 Found 4338 hits with Last Name = 'miller' and Initial = 'd'
Found 4338 hits with Last Name = 'miller' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | -50.7 | n/a | n/a | 1 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354983
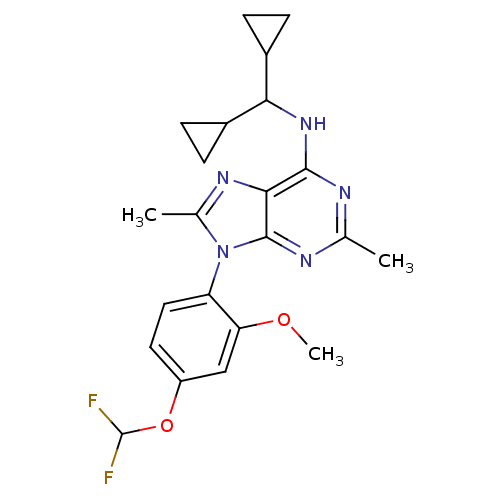
(CHEMBL1836947)Show SMILES COc1cc(OC(F)F)ccc1-n1c(C)nc2c(NC(C3CC3)C3CC3)nc(C)nc12 |(44.5,-44.78,;42.97,-44.81,;42.22,-46.16,;43.02,-47.47,;42.27,-48.83,;43.06,-50.15,;42.32,-51.49,;43.11,-52.81,;40.78,-51.52,;40.73,-48.85,;39.94,-47.53,;40.69,-46.19,;39.9,-44.87,;40.81,-43.62,;42.35,-43.62,;39.9,-42.36,;38.42,-42.84,;37.09,-42.08,;37.08,-40.54,;35.75,-39.77,;35.74,-38.23,;36.51,-36.91,;34.97,-36.91,;34.42,-40.55,;32.88,-40.55,;33.65,-41.88,;35.76,-42.85,;35.76,-44.39,;34.42,-45.16,;37.09,-45.16,;38.42,-44.39,)| Show InChI InChI=1S/C22H25F2N5O2/c1-11-25-20(28-18(13-4-5-13)14-6-7-14)19-21(26-11)29(12(2)27-19)16-9-8-15(31-22(23)24)10-17(16)30-3/h8-10,13-14,18,22H,4-7H2,1-3H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18699
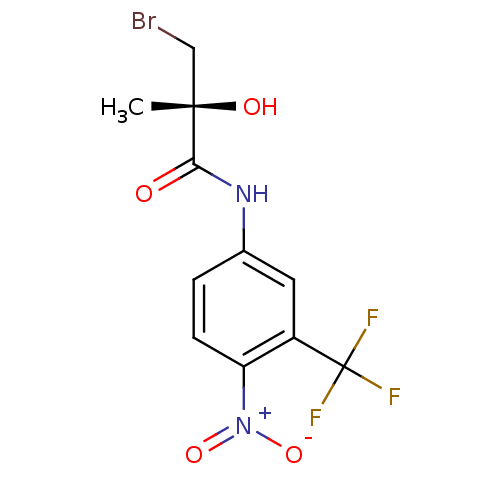
((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | -50.5 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699

((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM82517
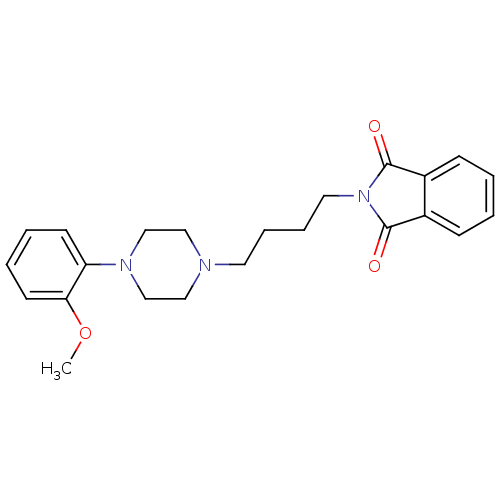
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699

((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50470923
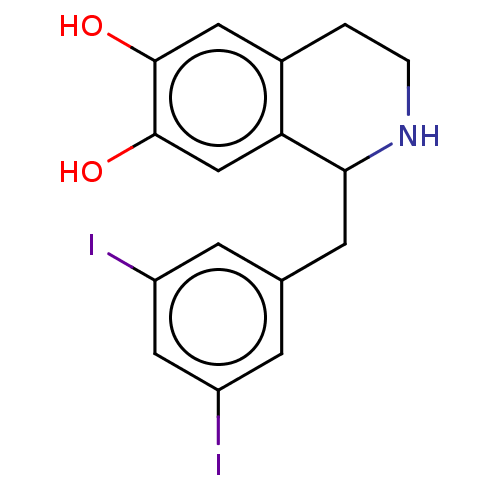
(CHEMBL122757)Show InChI InChI=1S/C16H15I2NO2/c17-11-3-9(4-12(18)7-11)5-14-13-8-16(21)15(20)6-10(13)1-2-19-14/h3-4,6-8,14,19-21H,1-2,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis
Curated by ChEMBL
| Assay Description
Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... |
J Med Chem 39: 3701-11 (1996)
