 Found 140 hits with Last Name = 'michejda' and Initial = 'cj'
Found 140 hits with Last Name = 'michejda' and Initial = 'cj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21737
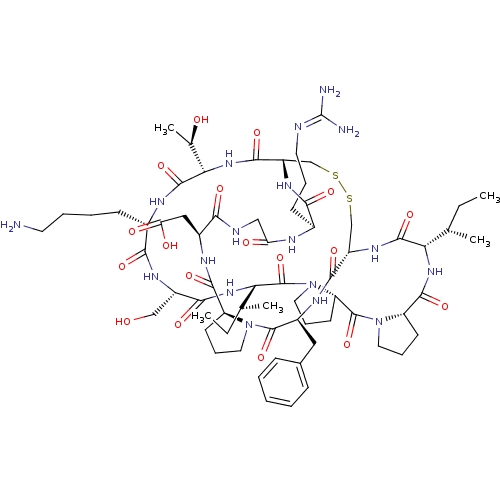
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21751
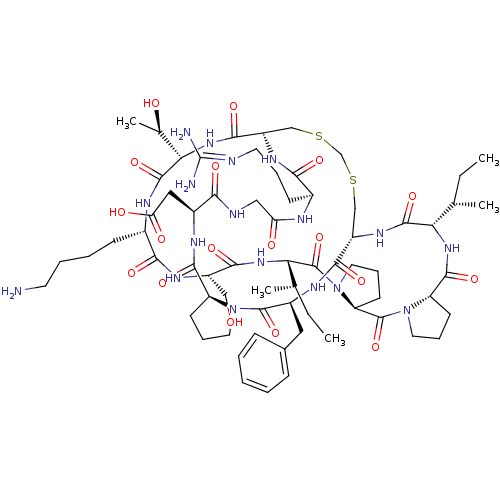
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21750
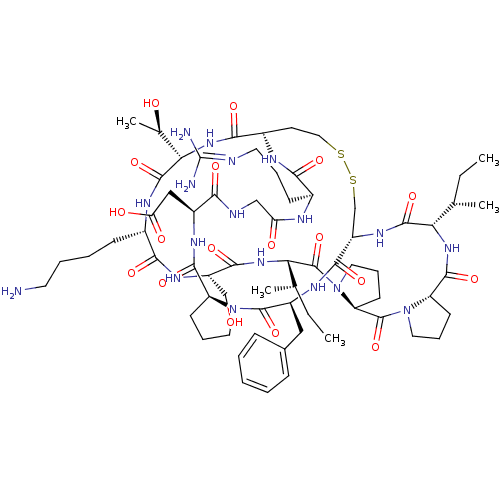
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31S,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-46-35-106-105-30-24-42(75-56(93)40(20-13-26-72-68(70)71)74-50(89)33-73-55(92)43(32-51(90)91)77-61(98)47-21-14-27-84(47)65(102)44(78-60(46)97)31-39-17-9-8-10-18-39)58(95)83-54(38(5)88)64(101)76-41(19-11-12-25-69)57(94)79-45(34-87)59(96)82-53(37(4)7-2)67(104)86-29-16-23-49(86)66(103)85-28-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,93)(H,76,101)(H,77,98)(H,78,97)(H,79,94)(H,80,100)(H,81,99)(H,82,96)(H,83,95)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | -36.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21746

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc3ccccc3c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H106N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-44(18-10-11-25-72)60(97)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-22-52(89)69(106)88-28-14-21-51(88)65(102)84-55)82-59(96)43(19-12-26-75-71(73)74)77-53(92)33-76-58(95)45(32-54(93)94)79-64(101)50-20-13-27-87(50)68(105)46(80-62(48)99)31-40-23-24-41-16-8-9-17-42(41)30-40/h8-9,16-17,23-24,30,37-39,43-52,55-57,90-91H,6-7,10-15,18-22,25-29,31-36,72H2,1-5H3,(H,76,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,97)(H,82,96)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,75)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | -35.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21749
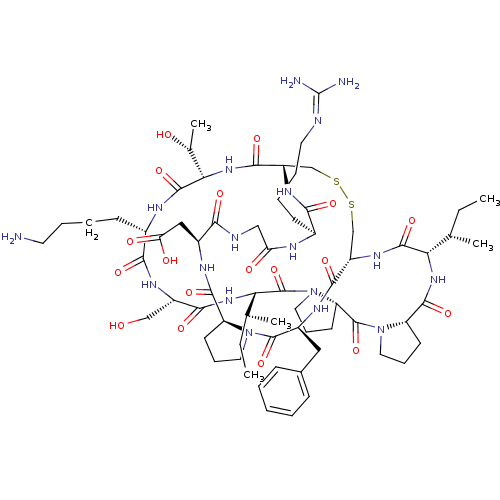
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-12-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-9-8-10-19-39)60(97)83-54(38(5)88)64(101)75-41(20-11-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8-10,18-19,36-38,40-49,52-54,87-88H,6-7,11-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 857 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21739
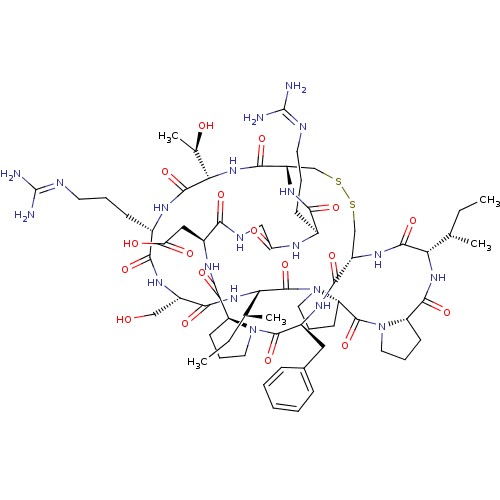
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-61(101)81-43-32-106-107-33-44(80-54(94)38(18-11-23-72-66(68)69)75-48(90)30-74-53(93)40(29-49(91)92)77-59(99)45-20-13-25-85(45)63(103)41(78-57(43)97)28-37-16-9-8-10-17-37)58(98)84-52(36(5)89)62(102)76-39(19-12-24-73-67(70)71)55(95)79-42(31-88)56(96)83-51(35(4)7-2)65(105)87-27-15-22-47(87)64(104)86-26-14-21-46(86)60(100)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,93)(H,75,90)(H,76,102)(H,77,99)(H,78,97)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,84,98)(H,91,92)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21748
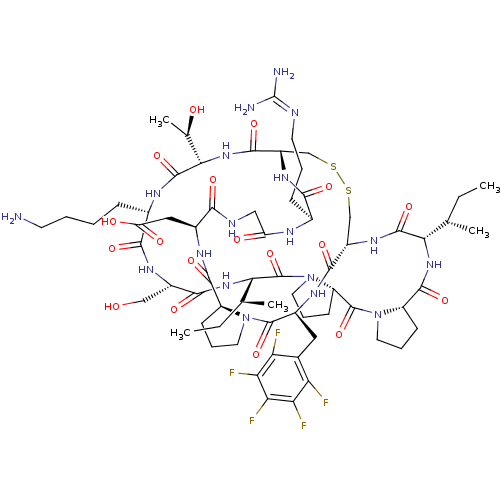
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1c(F)c(F)c(F)c(F)c1F)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H99F5N18O18S2/c1-6-30(3)51-62(104)84-39-28-109-110-29-40(59(101)87-53(32(5)92)63(105)79-35(14-8-9-19-73)56(98)82-38(27-91)57(99)86-52(31(4)7-2)66(108)90-23-13-18-43(90)65(107)89-22-12-17-42(89)61(103)85-51)83-55(97)34(15-10-20-76-67(74)75)78-44(93)26-77-54(96)36(25-45(94)95)80-60(102)41-16-11-21-88(41)64(106)37(81-58(39)100)24-33-46(68)48(70)50(72)49(71)47(33)69/h30-32,34-43,51-53,91-92H,6-29,73H2,1-5H3,(H,77,96)(H,78,93)(H,79,105)(H,80,102)(H,81,100)(H,82,98)(H,83,97)(H,84,104)(H,85,103)(H,86,99)(H,87,101)(H,94,95)(H4,74,75,76)/t30-,31-,32+,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | -34.2 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21747

