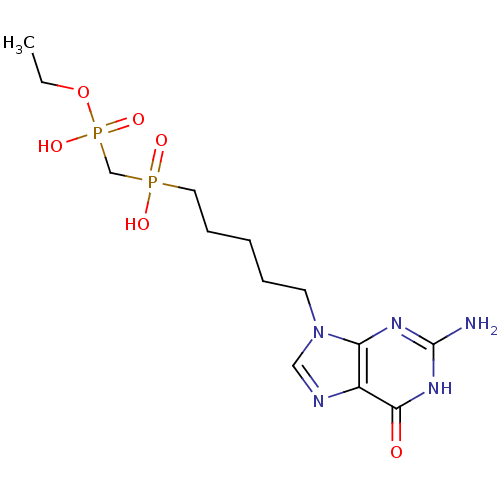 (CHEMBL268378 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033661 (CHEMBL7247 | {[7-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033662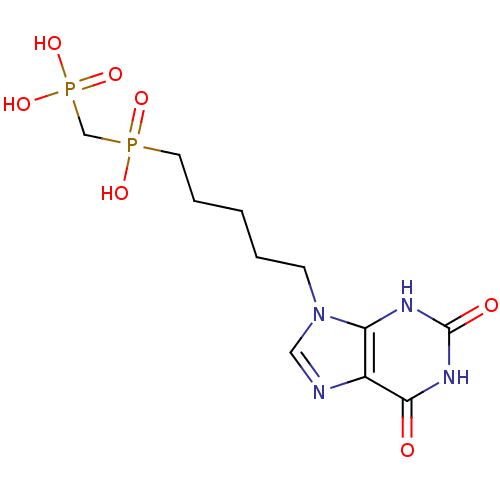 (CHEMBL7194 | {Hydroxy-[5-(2-hydroxy-6-oxo-1,6-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of zinc chloride | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033664 (CHEMBL7068 | {[4-(2-Amino-6-oxo-1,6-dihydro-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042360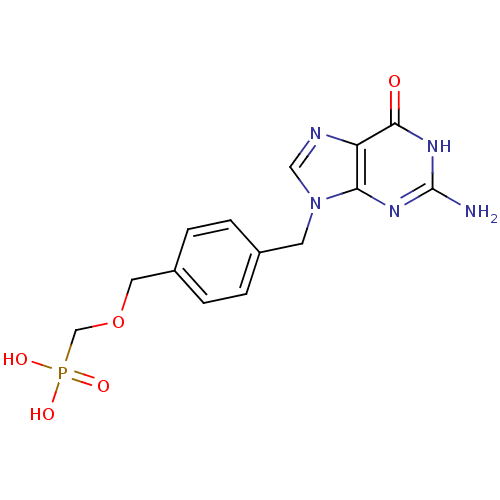 (CHEMBL117588 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042368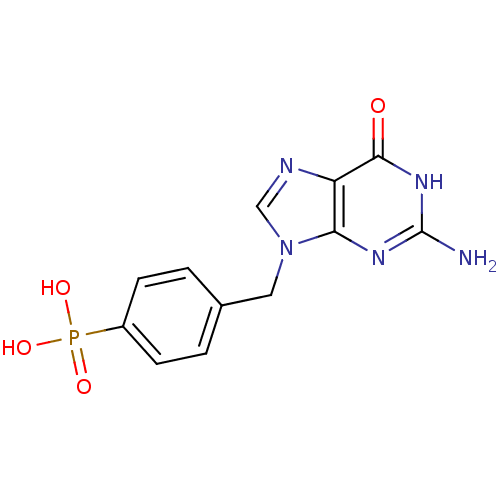 (CHEMBL446671 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033665 (CHEMBL268378 | {[5-(2-Amino-6-oxo-1,6-dihydro-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033662 (CHEMBL7194 | {Hydroxy-[5-(2-hydroxy-6-oxo-1,6-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte purine nucleoside phosphorylase (PNPase) in the presence of ethylenediaminetetraacetic acid (Na2 EDTA) | J Med Chem 38: 1005-14 (1995) BindingDB Entry DOI: 10.7270/Q2FF3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50042371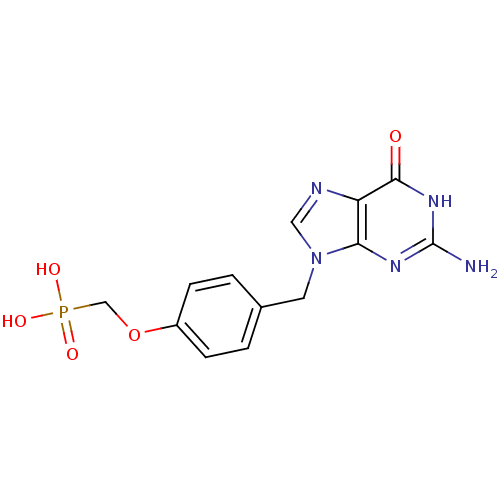 (CHEMBL327003 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description Inhibition of purine nucleoside phosphorylase of human erythrocytes in xanthine oxidase-coupled assay | J Med Chem 36: 3455-63 (1993) BindingDB Entry DOI: 10.7270/Q2PR7V1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50122955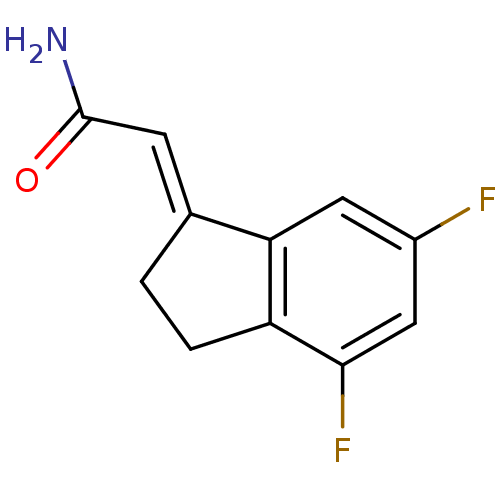 (2-(4,6-Difluoro-indan-1-ylidene)-acetamide | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Monoamine oxidase B (MAO-B) | J Med Chem 46: 399-408 (2003) Article DOI: 10.1021/jm020067s