

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128869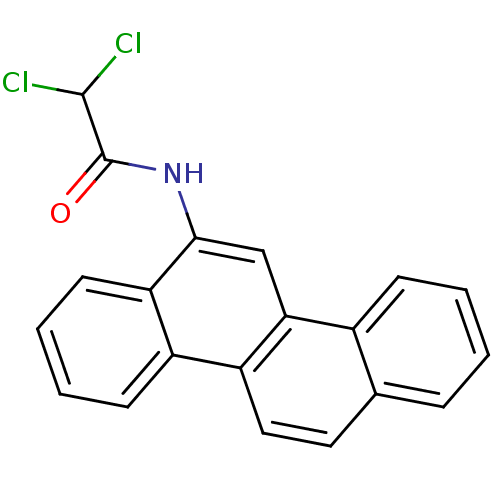 (2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128871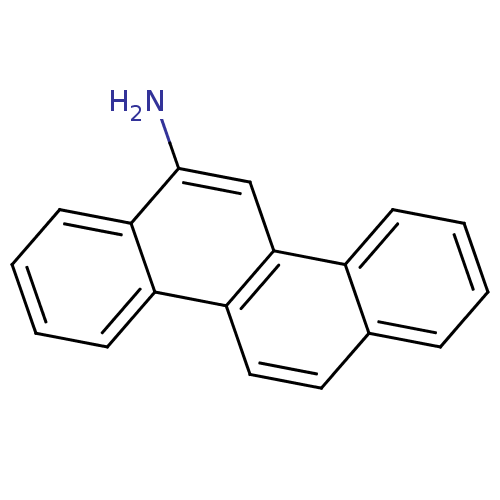 (CHEMBL313154 | Chrysen-6-ylamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128867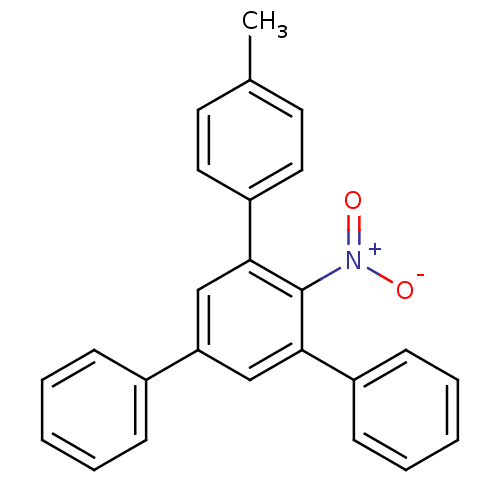 (3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128866 (2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128872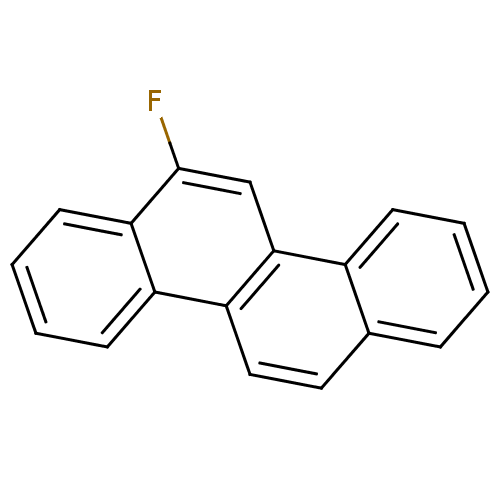 (6-Fluoro-chrysene | CHEMBL83242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128866 (2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128865 (6-Nitro-chrysene | CHEMBL82858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128870 (CHEMBL85685 | chrysene) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128867 (3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128868 (5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128867 (3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128869 (2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128869 (2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128868 (5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379241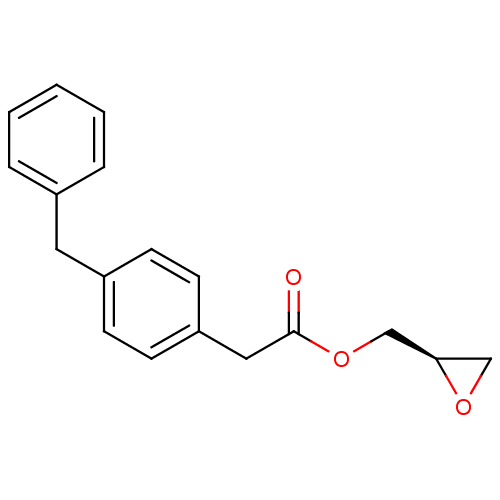 (CHEMBL2011479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant MAGL assessed as 7-hydroxycoumarin level using umbelliferyl arachidonate as substrate by luminescence... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50128868 (5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) | J Med Chem 46: 2548-50 (2003) Article DOI: 10.1021/jm030061i BindingDB Entry DOI: 10.7270/Q2CF9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379241 (CHEMBL2011479) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379241 (CHEMBL2011479) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379241 (CHEMBL2011479) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379241 (CHEMBL2011479) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379255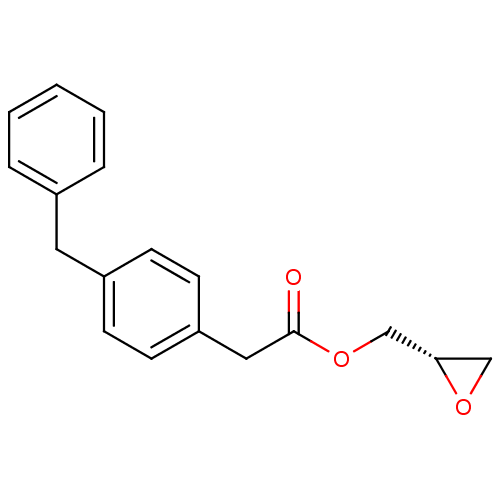 (CHEMBL2011480) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379255 (CHEMBL2011480) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379250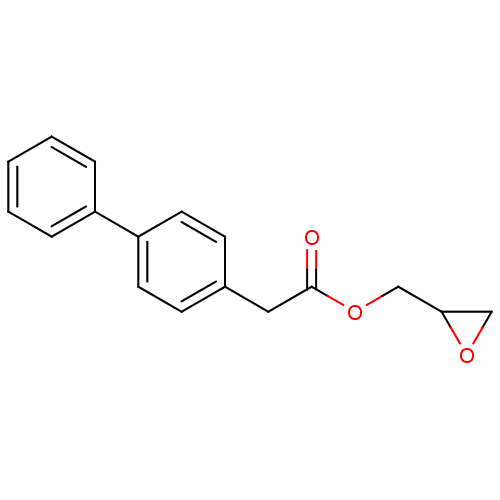 (CHEMBL2011307) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 589 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379250 (CHEMBL2011307) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379241 (CHEMBL2011479) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379241 (CHEMBL2011479) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379242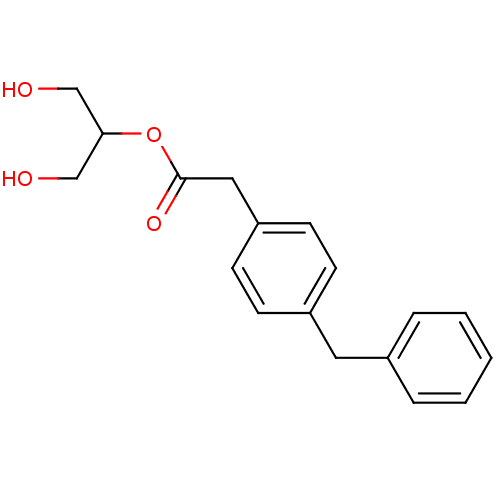 (CHEMBL2011482) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379248 (CHEMBL2011309) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379242 (CHEMBL2011482) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379248 (CHEMBL2011309) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379231 (CHEMBL2011319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379231 (CHEMBL2011319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379251 (CHEMBL2011308) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379251 (CHEMBL2011308) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 871 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379246 (CHEMBL2011481) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379246 (CHEMBL2011481) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379252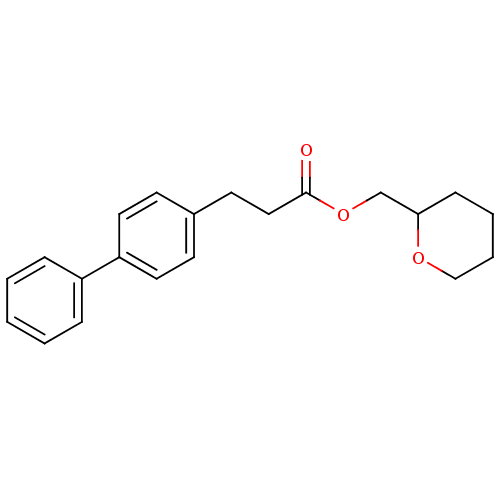 (CHEMBL2011314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379252 (CHEMBL2011314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379248 (CHEMBL2011309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379239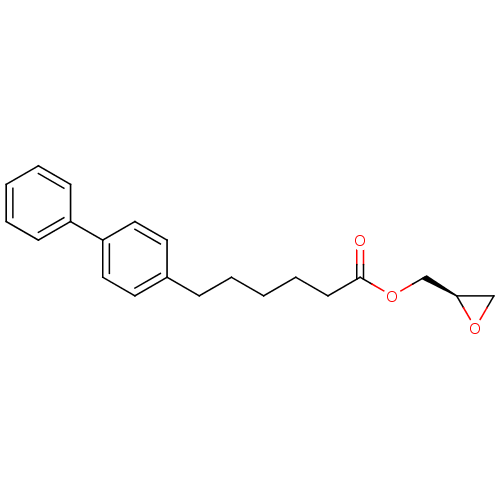 (CHEMBL2011477) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379246 (CHEMBL2011481) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379248 (CHEMBL2011309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379239 (CHEMBL2011477) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379246 (CHEMBL2011481) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50514962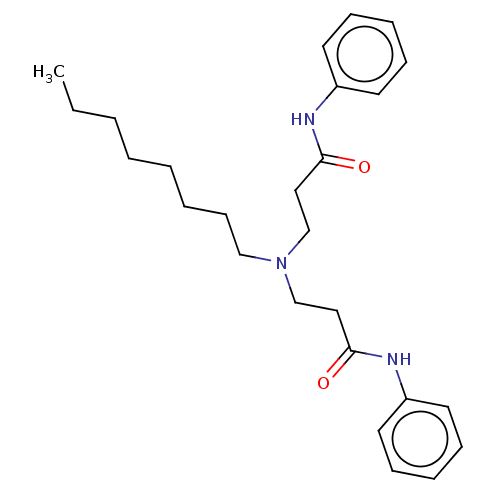 (CHEMBL4443226) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in Sf9 insect cell membranes using biotin-farnesyl-L-cysteine as substrate preincubated for 15 mins fo... | J Med Chem 62: 6035-6046 (2019) Article DOI: 10.1021/acs.jmedchem.9b00145 BindingDB Entry DOI: 10.7270/Q23X8B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379234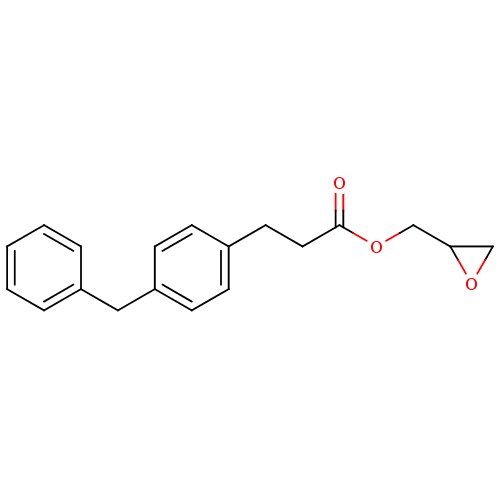 (CHEMBL2011323) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Rattus norvegicus (Rat)) | BDBM50379234 (CHEMBL2011323) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain cytosolic MAGL assessed as [3H]-2-arachidonoyl glycerol hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379241 (CHEMBL2011479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379241 (CHEMBL2011479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged MAGL expressed in Escherichia coli assessed as hydrolysis of 4-nitrophenyl acetate after 20 mins by microp... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50379247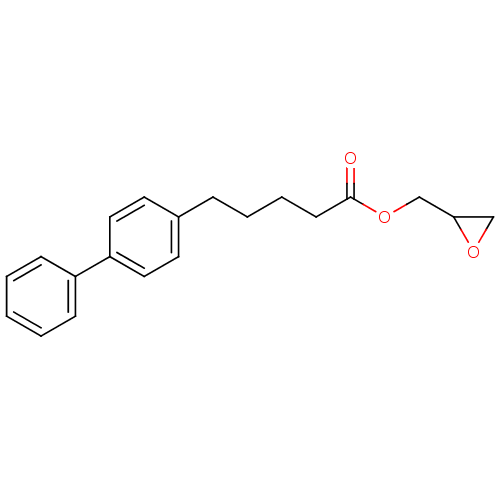 (CHEMBL2011310) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Inhibition of rat brain membrane FAAH assessed as [3H]-anandamide hydrolysis after 10 mins by liquid scintillation spectroscopy | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 180 total ) | Next | Last >> |