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C73H108N18O18S2/c1-6-39(3)57-68(105)85-50-37-110-111-38-51(65(102)88-59(41(5)93)69(106)80-46(19-11-12-28-74)62(99)83-49(36-92)63(100)87-58(40(4)7-2)72(109)91-32-16-23-54(91)71(108)90-31-15-22-53(90)67(104)86-57)84-61(98)45(20-13-29-77-73(75)76)79-55(94)35-78-60(97)47(34-56(95)96)81-66(103)52-21-14-30-89(52)70(107)48(82-64(50)101)33-42-24-26-44(27-25-42)43-17-9-8-10-18-43/h8-10,17-18,24-27,39-41,45-54,57-59,92-93H,6-7,11-16,19-23,28-38,74H2,1-5H3,(H,78,97)(H,79,94)(H,80,106)(H,81,103)(H,82,101)(H,83,99)(H,84,98)(H,85,105)(H,86,104)(H,87,100)(H,88,102)(H,95,96)(H4,75,76,77)/t39-,40-,41+,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21745
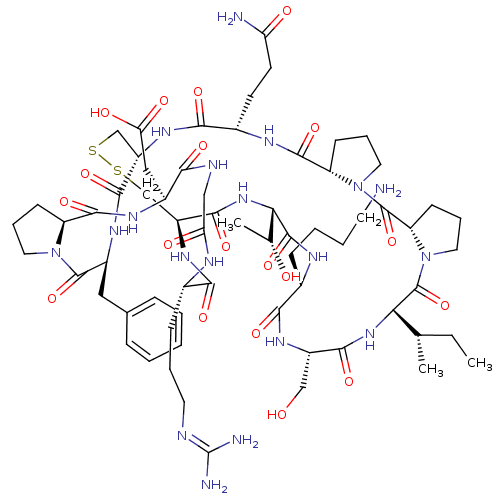
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8] |r| Show InChI InChI=1S/C66H101N19O19S2/c1-4-34(2)51-65(104)85-27-13-20-47(85)64(103)84-26-12-19-46(84)60(99)74-39(21-22-48(68)88)56(95)79-43-32-105-106-33-44(59(98)82-52(35(3)87)62(101)75-38(16-8-9-23-67)55(94)78-42(31-86)57(96)81-51)80-54(93)37(17-10-24-71-66(69)70)73-49(89)30-72-53(92)40(29-50(90)91)76-61(100)45-18-11-25-83(45)63(102)41(77-58(43)97)28-36-14-6-5-7-15-36/h5-7,14-15,34-35,37-47,51-52,86-87H,4,8-13,16-33,67H2,1-3H3,(H2,68,88)(H,72,92)(H,73,89)(H,74,99)(H,75,101)(H,76,100)(H,77,97)(H,78,94)(H,79,95)(H,80,93)(H,81,96)(H,82,98)(H,90,91)(H4,69,70,71)/t34-,35+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21739

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N20O18S2/c1-6-34(3)50-61(101)81-43-32-106-107-33-44(80-54(94)38(18-11-23-72-66(68)69)75-48(90)30-74-53(93)40(29-49(91)92)77-59(99)45-20-13-25-85(45)63(103)41(78-57(43)97)28-37-16-9-8-10-17-37)58(98)84-52(36(5)89)62(102)76-39(19-12-24-73-67(70)71)55(95)79-42(31-88)56(96)83-51(35(4)7-2)65(105)87-27-15-22-47(87)64(104)86-26-14-21-46(86)60(100)82-50/h8-10,16-17,34-36,38-47,50-52,88-89H,6-7,11-15,18-33H2,1-5H3,(H,74,93)(H,75,90)(H,76,102)(H,77,99)(H,78,97)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,84,98)(H,91,92)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.75E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21737

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21749

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-12-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-9-8-10-19-39)60(97)83-54(38(5)88)64(101)75-41(20-11-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8-10,18-19,36-38,40-49,52-54,87-88H,6-7,11-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.63E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21751

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-33-105-35-106-34-46(79-56(93)40(20-13-25-72-68(70)71)74-50(89)31-73-55(92)42(30-51(90)91)76-61(98)47-21-14-26-84(47)65(102)43(77-59(45)96)29-39-17-9-8-10-18-39)60(97)83-54(38(5)88)64(101)75-41(19-11-12-24-69)57(94)78-44(32-87)58(95)82-53(37(4)7-2)67(104)86-28-16-23-49(86)66(103)85-27-15-22-48(85)62(99)81-52/h8-10,17-18,36-38,40-49,52-54,87-88H,6-7,11-16,19-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21746

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc3ccccc3c1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H106N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-44(18-10-11-25-72)60(97)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-22-52(89)69(106)88-28-14-21-51(88)65(102)84-55)82-59(96)43(19-12-26-75-71(73)74)77-53(92)33-76-58(95)45(32-54(93)94)79-64(101)50-20-13-27-87(50)68(105)46(80-62(48)99)31-40-23-24-41-16-8-9-17-42(41)30-40/h8-9,16-17,23-24,30,37-39,43-52,55-57,90-91H,6-7,10-15,18-22,25-29,31-36,72H2,1-5H3,(H,76,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,97)(H,82,96)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,75)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.63E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21742
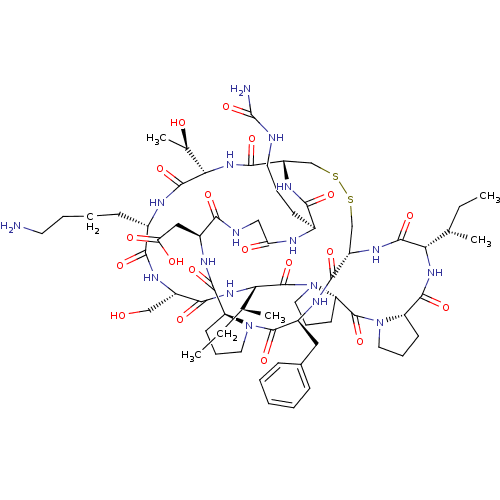
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H103N17O19S2/c1-6-35(3)51-62(98)78-44-33-104-105-34-45(77-55(91)39(20-13-25-70-67(69)103)72-49(87)31-71-54(90)41(30-50(88)89)74-60(96)46-21-14-26-82(46)64(100)42(75-58(44)94)29-38-17-9-8-10-18-38)59(95)81-53(37(5)86)63(99)73-40(19-11-12-24-68)56(92)76-43(32-85)57(93)80-52(36(4)7-2)66(102)84-28-16-23-48(84)65(101)83-27-15-22-47(83)61(97)79-51/h8-10,17-18,35-37,39-48,51-53,85-86H,6-7,11-16,19-34,68H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H3,69,70,103)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.25E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21747