Article DOI: 10.1021/jm960208o
BindingDB Entry DOI: 10.7270/Q2Q52SBM |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354981
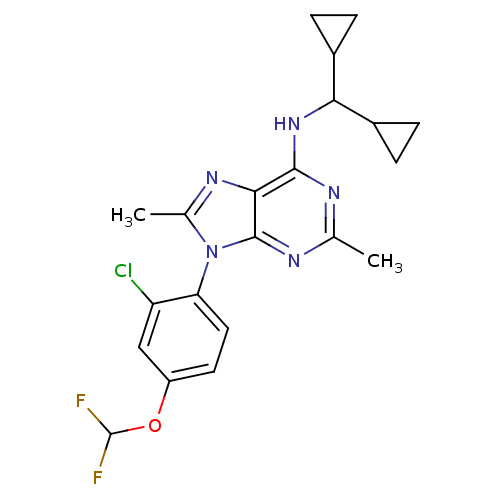
(CHEMBL1836945)Show SMILES Cc1nc2c(NC(C3CC3)C3CC3)nc(C)nc2n1-c1ccc(OC(F)F)cc1Cl |(10.94,-43.27,;9.4,-43.27,;8.49,-42.02,;7.02,-42.5,;5.68,-41.73,;5.68,-40.19,;4.34,-39.43,;4.34,-37.89,;5.1,-36.57,;3.56,-36.56,;3.01,-40.2,;1.47,-40.21,;2.24,-41.54,;4.35,-42.51,;4.35,-44.05,;3.02,-44.82,;5.69,-44.82,;7.02,-44.05,;8.49,-44.53,;9.28,-45.85,;8.53,-47.19,;9.32,-48.51,;10.86,-48.49,;11.66,-49.8,;10.91,-51.15,;11.7,-52.47,;9.37,-51.18,;11.61,-47.13,;10.82,-45.82,;11.56,-44.47,)| Show InChI InChI=1S/C21H22ClF2N5O/c1-10-25-19(28-17(12-3-4-12)13-5-6-13)18-20(26-10)29(11(2)27-18)16-8-7-14(9-15(16)22)30-21(23)24/h7-9,12-13,17,21H,3-6H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087151
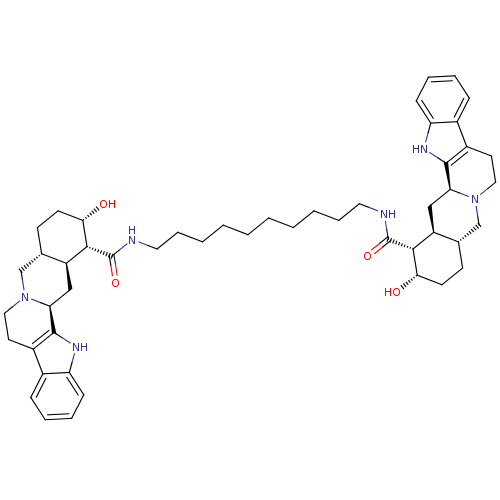
(1N-{10-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C50H68N6O4/c57-43-19-17-31-29-55-25-21-35-33-13-7-9-15-39(33)53-47(35)41(55)27-37(31)45(43)49(59)51-23-11-5-3-1-2-4-6-12-24-52-50(60)46-38-28-42-48-36(34-14-8-10-16-40(34)54-48)22-26-56(42)30-32(38)18-20-44(46)58/h7-10,13-16,31-32,37-38,41-46,53-54,57-58H,1-6,11-12,17-30H2,(H,51,59)(H,52,60)/t31-,32-,37-,38-,41-,42-,43-,44-,45+,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Sus scrofa) | BDBM50201438
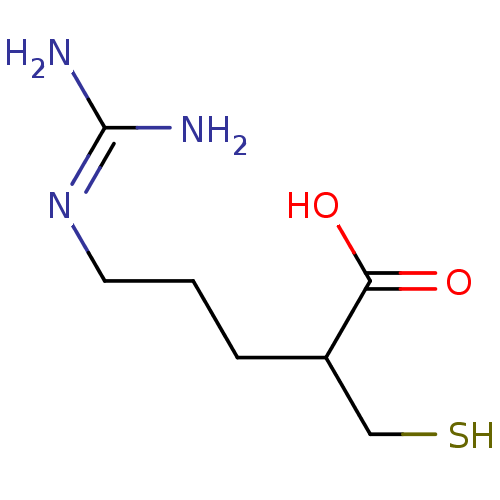
((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C7H15N3O2S/c8-7(9)10-3-1-2-5(4-13)6(11)12/h5,13H,1-4H2,(H,11,12)(H4,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of porcine pancreatic carboxypeptidase B |
J Med Chem 50: 6095-103 (2007)
Article DOI: 10.1021/jm0702433
BindingDB Entry DOI: 10.7270/Q2T153CG |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215713
