 Found 29 hits with Last Name = 'miller' and Initial = 'hb'
Found 29 hits with Last Name = 'miller' and Initial = 'hb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31822
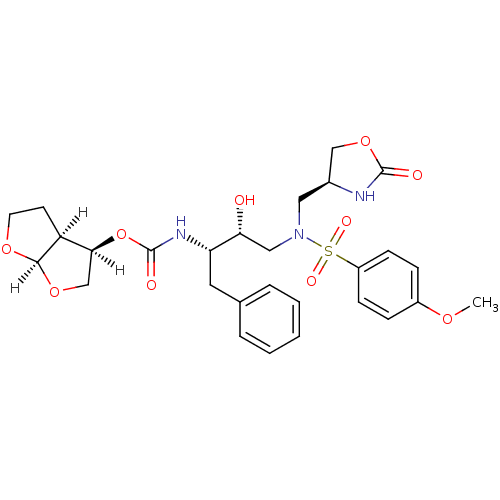
(oxazolidinone, 31)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1COC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C28H35N3O10S/c1-37-20-7-9-21(10-8-20)42(35,36)31(14-19-16-40-27(33)29-19)15-24(32)23(13-18-5-3-2-4-6-18)30-28(34)41-25-17-39-26-22(25)11-12-38-26/h2-10,19,22-26,32H,11-17H2,1H3,(H,29,33)(H,30,34)/t19-,22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0990 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31816
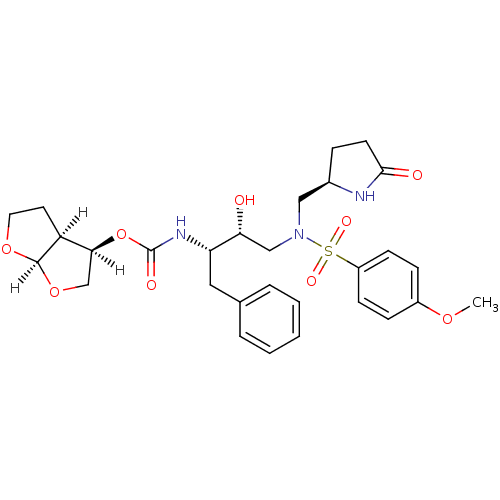
(methyl-2-pyrrolidinone, 19a)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H37N3O9S/c1-38-21-8-10-22(11-9-21)42(36,37)32(16-20-7-12-27(34)30-20)17-25(33)24(15-19-5-3-2-4-6-19)31-29(35)41-26-18-40-28-23(26)13-14-39-28/h2-6,8-11,20,23-26,28,33H,7,12-18H2,1H3,(H,30,34)(H,31,35)/t20-,23+,24+,25-,26+,28-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231938
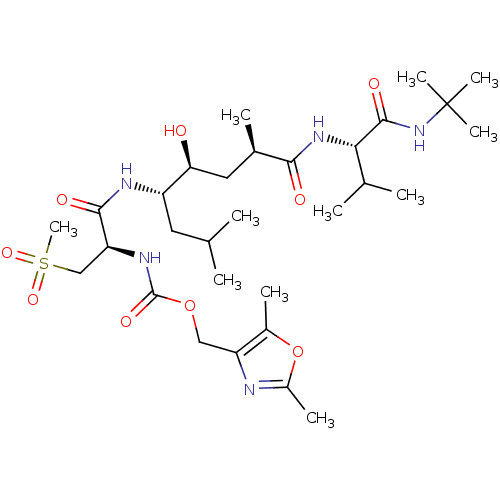
((2,5-dimethyloxazol-4-yl)methyl (R)-1-((4S,5S,7R)-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CS(C)(=O)=O)NC(=O)OCc1nc(C)oc1C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H53N5O9S/c1-16(2)12-21(24(36)13-18(5)26(37)34-25(17(3)4)28(39)35-30(8,9)10)32-27(38)23(15-45(11,41)42)33-29(40)43-14-22-19(6)44-20(7)31-22/h16-18,21,23-25,36H,12-15H2,1-11H3,(H,32,38)(H,33,40)(H,34,37)(H,35,39)/t18-,21+,23+,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31823
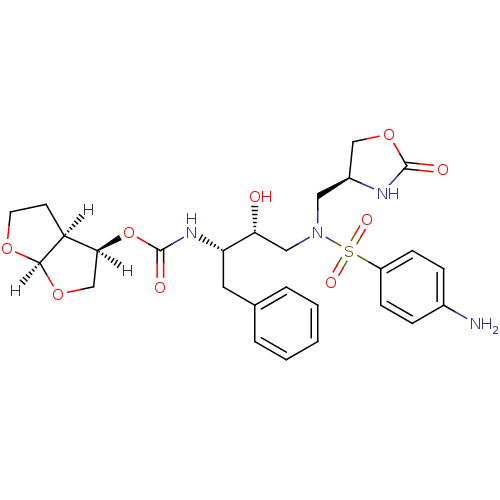
(oxazolidinone, 32)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1COC(=O)N1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H34N4O9S/c28-18-6-8-20(9-7-18)41(35,36)31(13-19-15-39-26(33)29-19)14-23(32)22(12-17-4-2-1-3-5-17)30-27(34)40-24-16-38-25-21(24)10-11-37-25/h1-9,19,21-25,32H,10-16,28H2,(H,29,33)(H,30,34)/t19-,21-,22-,23+,24-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | -54.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31814