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C73H108N18O18S2/c1-6-39(3)57-68(105)85-50-37-110-111-38-51(65(102)88-59(41(5)93)69(106)80-46(19-11-12-28-74)62(99)83-49(36-92)63(100)87-58(40(4)7-2)72(109)91-32-16-23-54(91)71(108)90-31-15-22-53(90)67(104)86-57)84-61(98)45(20-13-29-77-73(75)76)79-55(94)35-78-60(97)47(34-56(95)96)81-66(103)52-21-14-30-89(52)70(107)48(82-64(50)101)33-42-24-26-44(27-25-42)43-17-9-8-10-18-43/h8-10,17-18,24-27,39-41,45-54,57-59,92-93H,6-7,11-16,19-23,28-38,74H2,1-5H3,(H,78,97)(H,79,94)(H,80,106)(H,81,103)(H,82,101)(H,83,99)(H,84,98)(H,85,105)(H,86,104)(H,87,100)(H,88,102)(H,95,96)(H4,75,76,77)/t39-,40-,41+,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,57-,58-,59-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.56E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21743
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-10-8-11-19-39)60(97)83-54(38(5)88)64(101)75-41(20-12-9-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8,10-11,18-19,36-38,40-49,52-54,87-88H,6-7,9,12-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21748

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1c(F)c(F)c(F)c(F)c1F)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H99F5N18O18S2/c1-6-30(3)51-62(104)84-39-28-109-110-29-40(59(101)87-53(32(5)92)63(105)79-35(14-8-9-19-73)56(98)82-38(27-91)57(99)86-52(31(4)7-2)66(108)90-23-13-18-43(90)65(107)89-22-12-17-42(89)61(103)85-51)83-55(97)34(15-10-20-76-67(74)75)78-44(93)26-77-54(96)36(25-45(94)95)80-60(102)41-16-11-21-88(41)64(106)37(81-58(39)100)24-33-46(68)48(70)50(72)49(71)47(33)69/h30-32,34-43,51-53,91-92H,6-29,73H2,1-5H3,(H,77,96)(H,78,93)(H,79,105)(H,80,102)(H,81,100)(H,82,98)(H,83,97)(H,84,104)(H,85,103)(H,86,99)(H,87,101)(H,94,95)(H4,74,75,76)/t30-,31-,32+,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.36E+4 | -25.3 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21744
(2-[(1S,7S,10S,13S,16S,22S,25S,31S,34S,37S,40S,43S,...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H106N18O20/c1-6-35(3)51-62(101)79-43(32-86)57(96)76-42(29-38-17-9-8-10-18-38)64(103)83-26-14-21-46(83)60(99)75-41(30-50(91)92)54(93)72-31-49(90)73-39(20-13-25-71-67(69)70)55(94)77-45(34-88)59(98)82-53(37(5)89)63(102)74-40(19-11-12-24-68)56(95)78-44(33-87)58(97)81-52(36(4)7-2)66(105)85-28-16-23-48(85)65(104)84-27-15-22-47(84)61(100)80-51/h8-10,17-18,35-37,39-48,51-53,86-89H,6-7,11-16,19-34,68H2,1-5H3,(H,72,93)(H,73,90)(H,74,102)(H,75,99)(H,76,96)(H,77,94)(H,78,95)(H,79,101)(H,80,100)(H,81,97)(H,82,98)(H,91,92)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.04E+4 | -24.8 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21743

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C68H106N18O18S2/c1-6-36(3)52-63(100)80-45-34-105-106-35-46(79-56(93)40(21-14-26-72-68(70)71)74-50(89)32-73-55(92)42(31-51(90)91)76-61(98)47-22-15-27-84(47)65(102)43(77-59(45)96)30-39-18-10-8-11-19-39)60(97)83-54(38(5)88)64(101)75-41(20-12-9-13-25-69)57(94)78-44(33-87)58(95)82-53(37(4)7-2)67(104)86-29-17-24-49(86)66(103)85-28-16-23-48(85)62(99)81-52/h8,10-11,18-19,36-38,40-49,52-54,87-88H,6-7,9,12-17,20-35,69H2,1-5H3,(H,73,92)(H,74,89)(H,75,101)(H,76,98)(H,77,96)(H,78,94)(H,79,93)(H,80,100)(H,81,99)(H,82,95)(H,83,97)(H,90,91)(H4,70,71,72)/t36-,37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.56E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21741

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H104N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-43(18-11-12-26-72)59(96)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-21-52(89)69(106)88-28-14-20-51(88)65(102)84-55)82-60(97)44(30-41-22-24-42(25-23-41)76-71(73)74)77-53(92)33-75-58(95)45(32-54(93)94)79-64(101)50-19-13-27-87(50)68(105)46(80-62(48)99)31-40-16-9-8-10-17-40/h8-10,16-17,22-25,37-39,43-52,55-57,90-91H,6-7,11-15,18-21,26-36,72H2,1-5H3,(H,75,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,96)(H,82,97)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,76)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | -22.9 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21744

(2-[(1S,7S,10S,13S,16S,22S,25S,31S,34S,37S,40S,43S,...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C67H106N18O20/c1-6-35(3)51-62(101)79-43(32-86)57(96)76-42(29-38-17-9-8-10-18-38)64(103)83-26-14-21-46(83)60(99)75-41(30-50(91)92)54(93)72-31-49(90)73-39(20-13-25-71-67(69)70)55(94)77-45(34-88)59(98)82-53(37(5)89)63(102)74-40(19-11-12-24-68)56(95)78-44(33-87)58(97)81-52(36(4)7-2)66(105)85-28-16-23-48(85)65(104)84-27-15-22-47(84)61(100)80-51/h8-10,17-18,35-37,39-48,51-53,86-89H,6-7,11-16,19-34,68H2,1-5H3,(H,72,93)(H,73,90)(H,74,102)(H,75,99)(H,76,96)(H,77,94)(H,78,95)(H,79,101)(H,80,100)(H,81,97)(H,82,98)(H,91,92)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+5 | -22.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21741