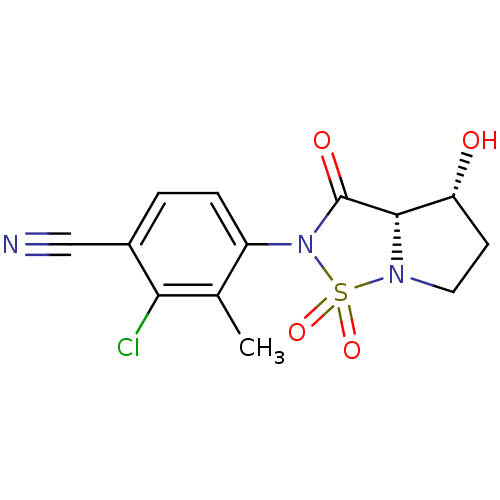
(2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...)Show SMILES Cc1c(Cl)c(ccc1N1C(=O)[C@@H]2[C@H](O)CCN2S1(=O)=O)C#N |r| Show InChI InChI=1S/C13H12ClN3O4S/c1-7-9(3-2-8(6-15)11(7)14)17-13(19)12-10(18)4-5-16(12)22(17,20)21/h2-3,10,12,18H,4-5H2,1H3/t10-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26260
((2S)-N-(4-cyano-3-iodophenyl)-3-(4-cyanophenoxy)-2...)Show SMILES C[C@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(I)c1 |r| Show InChI InChI=1S/C18H14IN3O3/c1-18(24,11-25-15-6-2-12(9-20)3-7-15)17(23)22-14-5-4-13(10-21)16(19)8-14/h2-8,24H,11H2,1H3,(H,22,23)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.540 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25768
(1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(pro...)Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM82487
(TMQ, 3',5'-Diiodo)Show InChI InChI=1S/C17H17I2NO3/c1-23-17-12(18)4-9(5-13(17)19)6-14-11-8-16(22)15(21)7-10(11)2-3-20-14/h4-5,7-8,14,20-22H,2-3,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18681

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18681

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25768

(1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(pro...)Show InChI InChI=1S/C17H27NO2/c1-11(2)18-13(4)16(19)10-20-17-9-8-12(3)14-6-5-7-15(14)17/h8-9,11,13,16,18-19H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50054820
(Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34C5C[C@H]6[C@H](OC)C5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,42.46,36.39,44.48,39.43,wD:28.55,32.34,25.27,31.32,TLB:28:29:32:36.38.39,1:2:47:25.23.24,25:28:42.44:4.2.3,THB:42:48:47:25.23.24,29:28:42.44:4.2.3,2:48:36.35.29:47.44,(3.83,-6.7,;5.61,-4.92,;8.1,-4.92,;7.12,-7.5,;8.22,-6.42,