

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50385902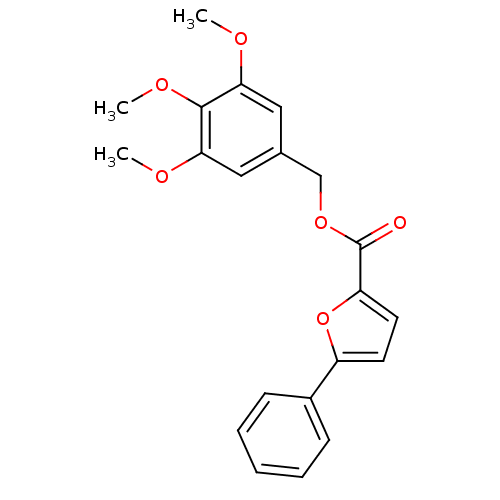 (CHEMBL2041596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PDE7A1 using 5 nM to 2 uM cAMP as substrate by Lineweaver-Burk plot analysis | J Med Chem 55: 3274-84 (2012) Article DOI: 10.1021/jm201720d BindingDB Entry DOI: 10.7270/Q2ZS2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50385903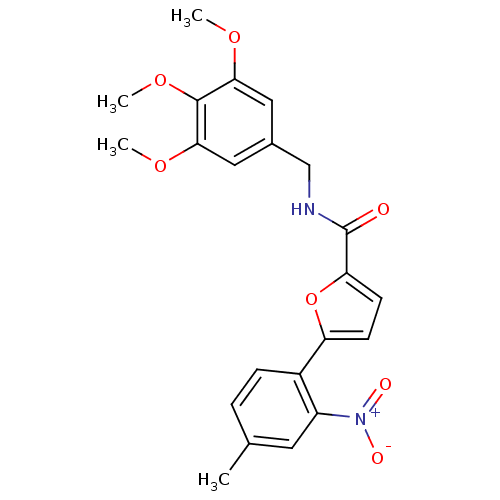 (CHEMBL2041614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PDE7A1 using 5 nM to 2 uM cAMP as substrate by Lineweaver-Burk plot analysis | J Med Chem 55: 3274-84 (2012) Article DOI: 10.1021/jm201720d BindingDB Entry DOI: 10.7270/Q2ZS2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50524502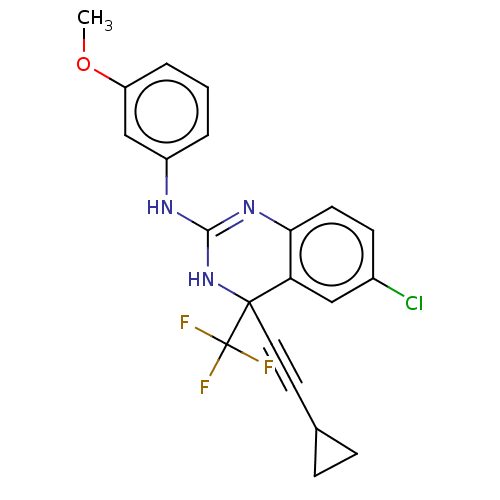 (CHEMBL4561610) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131550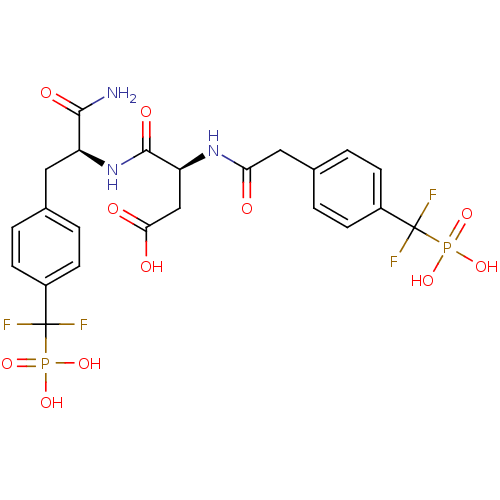 ((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 122: 756-769 (2016) Article DOI: 10.1016/j.ejmech.2016.05.060 BindingDB Entry DOI: 10.7270/Q25Q4Z2D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50524504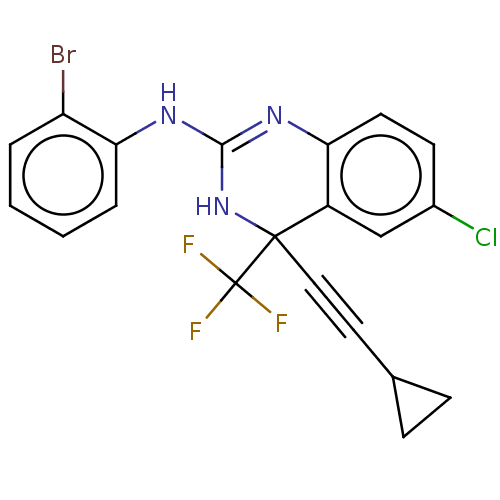 (CHEMBL4440982) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50524503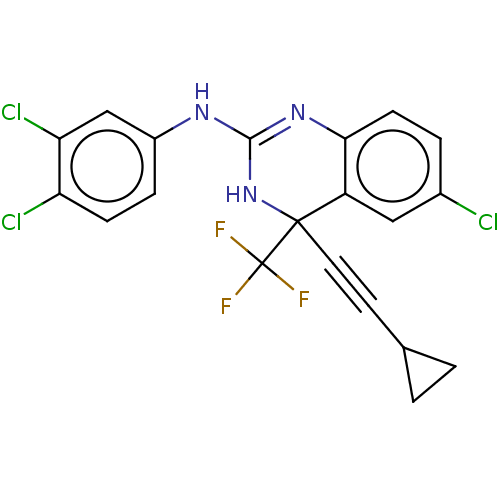 (CHEMBL4555651) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 GST-fused reverse transcriptase p66/p51 RNA-dependent DNA polymerase activity expressed in Escherichia coli ... | Bioorg Med Chem Lett 28: 3491-3495 (2018) Article DOI: 10.1016/j.bmcl.2018.10.010 BindingDB Entry DOI: 10.7270/Q24170RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50524501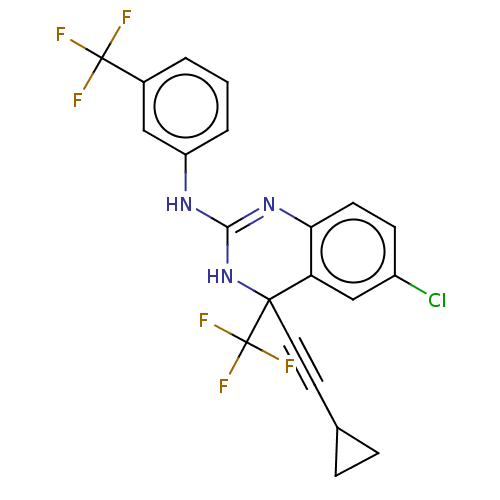 (CHEMBL4584006) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50308854 (CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 122: 756-769 (2016) Article DOI: 10.1016/j.ejmech.2016.05.060 BindingDB Entry DOI: 