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C71H104N18O18S2/c1-6-37(3)55-66(103)83-48-35-108-109-36-49(63(100)86-57(39(5)91)67(104)78-43(18-11-12-26-72)59(96)81-47(34-90)61(98)85-56(38(4)7-2)70(107)89-29-15-21-52(89)69(106)88-28-14-20-51(88)65(102)84-55)82-60(97)44(30-41-22-24-42(25-23-41)76-71(73)74)77-53(92)33-75-58(95)45(32-54(93)94)79-64(101)50-19-13-27-87(50)68(105)46(80-62(48)99)31-40-16-9-8-10-17-40/h8-10,16-17,22-25,37-39,43-52,55-57,90-91H,6-7,11-15,18-21,26-36,72H2,1-5H3,(H,75,95)(H,77,92)(H,78,104)(H,79,101)(H,80,99)(H,81,96)(H,82,97)(H,83,103)(H,84,102)(H,85,98)(H,86,100)(H,93,94)(H4,73,74,76)/t37-,38-,39+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,55-,56-,57-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM21740
(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] |r| Show InChI InChI=1S/C66H102N18O18S2/c1-6-34(3)50-61(98)78-43-32-103-104-33-44(77-54(91)38(19-12-24-70-66(68)69)72-48(87)30-71-53(90)40(29-49(88)89)74-59(96)45-20-13-25-82(45)63(100)41(75-57(43)94)28-37-16-9-8-10-17-37)58(95)81-52(36(5)86)62(99)73-39(18-11-23-67)55(92)76-42(31-85)56(93)80-51(35(4)7-2)65(102)84-27-15-22-47(84)64(101)83-26-14-21-46(83)60(97)79-50/h8-10,16-17,34-36,38-47,50-52,85-86H,6-7,11-15,18-33,67H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H4,68,69,70)/t34-,35-,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,50-,51-,52-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+5 | >-20.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21742

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H103N17O19S2/c1-6-35(3)51-62(98)78-44-33-104-105-34-45(77-55(91)39(20-13-25-70-67(69)103)72-49(87)31-71-54(90)41(30-50(88)89)74-60(96)46-21-14-26-82(46)64(100)42(75-58(44)94)29-38-17-9-8-10-18-38)59(95)81-53(37(5)86)63(99)73-40(19-11-12-24-68)56(92)76-43(32-85)57(93)80-52(36(4)7-2)66(102)84-28-16-23-48(84)65(101)83-27-15-22-47(83)61(97)79-51/h8-10,17-18,35-37,39-48,51-53,85-86H,6-7,11-16,19-34,68H2,1-5H3,(H,71,90)(H,72,87)(H,73,99)(H,74,96)(H,75,94)(H,76,92)(H,77,91)(H,78,98)(H,79,97)(H,80,93)(H,81,95)(H,88,89)(H3,69,70,103)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.27E+5 | -17.4 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM21745

(2-[(1R,4S,7S,13S,19S,22S,25S,28S,31R,34S,40S,43S,4...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6](=O)-[#6@@H]-3-[#6]-[#6]-[#6]-[#7]-3-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8] |r| Show InChI InChI=1S/C66H101N19O19S2/c1-4-34(2)51-65(104)85-27-13-20-47(85)64(103)84-26-12-19-46(84)60(99)74-39(21-22-48(68)88)56(95)79-43-32-105-106-33-44(59(98)82-52(35(3)87)62(101)75-38(16-8-9-23-67)55(94)78-42(31-86)57(96)81-51)80-54(93)37(17-10-24-71-66(69)70)73-49(89)30-72-53(92)40(29-50(90)91)76-61(100)45-18-11-25-83(45)63(102)41(77-58(43)97)28-36-14-6-5-7-15-36/h5-7,14-15,34-35,37-47,51-52,86-87H,4,8-13,16-33,67H2,1-3H3,(H2,68,88)(H,72,92)(H,73,89)(H,74,99)(H,75,101)(H,76,100)(H,77,97)(H,78,94)(H,79,95)(H,80,93)(H,81,96)(H,82,98)(H,90,91)(H4,69,70,71)/t34-,35+,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+6 | >-14.7 | n/a | n/a | n/a | n/a | n/a | 8.5 | 22 |
Georgetown University Medical Center
| Assay Description
The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ... |
J Med Chem 50: 5976-83 (2007)
Article DOI: 10.1021/jm0704898
BindingDB Entry DOI: 10.7270/Q2S75DNJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17667
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES COc1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,7.81,;1.21,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C21H14F4N2O/c1-28-18-10-4-9-17-20(18)26-21(19-15(24)7-3-8-16(19)25)27(17)11-12-13(22)5-2-6-14(12)23/h2-10H,11H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17666
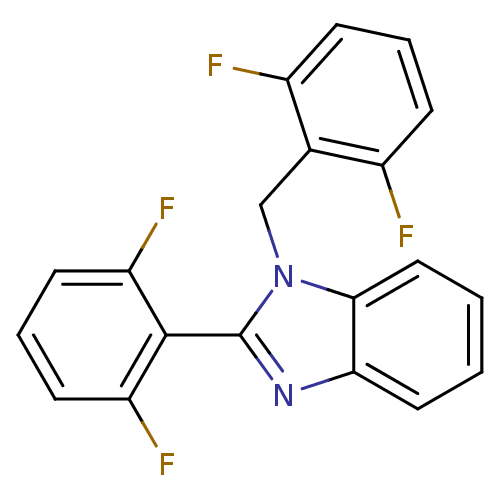
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES Fc1cccc(F)c1Cn1c(nc2ccccc12)-c1c(F)cccc1F |(-3.45,3.05,;-4.22,1.72,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;-3.45,.39,;-1.91,.34,;-1.14,1.68,;.39,1.52,;1.02,2.92,;-.12,3.95,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C20H12F4N2/c21-13-5-3-6-14(22)12(13)11-26-18-10-2-1-9-17(18)25-20(26)19-15(23)7-4-8-16(19)24/h1-10H,11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17665