;8.22,-7.94,;7.82,-9.45,;8.61,-10.78,;9.94,-10.01,;8.62,-12.33,;7.29,-13.1,;7.29,-14.64,;8.62,-15.41,;9.95,-14.64,;9.95,-13.1,;11.49,-13.09,;12.9,-13.7,;13.3,-15.19,;13.93,-12.55,;13.14,-11.22,;13.91,-9.88,;11.64,-11.55,;10.86,-10.2,;6.88,-5.65,;6.87,-4.11,;8.21,-3.34,;8.2,-1.8,;6.87,-1.03,;9.54,-4.11,;10.5,-3.18,;10.3,-1.71,;11.64,-1.06,;12.67,-2.15,;14.21,-2.15,;14.96,-3.46,;11.97,-3.45,;12.65,-4.9,;13.98,-5.67,;16.6,-3.1,;16.6,-1.56,;17.69,-.47,;19.02,-1.24,;11.93,-6.28,;12.7,-7.59,;10.46,-6.53,;10.06,-8.01,;11.39,-8.78,;9.55,-5.63,;9.53,-2.57,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22?,24+,25+,27?,28+,29?,30+,33?,34+,35-,36+,37+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50167573
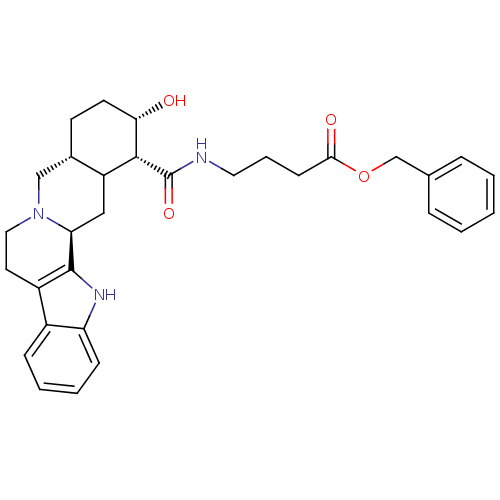
(4-[((1R,2S,4aR,13bS)-2-Hydroxy-1,2,3,4,4a,5,7,8,13...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3CC2[C@H]1C(=O)NCCCC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H37N3O4/c35-27-13-12-21-18-34-16-14-23-22-9-4-5-10-25(22)33-30(23)26(34)17-24(21)29(27)31(37)32-15-6-11-28(36)38-19-20-7-2-1-3-8-20/h1-5,7-10,21,24,26-27,29,33,35H,6,11-19H2,(H,32,37)/t21-,24?,26-,27-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
In vitro binding affinity measured by displacement of [3H]rauwolscine from alpha-2c adrenergic receptor expressed in CHO cells in presence of phentol... |
Bioorg Med Chem Lett 15: 2758-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.116
BindingDB Entry DOI: 10.7270/Q2KS6R23 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM82487

(TMQ, 3',5'-Diiodo)Show InChI InChI=1S/C17H17I2NO3/c1-23-17-12(18)4-9(5-13(17)19)6-14-11-8-16(22)15(21)7-10(11)2-3-20-14/h4-5,7-8,14,20-22H,2-3,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087149
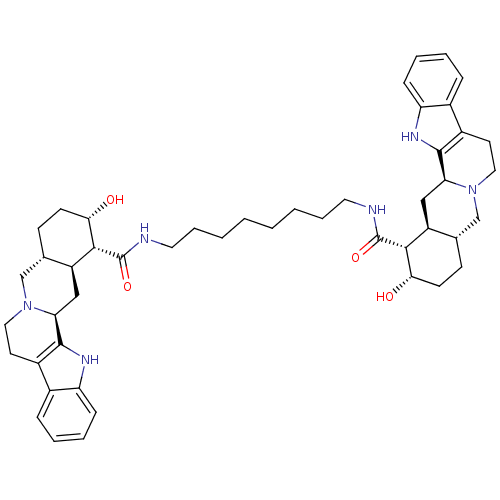