10.7270/Q25Q4Z2D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291987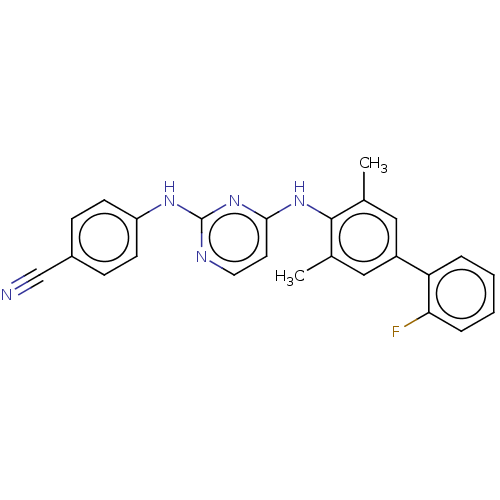 (CHEMBL4160562) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50524500 (CHEMBL4475694) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 BindingDB Entry DOI: 10.7270/Q2F47SJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291965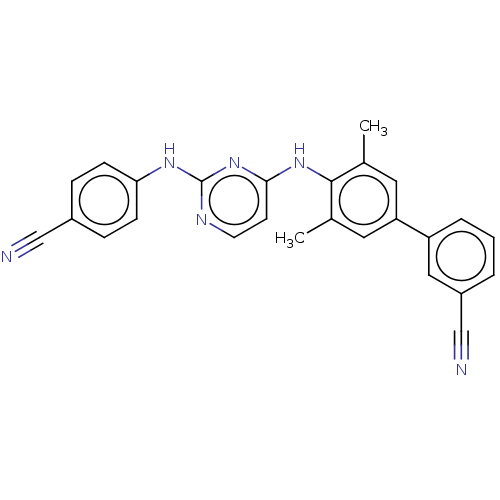 (CHEMBL4163356) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 GST-fused reverse transcriptase p66/p51 RNA-dependent DNA polymerase activity expressed in Escherichia coli ... | Bioorg Med Chem Lett 28: 3491-3495 (2018) Article DOI: 10.1016/j.bmcl.2018.10.010 BindingDB Entry DOI: 10.7270/Q24170RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291986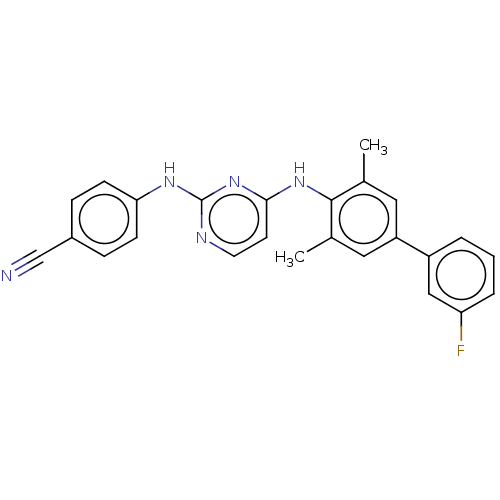 (CHEMBL4167795) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291964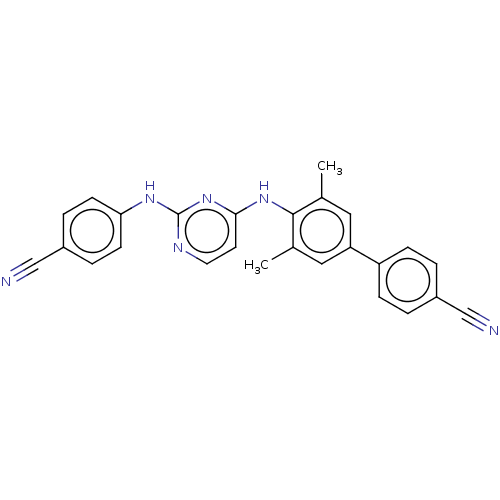 (CHEMBL4174712) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291963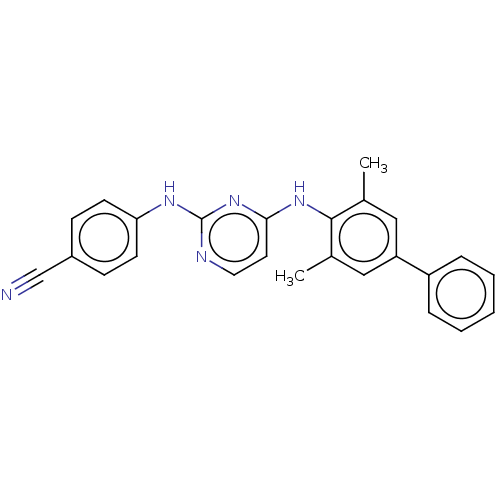 (CHEMBL4166811) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291982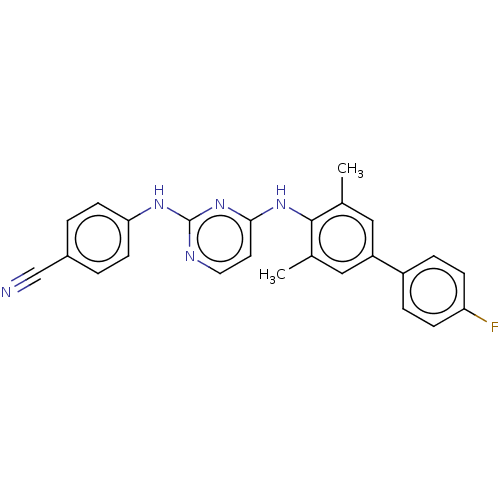 (CHEMBL4175661) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291992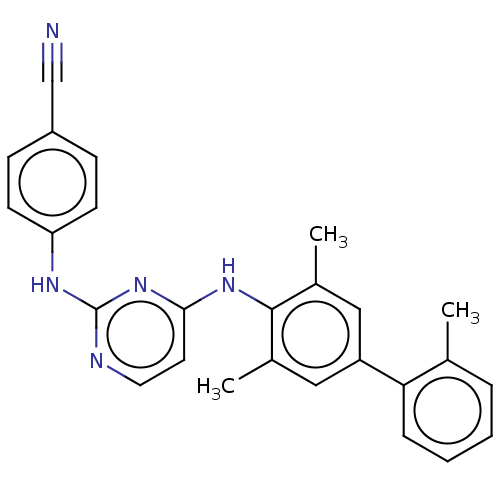 (CHEMBL4160951) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of VCAM (vascular cell adhesion molecule) adhesion to alpha4-beta1 integrin of leukocyte cells | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291988 (CHEMBL4171159) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291967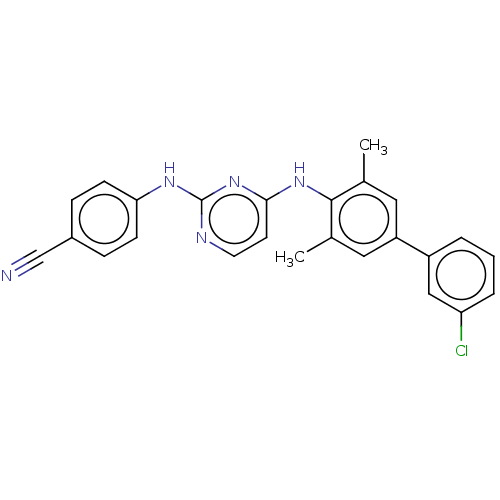 (CHEMBL4159993) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291975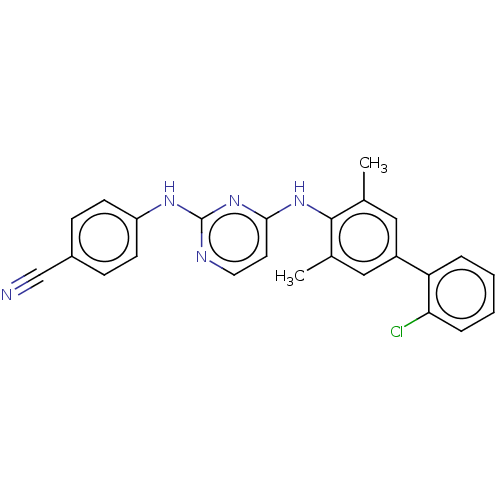 (CHEMBL4167914) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292173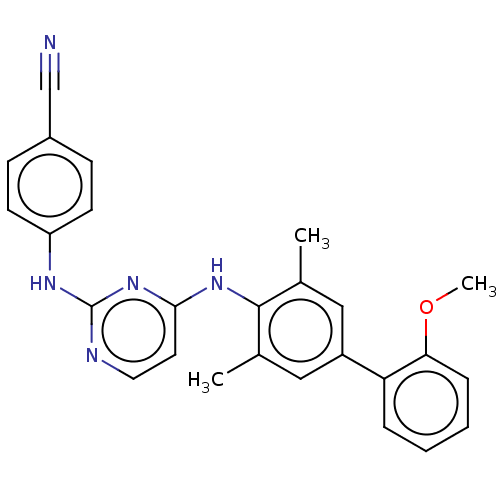 (CHEMBL4162019) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291966 (CHEMBL4171262) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292156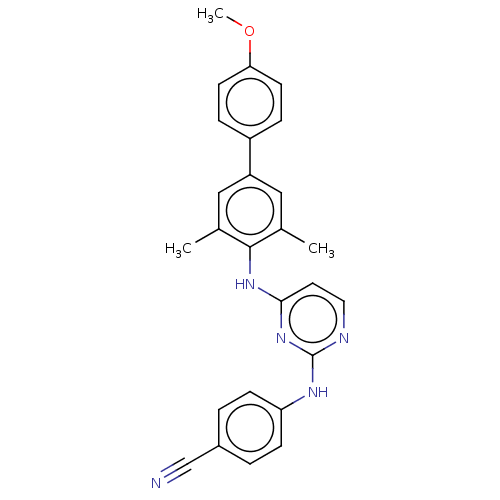 (CHEMBL4172245) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292172 (CHEMBL4164355) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50086955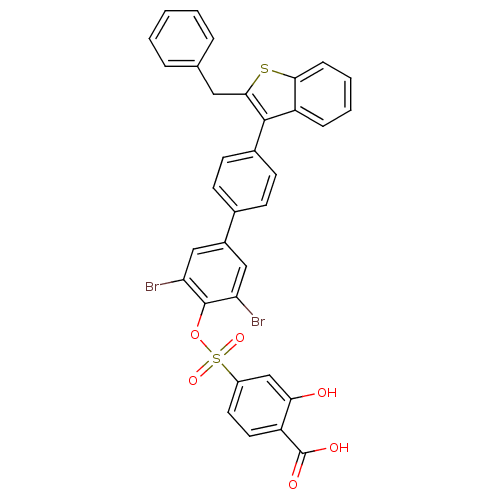 (4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibrom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 122: 756-769 (2016) Article DOI: 10.1016/j.ejmech.2016.05.060 BindingDB Entry DOI: 10.7270/Q25Q4Z2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50291991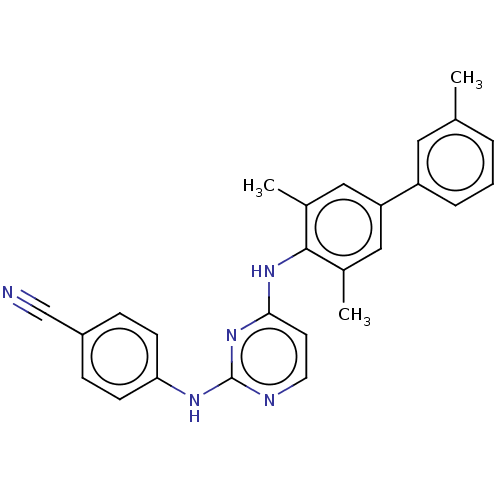 (CHEMBL4168889) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50308846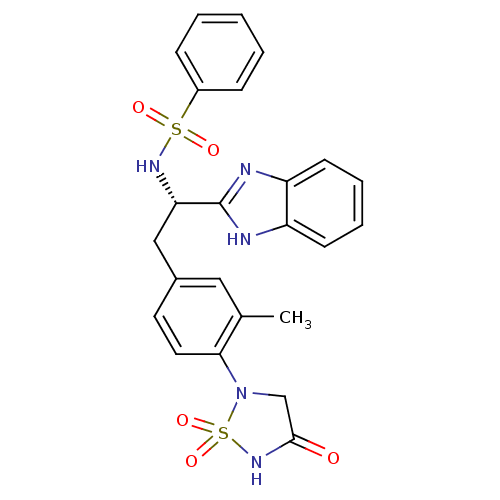 (CHEMBL592245 | N-{(S)-1-(1H-Benzoimidazol-2-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 122: 756-769 (2016) Article DOI: 10.1016/j.ejmech.2016.05.060 BindingDB Entry DOI: 10.7270/Q25Q4Z2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292234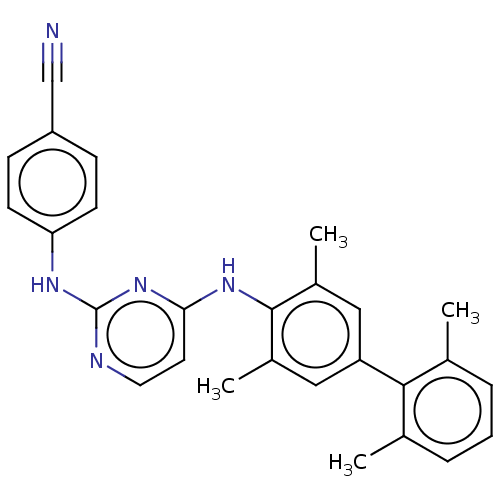 (CHEMBL4164636) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292232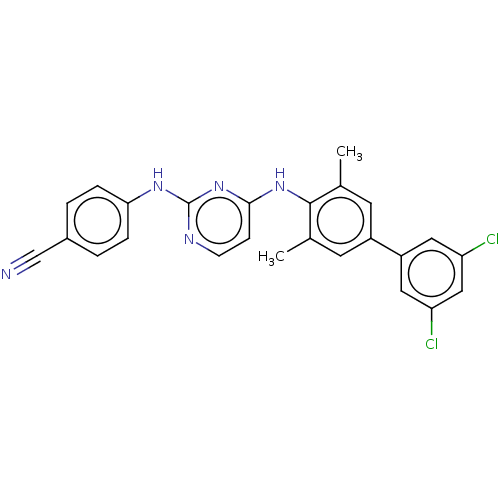 (CHEMBL4176144) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292218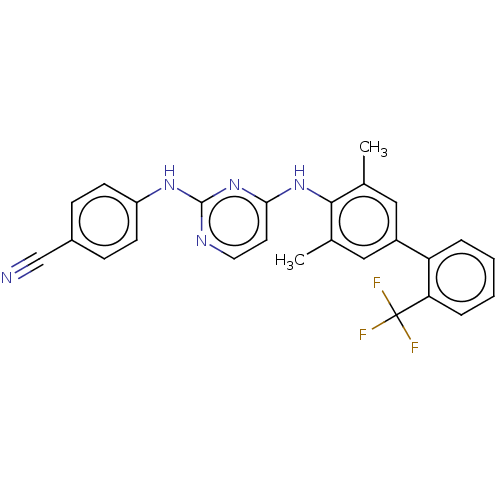 (CHEMBL4169134) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292233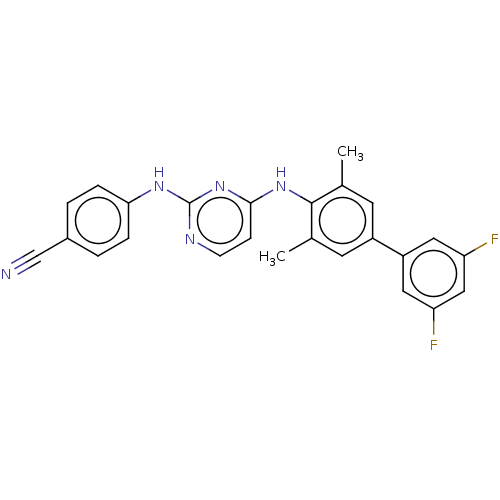 (CHEMBL4175919) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292206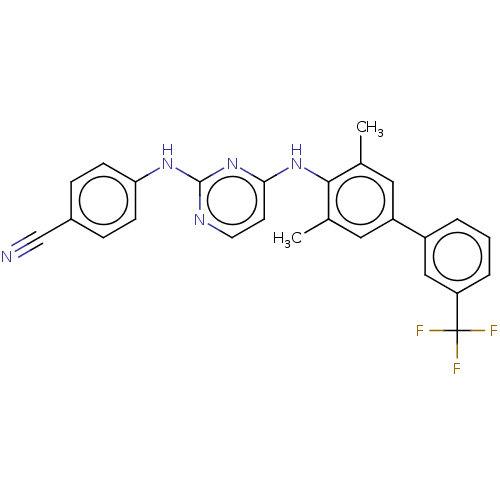 (CHEMBL4161215) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292181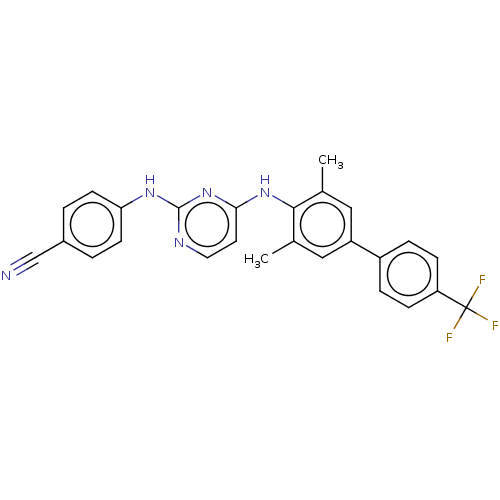 (CHEMBL4172683) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50277784 (CHEMBL484928 | N,N,2-trimethyl-5-nitrobenzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE7A1-mediated [3H]cAMP hydrolysis after 20 mins by scintillation proximity assay | J Med Chem 55: 3274-84 (2012) Article DOI: 10.1021/jm201720d BindingDB Entry DOI: 10.7270/Q2ZS2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292231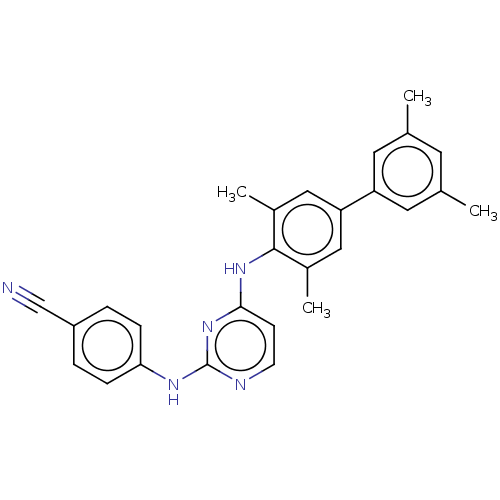 (CHEMBL4172516) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50539489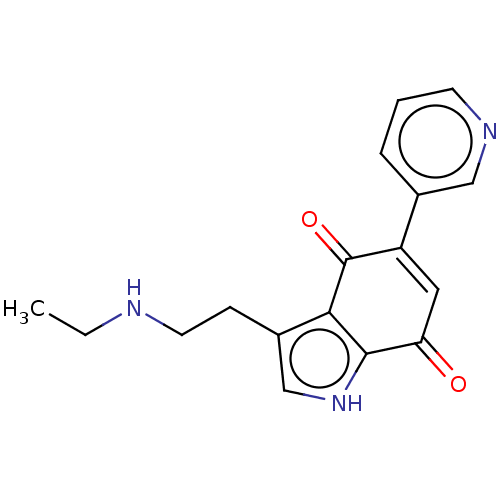 (CHEMBL4640334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 reverse transcriptase p66/p51 using poly (rA)/anoligo (dT)16 as template/primer assessed as inhibition of bi... | Eur J Med Chem 145: 726-734 (2018) Article DOI: 10.1016/j.ejmech.2018.01.016 BindingDB Entry DOI: 10.7270/Q21C20DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50201800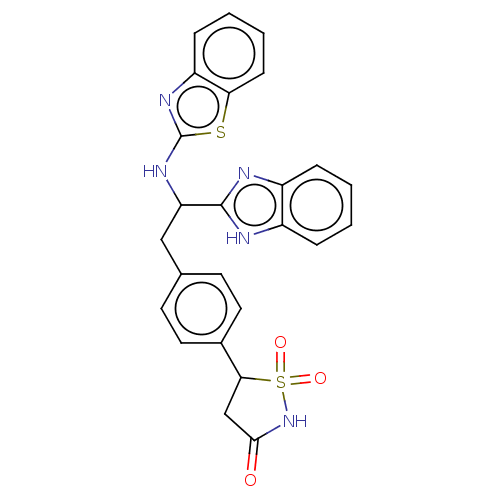 (CHEMBL3901092) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 122: 756-769 (2016) Article DOI: 10.1016/j.ejmech.2016.05.060 BindingDB Entry DOI: 10.7270/Q25Q4Z2D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 GST-fused reverse transcriptase p66/p51 RNA-dependent DNA polymerase activity expressed in Escherichia coli ... | Bioorg Med Chem Lett 28: 3491-3495 (2018) Article DOI: 10.1016/j.bmcl.2018.10.010 BindingDB Entry DOI: 