(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES Cc1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C21H14F4N2/c1-12-5-2-10-18-20(12)26-21(19-16(24)8-4-9-17(19)25)27(18)11-13-14(22)6-3-7-15(13)23/h2-10H,11H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17672
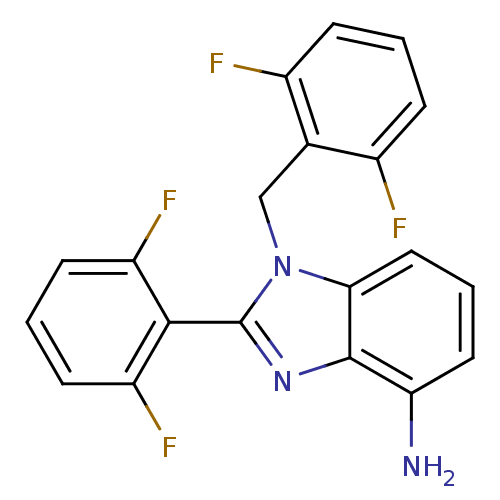
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES Nc1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C20H13F4N3/c21-12-4-1-5-13(22)11(12)10-27-17-9-3-8-16(25)19(17)26-20(27)18-14(23)6-2-7-15(18)24/h1-9H,10,25H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17668
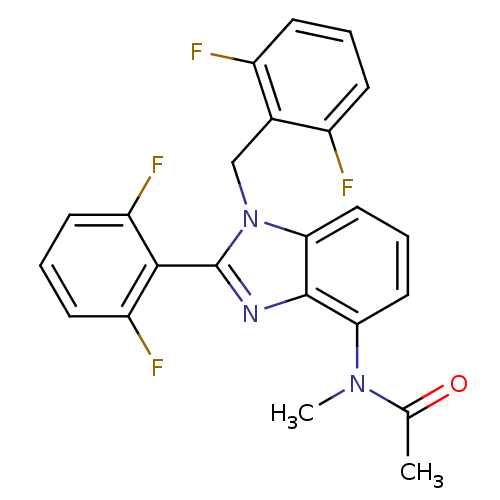
(CHEMBL58494 | N-[2-(2,6-difluorophenyl)-1-[(2,6-di...)Show SMILES CN(C(C)=O)c1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,7.81,;1.21,6.27,;2.54,5.49,;3.88,6.26,;2.54,3.95,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C23H17F4N3O/c1-13(31)29(2)19-10-5-11-20-22(19)28-23(21-17(26)8-4-9-18(21)27)30(20)12-14-15(24)6-3-7-16(14)25/h3-11H,12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM10245
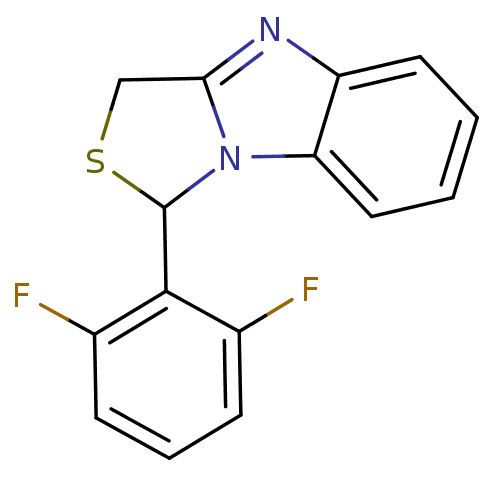
(3-(2,6-difluorophenyl)-4-thia-2,7-diazatricyclo[6....)Show InChI InChI=1S/C15H10F2N2S/c16-9-4-3-5-10(17)14(9)15-19-12-7-2-1-6-11(12)18-13(19)8-20-15/h1-7,15H,8H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Cancer Research and Development Center
Curated by ChEMBL
| Assay Description
The quantity of compound required to reduce WT Reverse transcriptase activity by 50% |
J Med Chem 40: 4199-207 (1998)
Article DOI: 10.1021/jm970096g
BindingDB Entry DOI: 10.7270/Q25B01KN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50061574
(1-Benzyl-2-(2,6-difluoro-phenyl)-4-methyl-1H-benzo...)Show SMILES Cc1cccc2n(Cc3ccccc3)c(nc12)-c1c(F)cccc1F |(7.92,-7.8,;7.93,-9.34,;6.6,-10.13,;6.6,-11.67,;7.93,-12.44,;9.27,-11.65,;10.74,-12.12,;11.23,-13.59,;10.2,-14.73,;8.7,-14.42,;7.68,-15.57,;8.17,-17.03,;9.68,-17.34,;10.69,-16.19,;11.65,-10.87,;10.73,-9.62,;9.27,-10.11,;13.18,-10.86,;13.96,-12.19,;13.19,-13.53,;15.5,-12.19,;16.26,-10.85,;15.48,-9.5,;13.94,-9.53,;13.16,-8.2,)| Show InChI InChI=1S/C21H16F2N2/c1-14-7-5-12-18-20(14)24-21(19-16(22)10-6-11-17(19)23)25(18)13-15-8-3-2-4-9-15/h2-12H,13H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Cancer Research and Development Center
Curated by ChEMBL
| Assay Description
The quantity of compound required to reduce WT Reverse transcriptase activity by 50% |
J Med Chem 40: 4199-207 (1998)
Article DOI: 10.1021/jm970096g
BindingDB Entry DOI: 10.7270/Q25B01KN |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17673
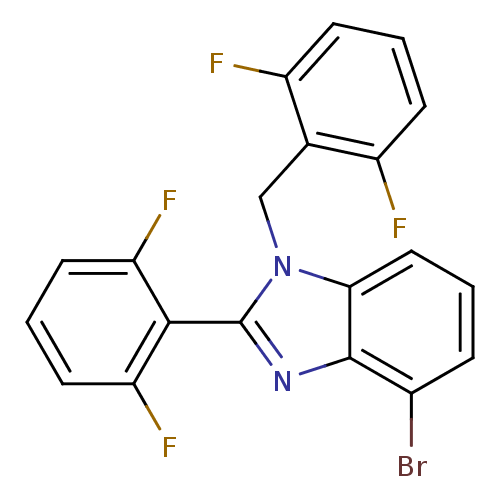
(4-bromo-2-(2,6-difluorophenyl)-1-[(2,6-difluorophe...)Show SMILES Fc1cccc(F)c1Cn1c(nc2c(Br)cccc12)-c1c(F)cccc1F |(-3.45,3.05,;-4.22,1.72,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;-3.45,.39,;-1.91,.34,;-1.14,1.68,;.39,1.52,;1.02,2.92,;-.12,3.95,;-.12,5.5,;1.21,6.27,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C20H11BrF4N2/c21-12-4-1-9-17-19(12)26-20(18-15(24)7-3-8-16(18)25)27(17)10-11-13(22)5-2-6-14(11)23/h1-9H,10H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17670
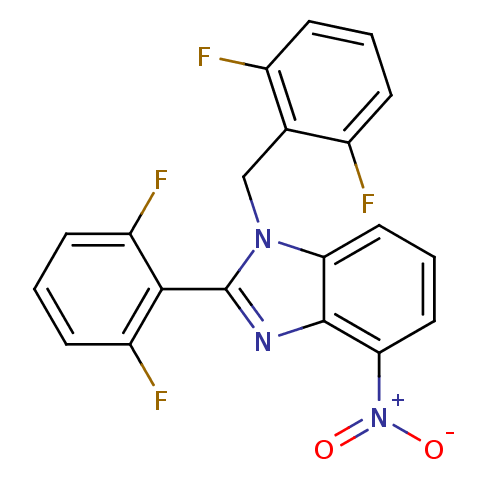
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES [O-][N+](=O)c1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,7.81,;1.21,6.27,;2.75,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C20H11F4N3O2/c21-12-4-1-5-13(22)11(12)10-26-16-8-3-9-17(27(28)29)19(16)25-20(26)18-14(23)6-2-7-15(18)24/h1-9H,10H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17674
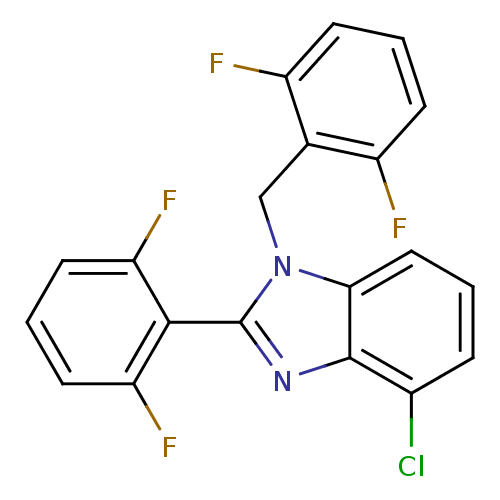
(4-chloro-2-(2,6-difluorophenyl)-1-[(2,6-difluoroph...)Show SMILES Fc1cccc(F)c1Cn1c(nc2c(Cl)cccc12)-c1c(F)cccc1F |(-3.45,3.05,;-4.22,1.72,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;-3.45,.39,;-1.91,.34,;-1.14,1.68,;.39,1.52,;1.02,2.92,;-.12,3.95,;-.12,5.5,;1.21,6.27,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C20H11ClF4N2/c21-12-4-1-9-17-19(12)26-20(18-15(24)7-3-8-16(18)25)27(17)10-11-13(22)5-2-6-14(11)23/h1-9H,10H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50369418