(1N-{8-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C48H64N6O4/c55-41-17-15-29-27-53-23-19-33-31-11-5-7-13-37(31)51-45(33)39(53)25-35(29)43(41)47(57)49-21-9-3-1-2-4-10-22-50-48(58)44-36-26-40-46-34(32-12-6-8-14-38(32)52-46)20-24-54(40)28-30(36)16-18-42(44)56/h5-8,11-14,29-30,35-36,39-44,51-52,55-56H,1-4,9-10,15-28H2,(H,49,57)(H,50,58)/t29-,30-,35-,36-,39-,40-,41-,42-,43+,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18701
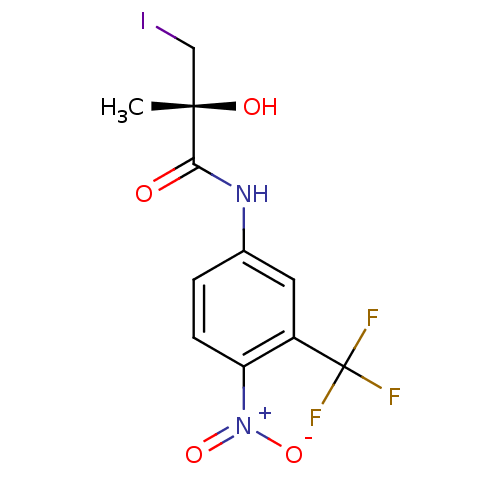
((2R)-2-hydroxy-3-iodo-2-methyl-N-[4-nitro-3-(trifl...)Show SMILES C[C@](O)(CI)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10F3IN2O4/c1-10(19,5-15)9(18)16-6-2-3-8(17(20)21)7(4-6)11(12,13)14/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | -48.1 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087152
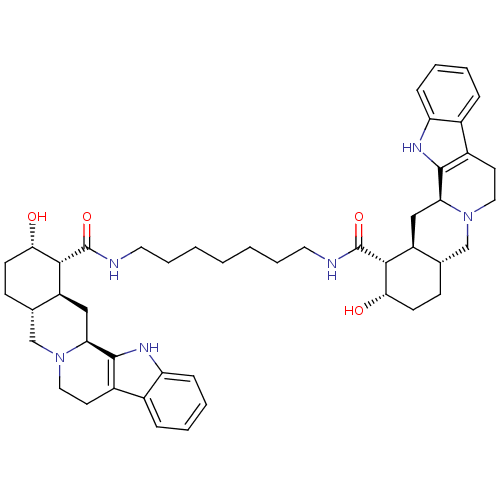
(1N-{7-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C47H62N6O4/c54-40-16-14-28-26-52-22-18-32-30-10-4-6-12-36(30)50-44(32)38(52)24-34(28)42(40)46(56)48-20-8-2-1-3-9-21-49-47(57)43-35-25-39-45-33(31-11-5-7-13-37(31)51-45)19-23-53(39)27-29(35)15-17-41(43)55/h4-7,10-13,28-29,34-35,38-43,50-51,54-55H,1-3,8-9,14-27H2,(H,48,56)(H,49,57)/t28-,29-,34-,35-,38-,39-,40-,41-,42+,43+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50167576
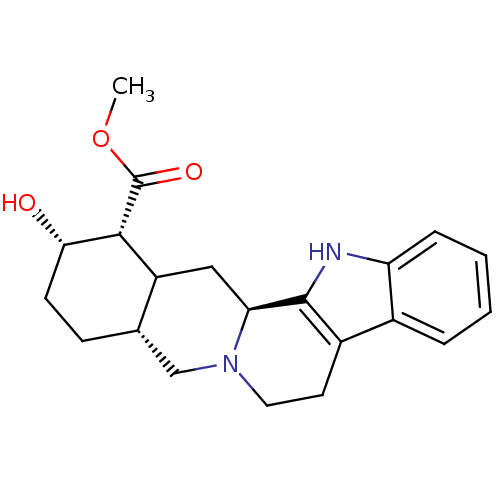
((1R,2S,4aR,13bS)-2-Hydroxy-1,2,3,4,4a,5,7,8,13,13b...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3CC12 Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15?,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
In vitro binding affinity measured by displacement of [3H]rauwolscine from alpha-2c adrenergic receptor expressed in CHO cells in presence of phentol... |
Bioorg Med Chem Lett 15: 2758-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.116
