

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566635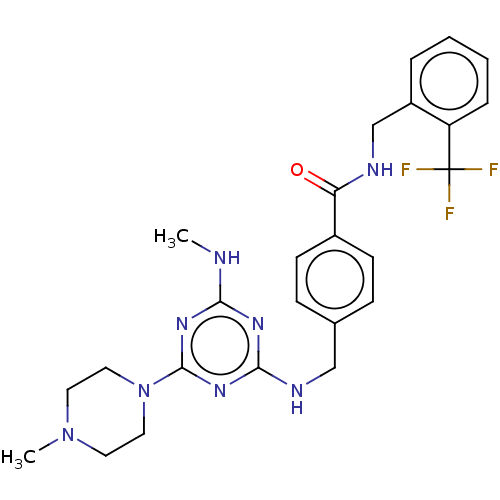 (CHEMBL4875337) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566638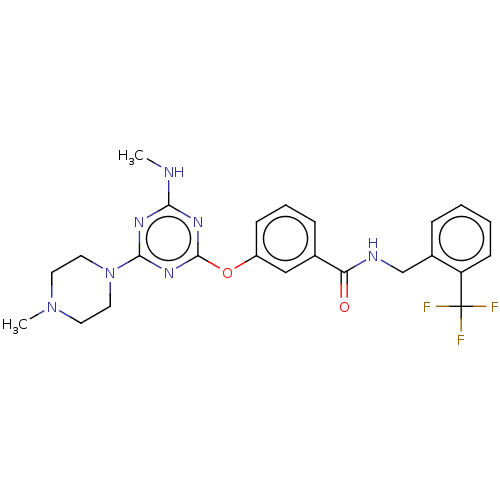 (CHEMBL4862566) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36463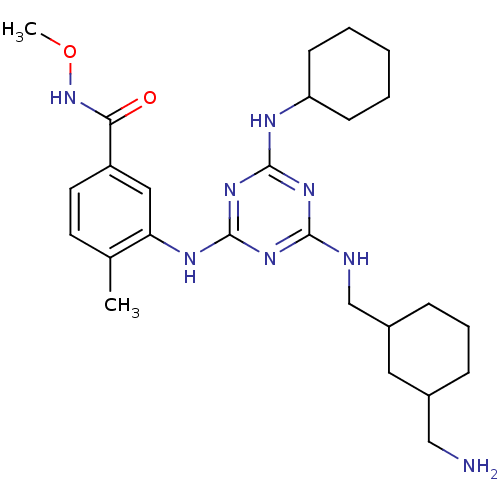 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36462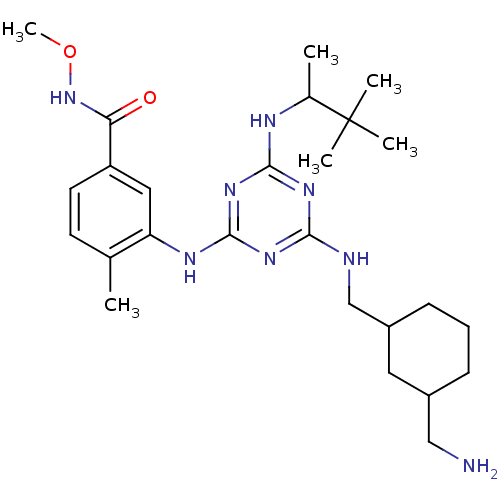 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448867 (CHEMBL3125235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448871 (CHEMBL3125101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514606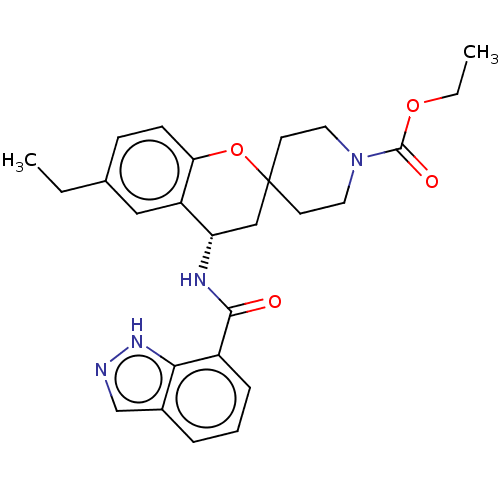 (CHEMBL4517539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448888 (CHEMBL3125253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566636 (CHEMBL4870025) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539700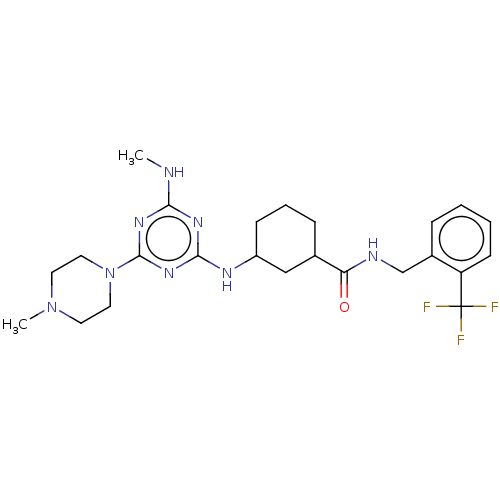 (CHEMBL4637413) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448868 (CHEMBL3125104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448875 (CHEMBL3125266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448880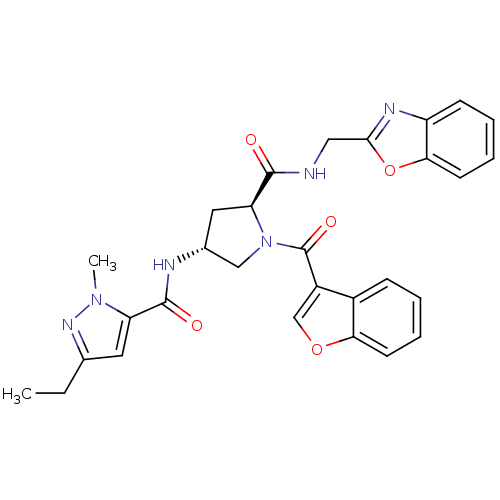 (CHEMBL3125261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448866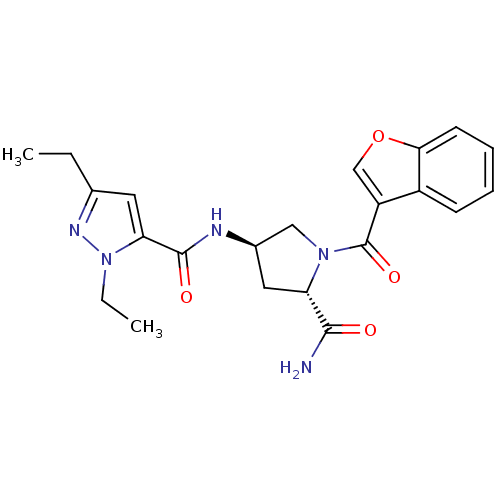 (CHEMBL3125270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448870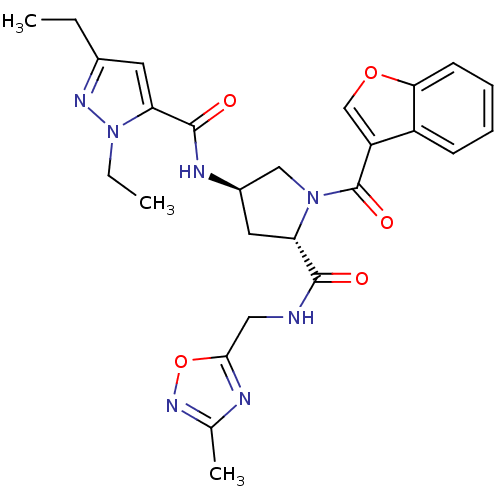 (CHEMBL3125102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448869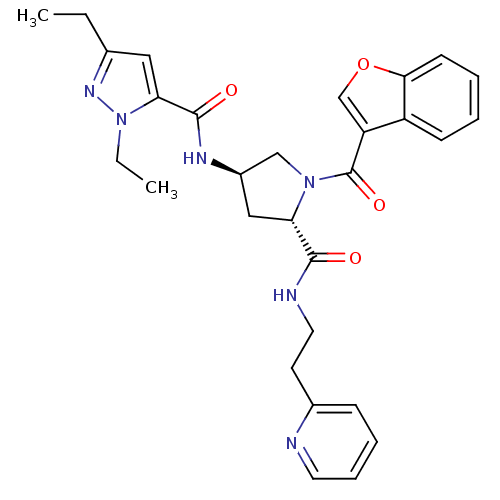 (CHEMBL3125103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448885 (CHEMBL3125256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514614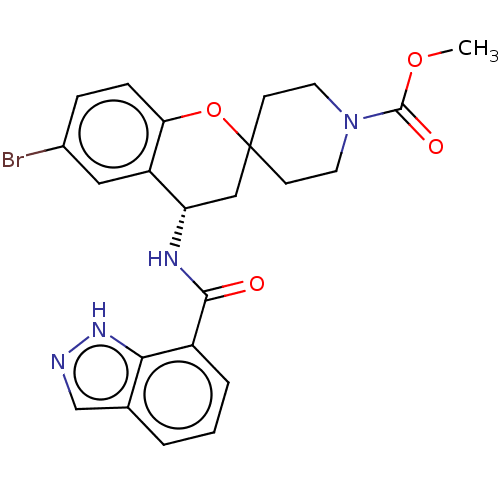 (CHEMBL4533779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514621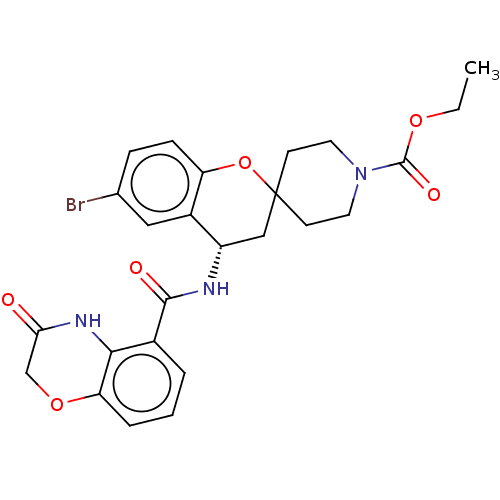 (CHEMBL4443475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514615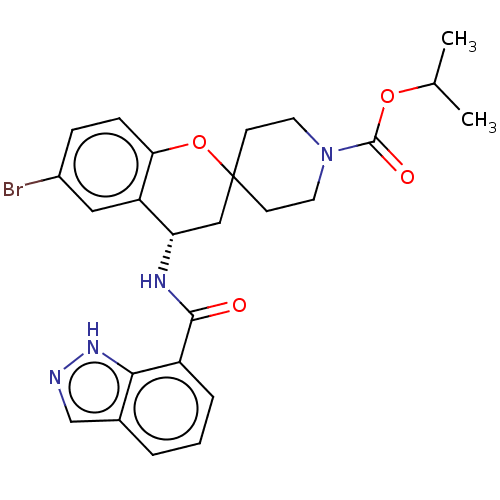 (CHEMBL4520765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448886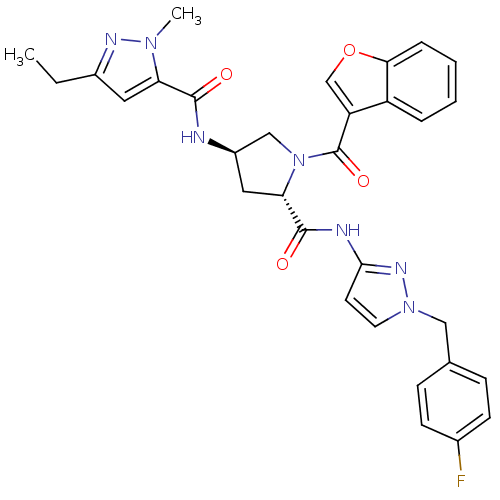 (CHEMBL3125255) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448845 (CHEMBL3125240) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448882 (CHEMBL3125259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448864 (CHEMBL3125276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514603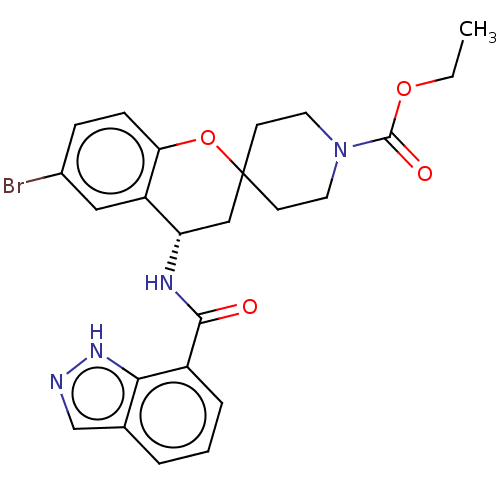 (CHEMBL4550298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514623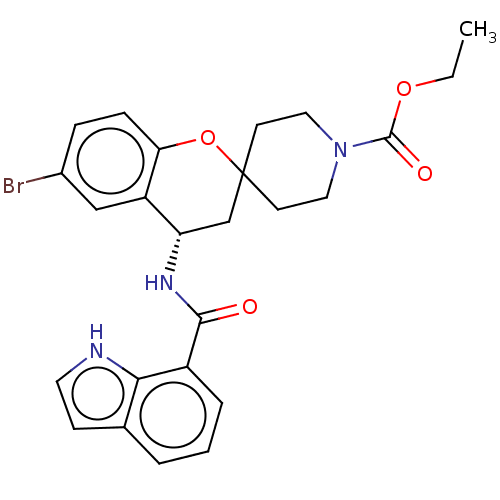 (CHEMBL4457477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566637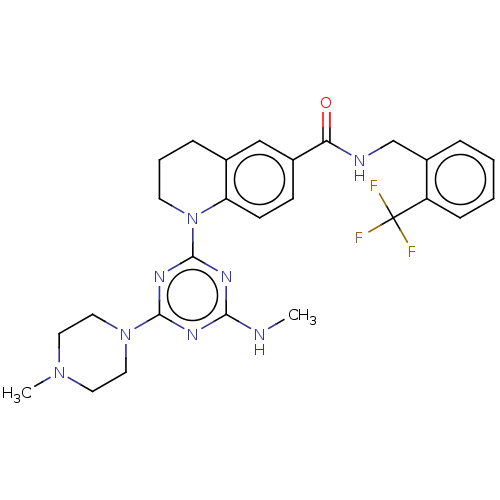 (CHEMBL4871885) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448838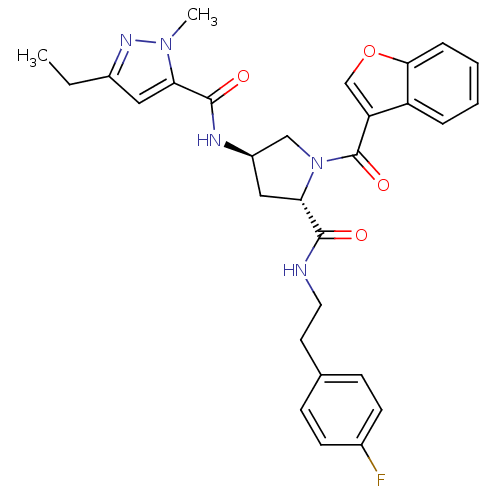 (CHEMBL3125247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122453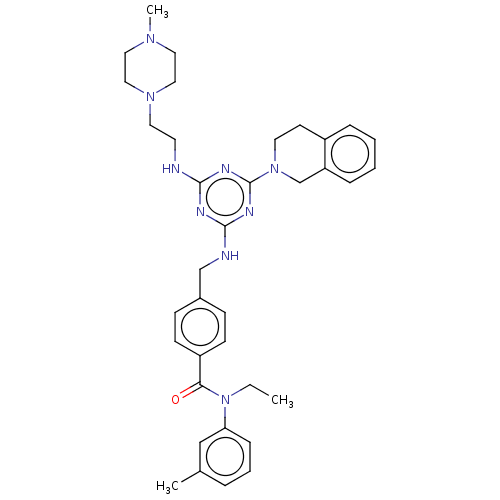 (CHEMBL3622491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514613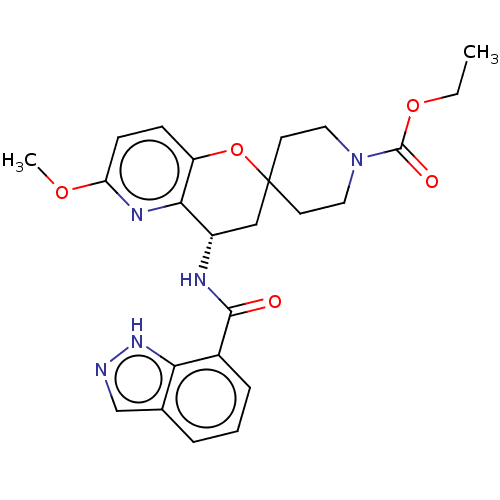 (CHEMBL4459934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448881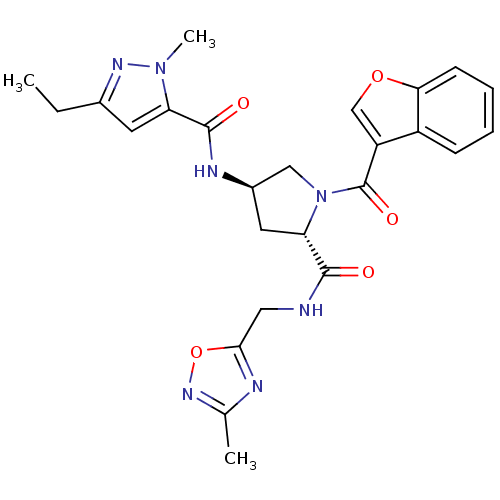 (CHEMBL3125260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448889 (CHEMBL3125252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514603 (CHEMBL4550298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122451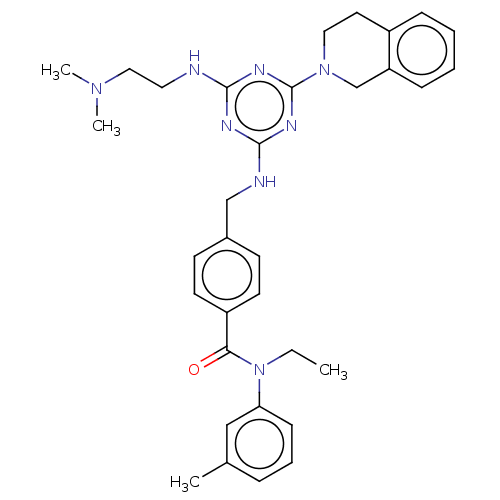 (CHEMBL3622492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514625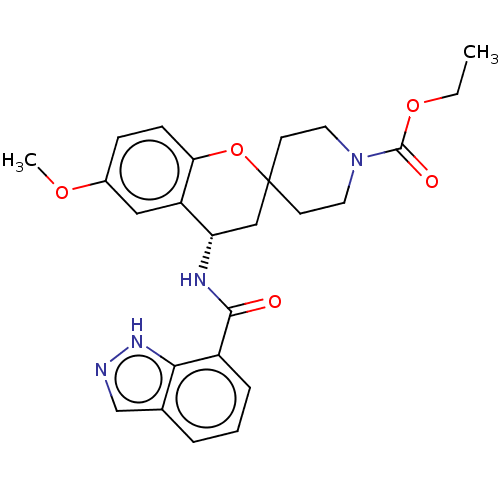 (CHEMBL4521139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566632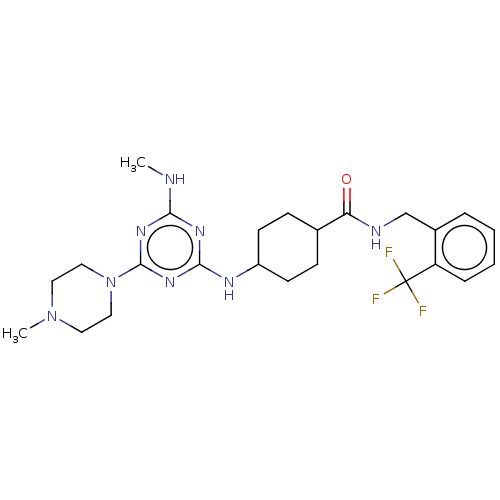 (CHEMBL4850549) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514620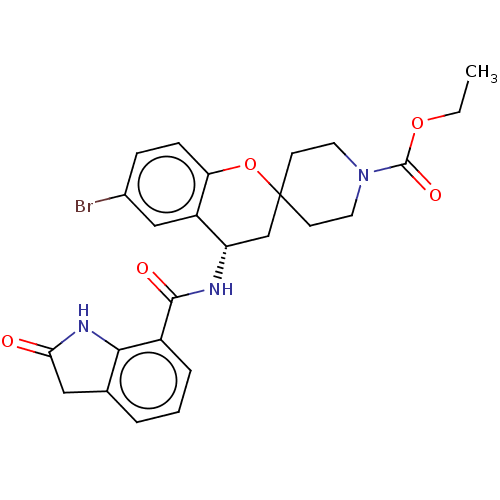 (CHEMBL4529084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448890 (CHEMBL3125251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448843 (CHEMBL3125242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122449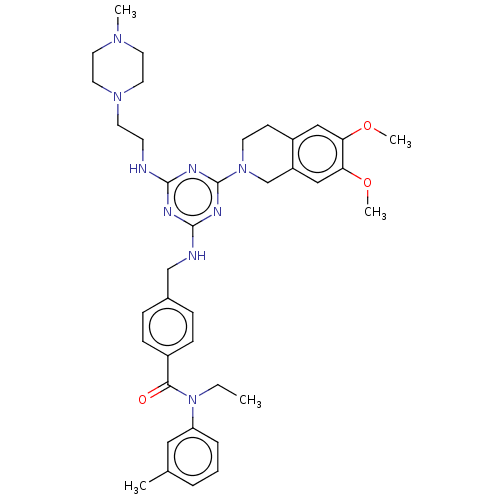 (CHEMBL3622483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448874 (CHEMBL3125267) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448841 (CHEMBL3125244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435763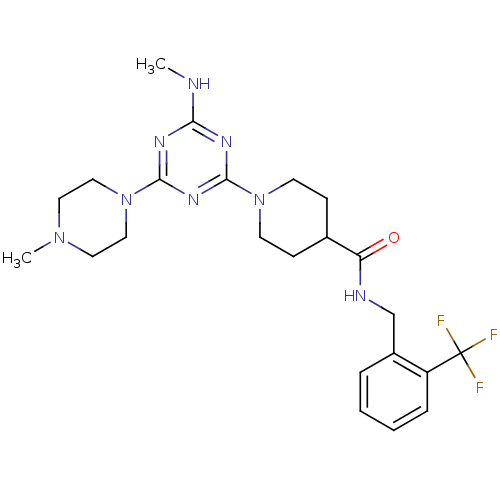 (CHEMBL2392711) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448857 (CHEMBL3125279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514630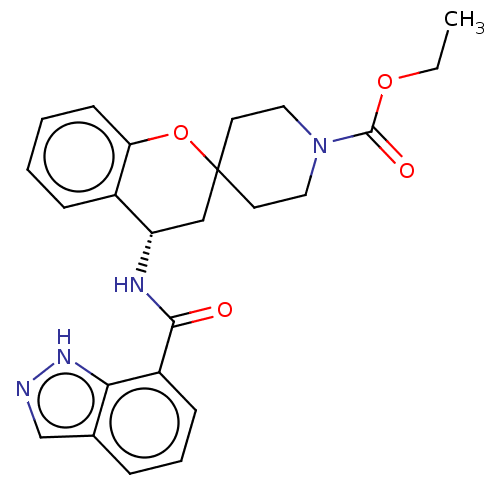 (CHEMBL4463872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514631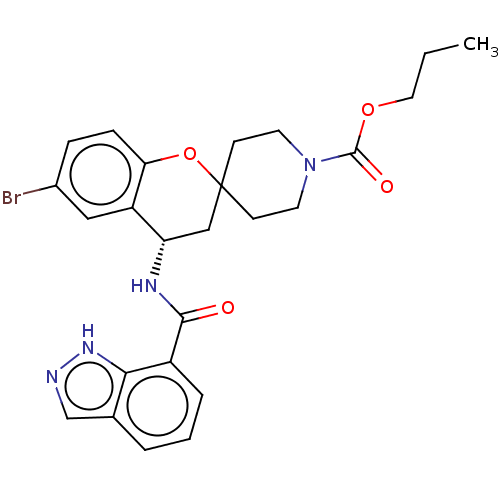 (CHEMBL4456038) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 