(CHEMBL58711 | R-87027)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)c(Cl)ccc3[nH]c2=S |r,wD:1.0,(3.24,.55,;1.7,.51,;1,1.88,;-.51,2.18,;-1.69,1.19,;-1.65,-.35,;-.42,-1.28,;1.07,-.89,;2.07,-2.07,;3.58,-1.8,;4.58,-2.98,;6.09,-2.71,;4.05,-4.42,;-3.1,-1.07,;-3.17,-2.61,;-4.4,-.26,;-4.35,1.28,;-3,2,;-2.63,3.5,;-1.09,3.61,;-.28,4.92,)| Show InChI InChI=1S/C16H20ClN3S/c1-10(2)6-7-19-9-12-13(17)4-5-14-15(12)20(8-11(19)3)16(21)18-14/h4-6,11H,7-9H2,1-3H3,(H,18,21)/t11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17676
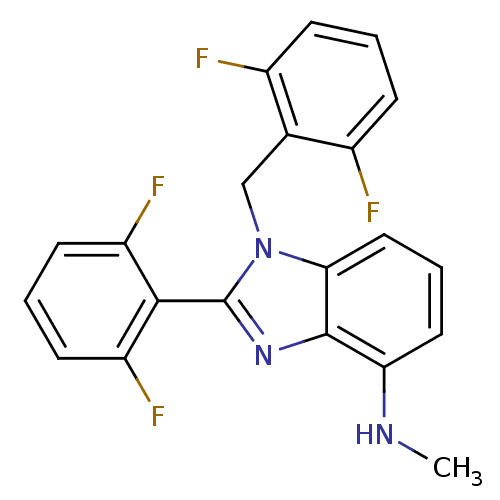
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES CNc1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,7.81,;1.21,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C21H15F4N3/c1-26-17-9-4-10-18-20(17)27-21(19-15(24)7-3-8-16(19)25)28(18)11-12-13(22)5-2-6-14(12)23/h2-10,26H,11H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50036586
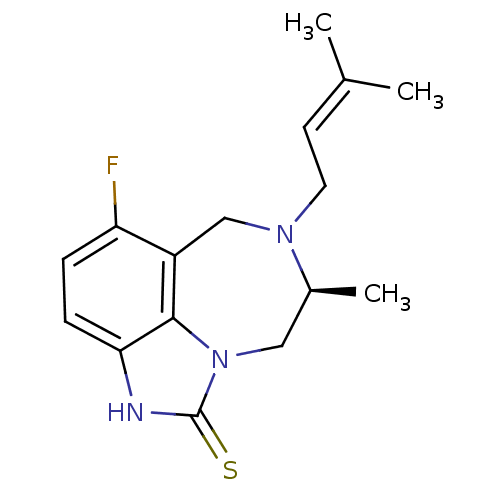
((S)-5-Fluoro-8-methyl-7-(3-methyl-but-2-enyl)-6,7,...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)c(F)ccc3[nH]c2=S |wD:1.0,(11.63,-15.32,;11.63,-13.78,;13.01,-13.12,;13.34,-11.61,;12.35,-10.4,;10.83,-10.41,;9.87,-11.63,;10.23,-13.12,;9.03,-14.11,;7.58,-13.56,;6.39,-14.53,;4.94,-13.98,;6.62,-16.05,;10.05,-9.08,;8.51,-9.1,;10.81,-7.75,;12.35,-7.75,;13.12,-9.08,;14.62,-9.41,;14.79,-10.93,;16.12,-11.69,)| Show InChI InChI=1S/C16H20FN3S/c1-10(2)6-7-19-9-12-13(17)4-5-14-15(12)20(8-11(19)3)16(21)18-14/h4-6,11H,7-9H2,1-3H3,(H,18,21)/t11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50036599
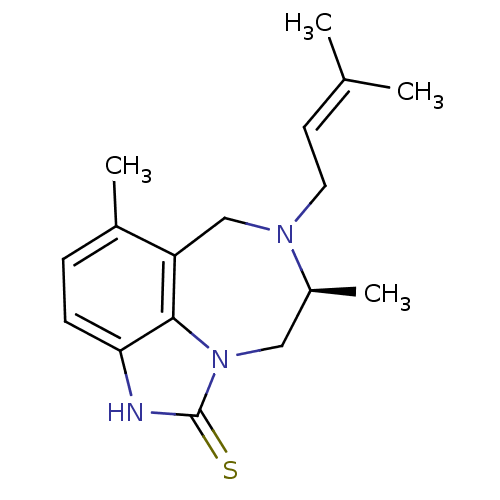
((S)-5,8-Dimethyl-7-(3-methyl-but-2-enyl)-6,7,8,9-t...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)c(C)ccc3[nH]c2=S |wD:1.0,(11.98,-15.32,;11.98,-13.78,;13.36,-13.12,;13.69,-11.61,;12.7,-10.4,;11.18,-10.41,;10.22,-11.63,;10.58,-13.12,;9.38,-14.11,;7.93,-13.56,;6.74,-14.53,;5.29,-13.98,;6.98,-16.05,;10.4,-9.08,;8.86,-9.1,;11.16,-7.75,;12.7,-7.75,;13.48,-9.08,;14.97,-9.41,;15.14,-10.93,;16.47,-11.69,)| Show InChI InChI=1S/C17H23N3S/c1-11(2)7-8-19-10-14-12(3)5-6-15-16(14)20(9-13(19)4)17(21)18-15/h5-7,13H,8-10H2,1-4H3,(H,18,21)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
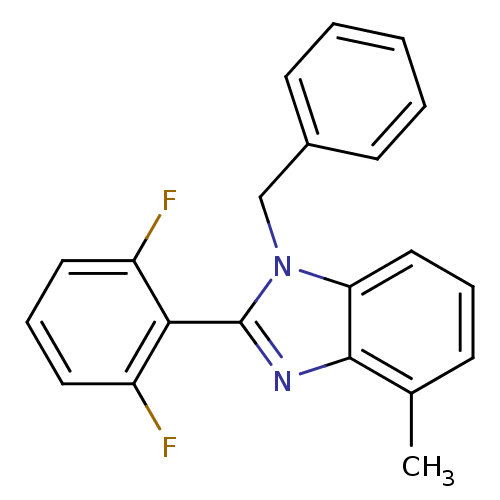
(Human immunodeficiency virus 1) | BDBM50061572
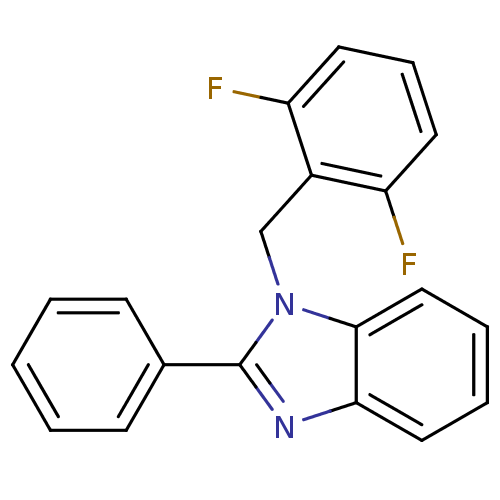
(1-(2,6-Difluoro-benzyl)-2-phenyl-1H-benzoimidazole...)Show InChI InChI=1S/C20H14F2N2/c21-16-9-6-10-17(22)15(16)13-24-19-12-5-4-11-18(19)23-20(24)14-7-2-1-3-8-14/h1-12H,13H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Cancer Research and Development Center
Curated by ChEMBL
| Assay Description
The quantity of compound required to reduce WT Reverse transcriptase activity by 50% |
J Med Chem 40: 4199-207 (1998)
Article DOI: 10.1021/jm970096g
BindingDB Entry DOI: 10.7270/Q25B01KN |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17669
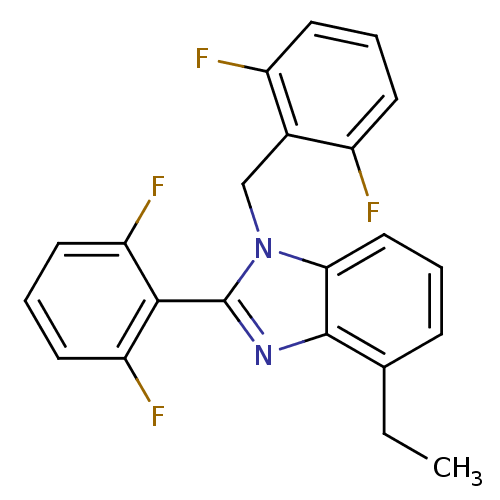
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES CCc1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,7.81,;1.21,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C22H16F4N2/c1-2-13-6-3-11-19-21(13)27-22(20-17(25)9-5-10-18(20)26)28(19)12-14-15(23)7-4-8-16(14)24/h3-11H,2,12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50061575
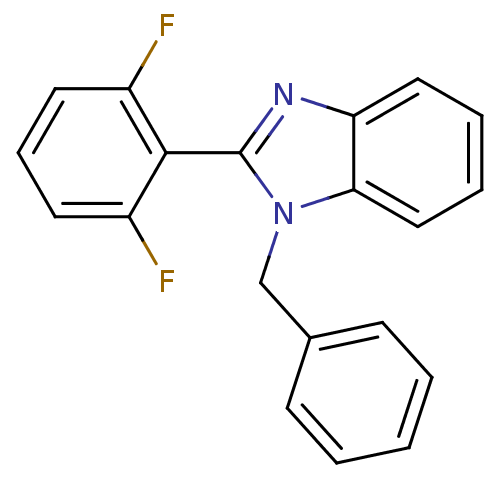
(1-Benzyl-2-(2,6-difluoro-phenyl)-1H-benzoimidazole...)Show SMILES Fc1cccc(F)c1-c1nc2ccccc2n1Cc1ccccc1 |(12.79,-8.1,;13.56,-6.77,;15.1,-6.77,;15.87,-5.44,;15.08,-4.11,;13.54,-4.11,;12.76,-2.78,;12.79,-5.44,;11.25,-5.44,;10.34,-4.21,;8.87,-4.7,;7.54,-3.92,;6.21,-4.7,;6.21,-6.25,;7.54,-7.01,;8.87,-6.24,;10.36,-6.7,;10.83,-8.17,;9.82,-9.32,;10.31,-10.76,;9.29,-11.92,;7.78,-11.6,;7.29,-10.15,;8.31,-8.99,)| Show InChI InChI=1S/C20H14F2N2/c21-15-9-6-10-16(22)19(15)20-23-17-11-4-5-12-18(17)24(20)13-14-7-2-1-3-8-14/h1-12H,13H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Cancer Research and Development Center
Curated by ChEMBL
| Assay Description
The quantity of compound required to reduce WT Reverse transcriptase activity by 50% |
J Med Chem 40: 4199-207 (1998)
Article DOI: 10.1021/jm970096g
BindingDB Entry DOI: 10.7270/Q25B01KN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50036570
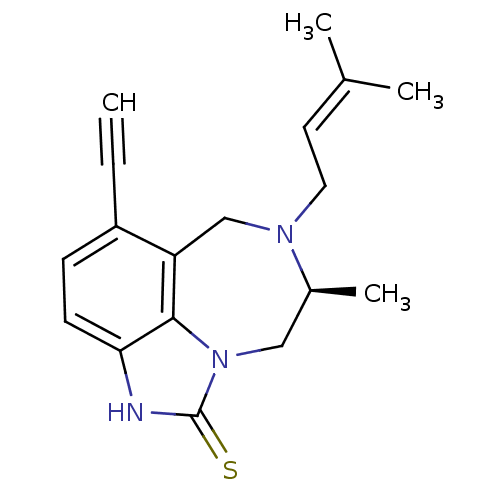
((S)-5-Ethynyl-8-methyl-7-(3-methyl-but-2-enyl)-6,7...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)c(ccc3[nH]c2=S)C#C |wD:1.0,(12.14,-15.83,;12.13,-14.29,;13.51,-13.61,;13.84,-12.11,;12.86,-10.9,;11.32,-10.92,;10.37,-12.13,;10.73,-13.63,;9.54,-14.61,;8.1,-14.05,;6.91,-15.03,;7.15,-16.55,;5.47,-14.47,;10.55,-9.6,;11.32,-8.27,;12.86,-8.26,;13.63,-9.59,;15.13,-9.92,;15.28,-11.43,;16.62,-12.19,;9.01,-9.6,;7.47,-9.6,)| Show InChI InChI=1S/C18H21N3S/c1-5-14-6-7-16-17-15(14)11-20(9-8-12(2)3)13(4)10-21(17)18(22)19-16/h1,6-8,13H,9-11H2,2-4H3,(H,19,22)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50036605