BindingDB Entry DOI: 10.7270/Q2KS6R23 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18177
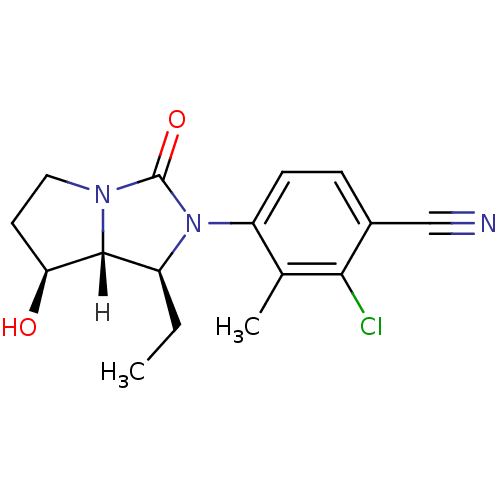
(4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H18ClN3O2/c1-3-11-15-13(21)6-7-19(15)16(22)20(11)12-5-4-10(8-18)14(17)9(12)2/h4-5,11,13,15,21H,3,6-7H2,1-2H3/t11-,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM82487

(TMQ, 3',5'-Diiodo)Show InChI InChI=1S/C17H17I2NO3/c1-23-17-12(18)4-9(5-13(17)19)6-14-11-8-16(22)15(21)7-10(11)2-3-20-14/h4-5,7-8,14,20-22H,2-3,6H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM82481
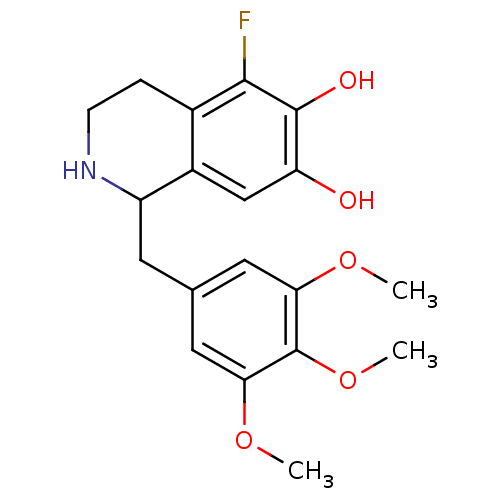
(CAS_128657 | NSC_128657 | TMQ, 5-Fluoro)Show InChI InChI=1S/C19H22FNO5/c1-24-15-7-10(8-16(25-2)19(15)26-3)6-13-12-9-14(22)18(23)17(20)11(12)4-5-21-13/h7-9,13,21-23H,4-6H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM82484
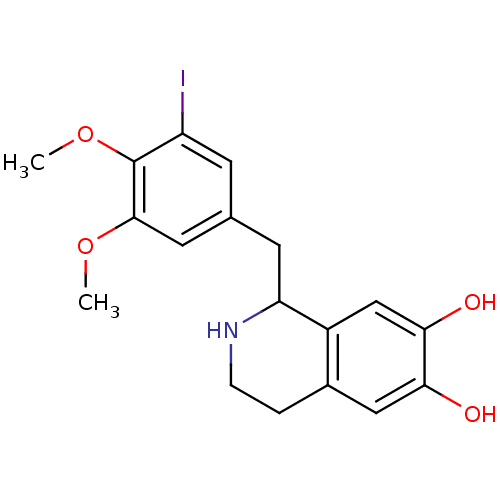
(TMQ, 3'-Iodo)Show InChI InChI=1S/C18H20INO4/c1-23-17-7-10(5-13(19)18(17)24-2)6-14-12-9-16(22)15(21)8-11(12)3-4-20-14/h5,7-9,14,20-22H,3-4,6H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50167578

(CHEMBL369938 | {3-[((1R,2S,4aR,13bS)-2-Hydroxy-1,2...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3CC2[C@H]1C(=O)NCCCNC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H38N4O4/c36-27-12-11-21-18-35-16-13-23-22-9-4-5-10-25(22)34-29(23)26(35)17-24(21)28(27)30(37)32-14-6-15-33-31(38)39-19-20-7-2-1-3-8-20/h1-5,7-10,21,24,26-28,34,36H,6,11-19H2,(H,32,37)(H,33,38)/t21-,24?,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
In vitro binding affinity measured by displacement of [3H]rauwolscine from alpha-2c adrenergic receptor expressed in CHO cells in presence of phentol... |
Bioorg Med Chem Lett 15: 2758-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.116