BindingDB Entry DOI: 10.7270/Q2M33045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50122484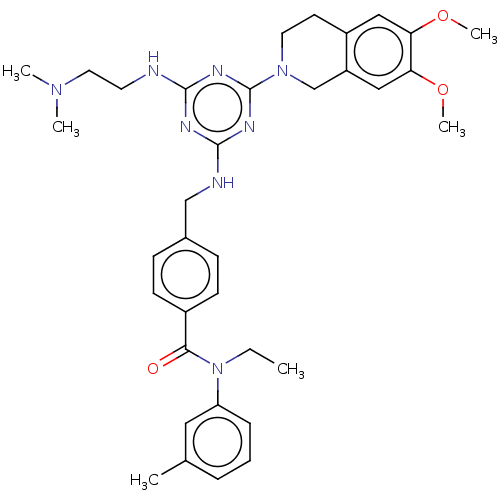 (CHEMBL3622484) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... | ACS Med Chem Lett 6: 888-93 (2015) Article DOI: 10.1021/acsmedchemlett.5b00138 BindingDB Entry DOI: 10.7270/Q2416ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448853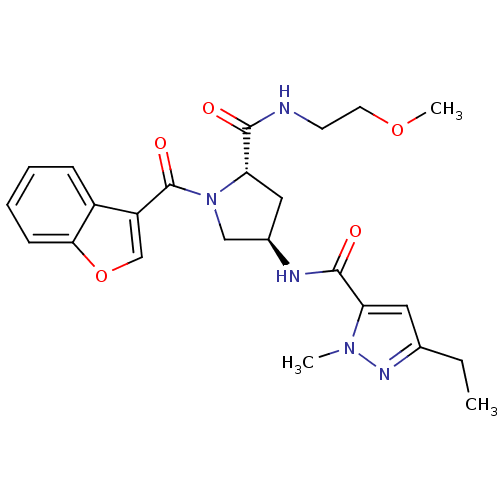 (CHEMBL3125283) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448876 (CHEMBL3125265) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36464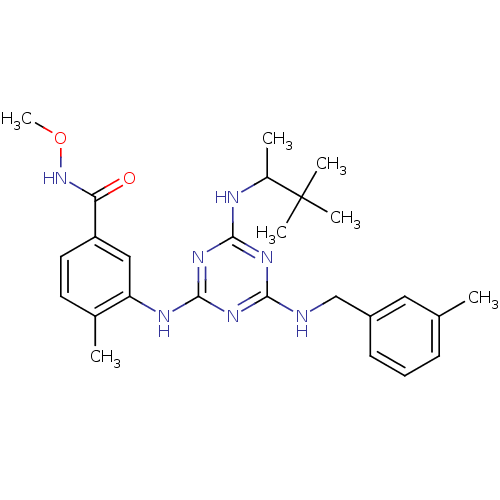 (3-(4-(3,3-Dimethylbutan-2-ylamino)-6-(3-methylbenz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 250 total ) | Next | Last >> |