BindingDB Entry DOI: 10.7270/Q2VH5N7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520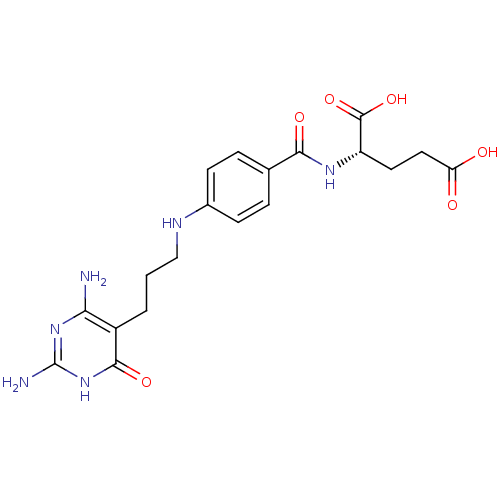 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Hexaglutamyl homologue inhibition activity against the AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against hog liver | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Triglutamyl homologue inhibition activity against AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against MOLT-4 | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50014844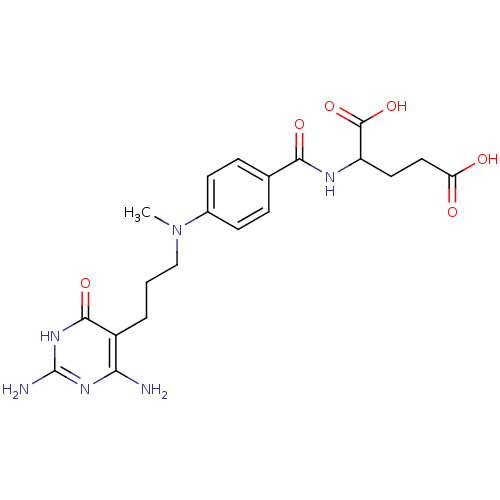 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) from L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50122955 (2-(4,6-Difluoro-indan-1-ylidene)-acetamide | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Monoamine oxidase A (MAO-A) | J Med Chem 46: 399-408 (2003) Article DOI: 10.1021/jm020067s BindingDB Entry DOI: 10.7270/Q2VH5N7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50014843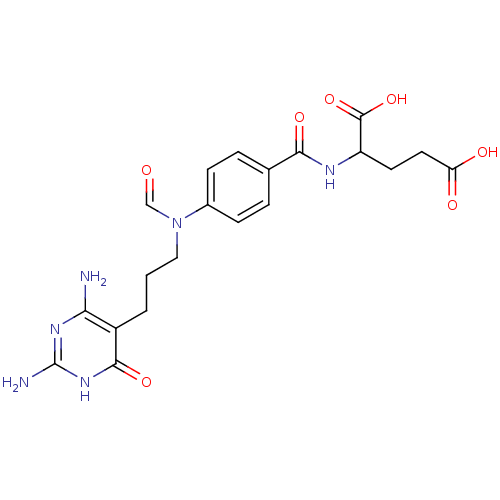 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM50122955 (2-(4,6-Difluoro-indan-1-ylidene)-acetamide | CHEMB...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against lysyl oxidase | J Med Chem 46: 399-408 (2003) Article DOI: 10.1021/jm020067s BindingDB Entry DOI: 10.7270/Q2VH5N7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014844 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase from hog liver | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against MOLT-4 | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against MOLT-4 | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Hexaglutamyl homologue inhibition activity against the AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||