(methyl-2-pyrrolidinone, 18a)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H]1CCC(=O)N1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H36N4O8S/c29-19-6-9-21(10-7-19)41(36,37)32(15-20-8-11-26(34)30-20)16-24(33)23(14-18-4-2-1-3-5-18)31-28(35)40-25-17-39-27-22(25)12-13-38-27/h1-7,9-10,20,22-25,27,33H,8,11-17,29H2,(H,30,34)(H,31,35)/t20-,22-,23-,24+,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31820
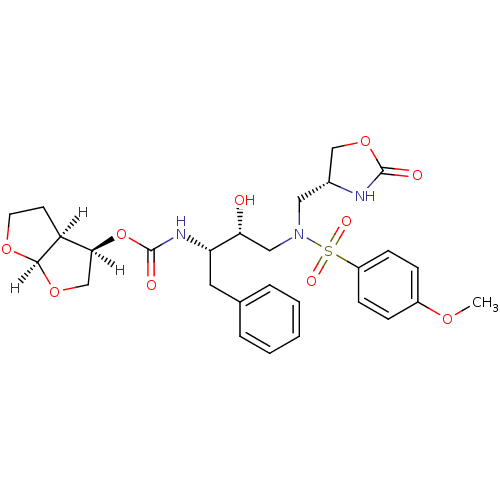
(oxazolidinone, 29)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H]1COC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C28H35N3O10S/c1-37-20-7-9-21(10-8-20)42(35,36)31(14-19-16-40-27(33)29-19)15-24(32)23(13-18-5-3-2-4-6-18)30-28(34)41-25-17-39-26-22(25)11-12-38-26/h2-10,19,22-26,32H,11-17H2,1H3,(H,29,33)(H,30,34)/t19-,22+,23+,24-,25+,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31819
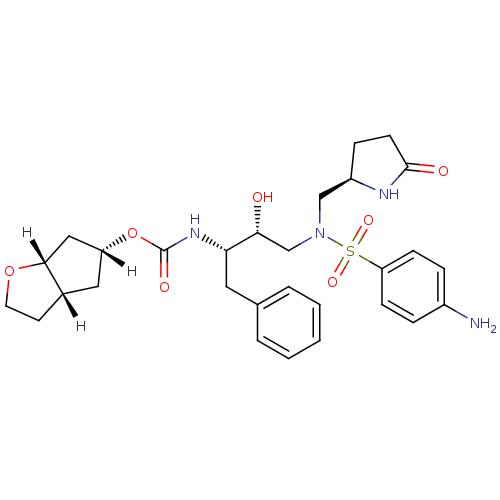
(methyl-2-pyrrolidinone, 20b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C29H38N4O7S/c30-21-6-9-24(10-7-21)41(37,38)33(17-22-8-11-28(35)31-22)18-26(34)25(14-19-4-2-1-3-5-19)32-29(36)40-23-15-20-12-13-39-27(20)16-23/h1-7,9-10,20,22-23,25-27,34H,8,11-18,30H2,(H,31,35)(H,32,36)/t20-,22+,23+,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31813
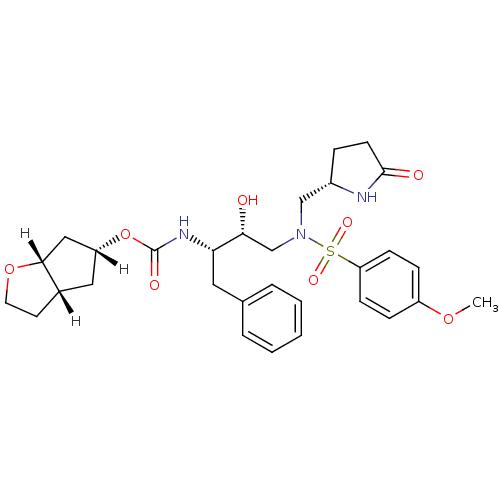
(methyl-2-pyrrolidinone, 17b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22-,24+,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31821

(oxazolidinone, 30)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H]1COC(=O)N1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H34N4O9S/c28-18-6-8-20(9-7-18)41(35,36)31(13-19-15-39-26(33)29-19)14-23(32)22(12-17-4-2-1-3-5-17)30-27(34)40-24-16-38-25-21(24)10-11-37-25/h1-9,19,21-25,32H,10-16,28H2,(H,29,33)(H,30,34)/t19-,21+,22+,23-,24+,25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31812