BindingDB Entry DOI: 10.7270/Q2KS6R23 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863
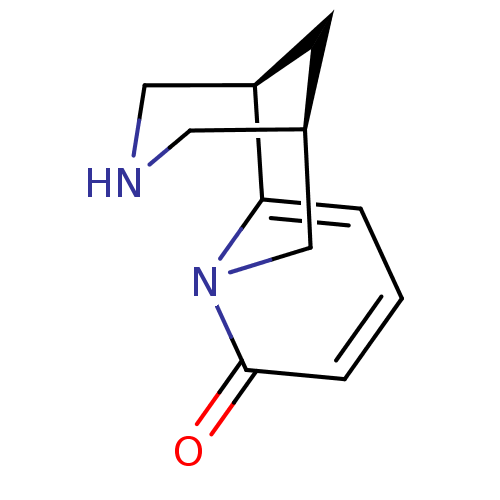
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50258751

((R)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-1,1,...)Show SMILES O[C@](C=C)(c1nc2cc(Cl)c(Cl)cc2[nH]1)C(F)(F)F |r| Show InChI InChI=1S/C11H7Cl2F3N2O/c1-2-10(19,11(14,15)16)9-17-7-3-5(12)6(13)4-8(7)18-9/h2-4,19H,1H2,(H,17,18)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50019443
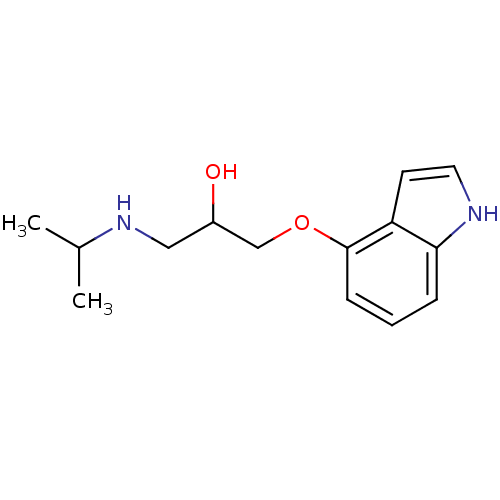
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087145
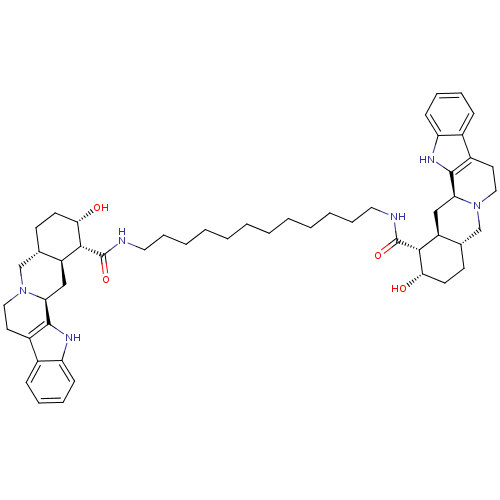
(1N-{12-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C52H72N6O4/c59-45-21-19-33-31-57-27-23-37-35-15-9-11-17-41(35)55-49(37)43(57)29-39(33)47(45)51(61)53-25-13-7-5-3-1-2-4-6-8-14-26-54-52(62)48-40-30-44-50-38(36-16-10-12-18-42(36)56-50)24-28-58(44)32-34(40)20-22-46(48)60/h9-12,15-18,33-34,39-40,43-48,55-56,59-60H,1-8,13-14,19-32H2,(H,53,61)(H,54,62)/t33-,34-,39-,40-,43-,44-,45-,46-,47+,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against the binding of [3H]-spiperone to Dopamine receptor D2 in rat striatal membranes |
J Med Chem 30: 1631-5 (1987)
BindingDB Entry DOI: 10.7270/Q2FB51ZZ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087148
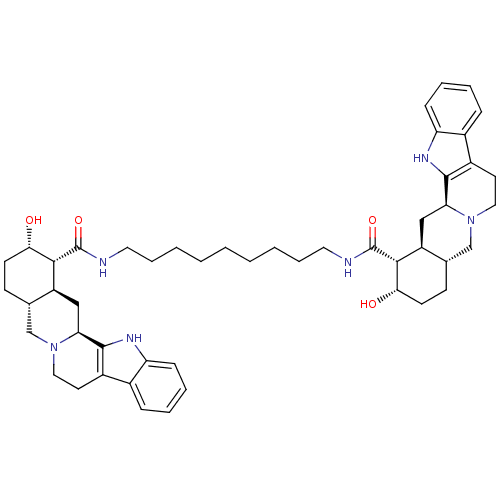
(1N-{9-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C49H66N6O4/c56-42-18-16-30-28-54-24-20-34-32-12-6-8-14-38(32)52-46(34)40(54)26-36(30)44(42)48(58)50-22-10-4-2-1-3-5-11-23-51-49(59)45-37-27-41-47-35(33-13-7-9-15-39(33)53-47)21-25-55(41)29-31(37)17-19-43(45)57/h6-9,12-15,30-31,36-37,40-45,52-53,56-57H,1-5,10-11,16-29H2,(H,50,58)(H,51,59)/t30-,31-,36-,37-,40-,41-,42-,43-,44+,45+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087147