10.7270/Q24170RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50209683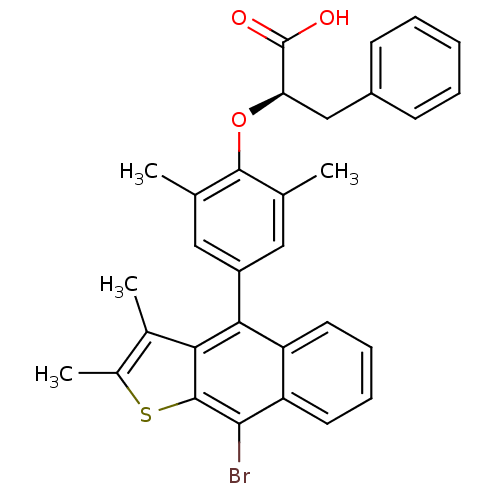 ((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) | Eur J Med Chem 122: 756-769 (2016) Article DOI: 10.1016/j.ejmech.2016.05.060 BindingDB Entry DOI: 10.7270/Q25Q4Z2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50539495 (CHEMBL4639719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50539488 (CHEMBL4646335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50539490 (CHEMBL4636464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50539492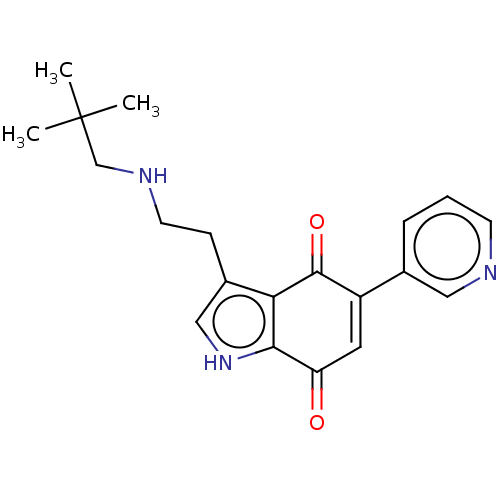 (CHEMBL4634284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50385909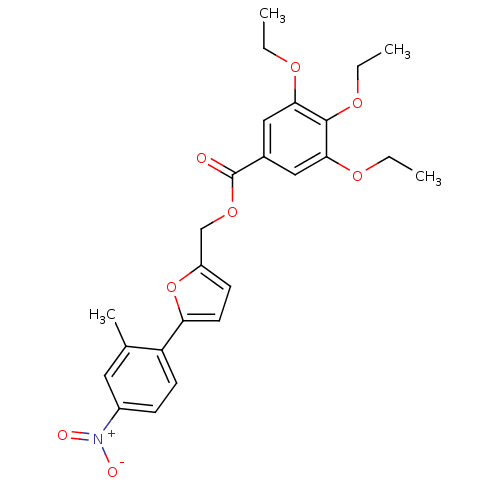 (CHEMBL2041586) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of PDE7A1 | J Med Chem 55: 3274-84 (2012) Article DOI: 10.1021/jm201720d BindingDB Entry DOI: 10.7270/Q2ZS2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 134 total ) | Next | Last >> |