((S)-5-Methoxy-8-methyl-7-(3-methyl-but-2-enyl)-6,7...)Show SMILES COc1ccc2[nH]c(=S)n3C[C@H](C)N(CC=C(C)C)Cc1c23 |wU:11.11,(6.69,-4.68,;6.69,-6.22,;8.02,-6.99,;8.02,-8.54,;9.35,-9.31,;10.68,-8.54,;12.14,-9.03,;13.05,-7.78,;14.59,-7.79,;12.15,-6.53,;12.7,-4.99,;11.76,-3.35,;12.7,-1.71,;9.95,-3.41,;9.24,-2.04,;10.07,-.73,;9.35,.63,;10.17,1.93,;7.81,.69,;8.96,-4.75,;9.35,-6.24,;10.69,-7.01,)| Show InChI InChI=1S/C17H23N3OS/c1-11(2)7-8-19-10-13-15(21-4)6-5-14-16(13)20(9-12(19)3)17(22)18-14/h5-7,12H,8-10H2,1-4H3,(H,18,22)/t12-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50279762
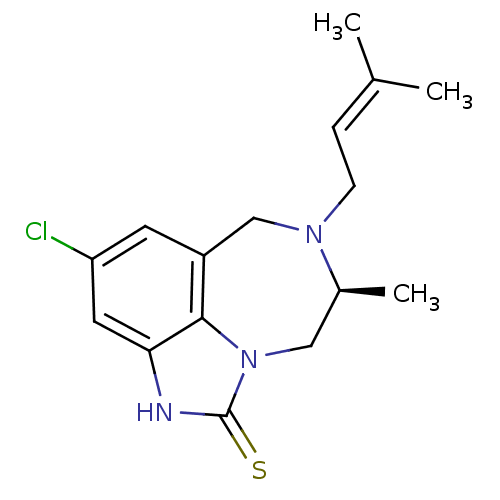
((5S)-9-chloro-5-methyl-6-(3-methylbut-2-enyl)-4,5,...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)cc(Cl)cc3[nH]c2=S |wD:1.0,(11.88,-16.19,;11.86,-14.64,;13.24,-13.98,;13.57,-12.47,;12.6,-11.27,;11.07,-11.28,;10.11,-12.49,;10.46,-13.99,;9.27,-14.97,;7.83,-14.43,;6.62,-15.39,;5.19,-14.85,;6.87,-16.91,;10.3,-9.95,;11.06,-8.61,;10.27,-7.28,;12.6,-8.61,;13.37,-9.95,;14.87,-10.27,;15.02,-11.79,;16.37,-12.56,)| Show InChI InChI=1S/C16H20ClN3S/c1-10(2)4-5-19-9-12-6-13(17)7-14-15(12)20(8-11(19)3)16(21)18-14/h4,6-7,11H,5,8-9H2,1-3H3,(H,18,21)/t11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17677
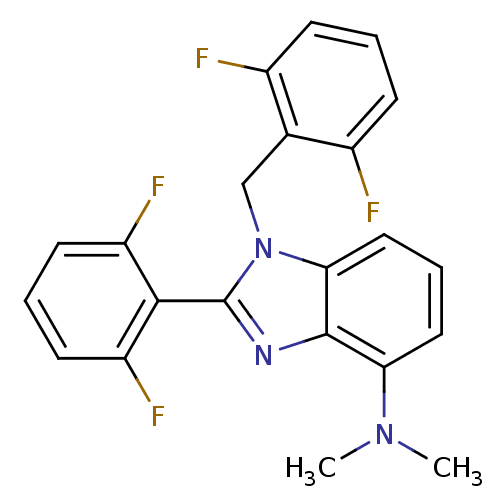
(2-(2,6-difluorophenyl)-1-[(2,6-difluorophenyl)meth...)Show SMILES CN(C)c1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(1.21,7.81,;1.21,6.27,;2.54,5.49,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C22H17F4N3/c1-28(2)18-10-5-11-19-21(18)27-22(20-16(25)8-4-9-17(20)26)29(19)12-13-14(23)6-3-7-15(13)24/h3-11H,12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1433