(1N-{6-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C46H60N6O4/c53-39-15-13-27-25-51-21-17-31-29-9-3-5-11-35(29)49-43(31)37(51)23-33(27)41(39)45(55)47-19-7-1-2-8-20-48-46(56)42-34-24-38-44-32(30-10-4-6-12-36(30)50-44)18-22-52(38)26-28(34)14-16-40(42)54/h3-6,9-12,27-28,33-34,37-42,49-50,53-54H,1-2,7-8,13-26H2,(H,47,55)(H,48,56)/t27-,28-,33-,34-,37-,38-,39-,40-,41+,42+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26262
((2S)-2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromet...)Show SMILES C[C@](O)(COc1c(F)c(F)c(F)c(F)c1F)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C17H10F8N2O5/c1-16(29,5-32-14-12(21)10(19)9(18)11(20)13(14)22)15(28)26-6-2-3-8(27(30)31)7(4-6)17(23,24)25/h2-4,29H,5H2,1H3,(H,26,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.40 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50167576

((1R,2S,4aR,13bS)-2-Hydroxy-1,2,3,4,4a,5,7,8,13,13b...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3CC12 Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15?,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards alpha-2a adrenergic receptor |
Bioorg Med Chem Lett 15: 2758-60 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.116
BindingDB Entry DOI: 10.7270/Q2KS6R23 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50470917
(CHEMBL120278)Show InChI InChI=1S/C16H14I3NO2/c17-11-3-8(4-12(18)16(11)19)5-13-10-7-15(22)14(21)6-9(10)1-2-20-13/h3-4,6-7,13,20-22H,1-2,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis
Curated by ChEMBL
| Assay Description
Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... |
J Med Chem 39: 3701-11 (1996)
Article DOI: 10.1021/jm960208o
BindingDB Entry DOI: 10.7270/Q2Q52SBM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data