((11S)-11-methyl-10-(3-methylbut-2-en-1-yl)-1,3,10-...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)cccc3[nH]c2=S |r,wD:1.0,(16.54,-10.87,;15.69,-9.78,;16.51,-8.45,;15.85,-7.02,;14.39,-6.55,;13.06,-7.32,;12.7,-8.76,;13.8,-9.86,;13.16,-11.23,;14.12,-12.51,;13.35,-13.93,;11.94,-14.42,;14.15,-15.25,;11.72,-6.55,;11.72,-5.01,;13.06,-4.24,;14.39,-5.01,;15.85,-4.53,;16.76,-5.78,;18.33,-5.78,)| Show InChI InChI=1S/C16H21N3S/c1-11(2)7-8-18-10-13-5-4-6-14-15(13)19(9-12(18)3)16(20)17-14/h4-7,12H,8-10H2,1-3H3,(H,17,20)/t12-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S]
(Human immunodeficiency virus type 1) | BDBM17675
(CHEMBL58358 | N-[2-(2,6-difluorophenyl)-1-[(2,6-di...)Show SMILES CC(=O)Nc1cccc2n(Cc3c(F)cccc3F)c(nc12)-c1c(F)cccc1F |(2.54,8.57,;1.21,7.81,;-.12,8.58,;1.21,6.27,;-.12,5.5,;-1.46,6.27,;-2.79,5.5,;-2.79,3.95,;-1.46,3.18,;-1.14,1.68,;-1.91,.34,;-3.45,.39,;-4.22,1.72,;-3.45,3.05,;-5.76,1.72,;-6.53,.39,;-5.76,-.95,;-4.22,-.95,;-3.45,-2.28,;.39,1.52,;1.02,2.92,;-.12,3.95,;1.16,.18,;2.7,.18,;3.47,1.52,;3.47,-1.15,;2.7,-2.48,;1.16,-2.48,;.39,-1.15,;-1.15,-1.15,)| Show InChI InChI=1S/C22H15F4N3O/c1-12(30)27-18-9-4-10-19-21(18)28-22(20-16(25)7-3-8-17(20)26)29(19)11-13-14(23)5-2-6-15(13)24/h2-10H,11H2,1H3,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.47E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
NCI-FCRDC
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 50: 4003-4015 (2007)
Article DOI: 10.1021/jm060103d
BindingDB Entry DOI: 10.7270/Q25M6409 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50036595

((S)-8-Methyl-7-(3-methyl-but-2-enyl)-1-thioxo-1,2,...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)c(ccc3[nH]c2=S)C#N |wD:1.0,(12.67,-15.73,;12.67,-14.19,;14.05,-13.51,;14.37,-12.01,;13.39,-10.8,;11.87,-10.81,;10.91,-12.03,;11.26,-13.53,;10.07,-14.5,;8.63,-13.95,;7.43,-14.93,;7.67,-16.45,;5.99,-14.37,;11.09,-9.49,;11.85,-8.16,;13.39,-8.15,;14.16,-9.48,;15.66,-9.81,;15.82,-11.33,;17.15,-12.08,;9.55,-9.49,;8.01,-9.49,)| Show InChI InChI=1S/C17H20N4S/c1-11(2)6-7-20-10-14-13(8-18)4-5-15-16(14)21(9-12(20)3)17(22)19-15/h4-6,12H,7,9-10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Western Maryland College
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
J Med Chem 41: 5272-86 (1999)
Article DOI: 10.1021/jm9804174
BindingDB Entry DOI: 10.7270/Q28S4QM9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




















