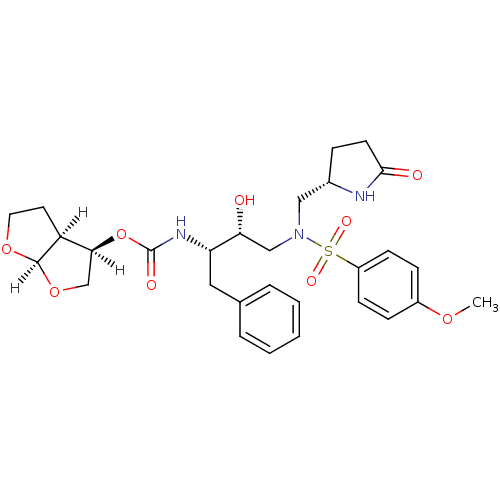
(methyl-2-pyrrolidinone, 17a)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H37N3O9S/c1-38-21-8-10-22(11-9-21)42(36,37)32(16-20-7-12-27(34)30-20)17-25(33)24(15-19-5-3-2-4-6-19)31-29(35)41-26-18-40-28-23(26)13-14-39-28/h2-6,8-11,20,23-26,28,33H,7,12-18H2,1H3,(H,30,34)(H,31,35)/t20-,23-,24-,25+,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | -51.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31818

(methyl-2-pyrrolidinone, 20a)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H36N4O8S/c29-19-6-9-21(10-7-19)41(36,37)32(15-20-8-11-26(34)30-20)16-24(33)23(14-18-4-2-1-3-5-18)31-28(35)40-25-17-39-27-22(25)12-13-38-27/h1-7,9-10,20,22-25,27,33H,8,11-17,29H2,(H,30,34)(H,31,35)/t20-,22+,23+,24-,25+,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | -51.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31815
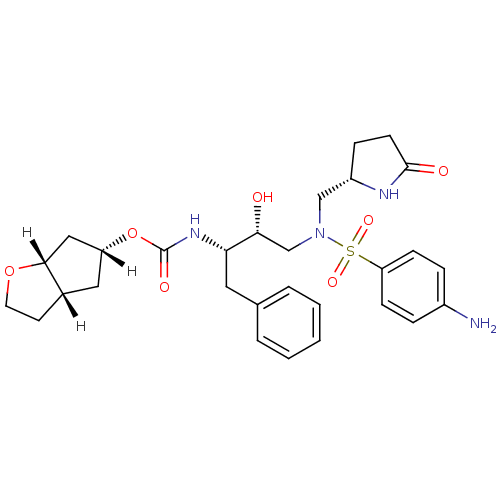
(methyl-2-pyrrolidinone, 18b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H]1CCC(=O)N1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C29H38N4O7S/c30-21-6-9-24(10-7-21)41(37,38)33(17-22-8-11-28(35)31-22)18-26(34)25(14-19-4-2-1-3-5-19)32-29(36)40-23-15-20-12-13-39-27(20)16-23/h1-7,9-10,20,22-23,25-27,34H,8,11-18,30H2,(H,31,35)(H,32,36)/t20-,22-,23+,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.27 | -50.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231937
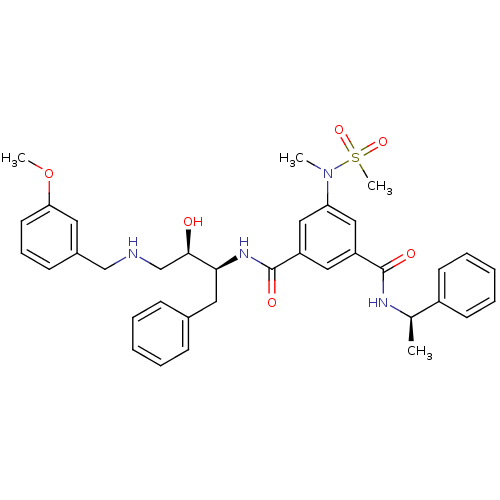
(CHEMBL403268 | N-{(1S,2R)-1-benzyl-2-hydroxy-3-[(3...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(cc(c2)C(=O)N[C@H](C)c2ccccc2)N(C)S(C)(=O)=O)c1 Show InChI InChI=1S/C36H42N4O6S/c1-25(28-15-9-6-10-16-28)38-35(42)29-20-30(22-31(21-29)40(2)47(4,44)45)36(43)39-33(19-26-12-7-5-8-13-26)34(41)24-37-23-27-14-11-17-32(18-27)46-3/h5-18,20-22,25,33-34,37,41H,19,23-24H2,1-4H3,(H,38,42)(H,39,43)/t25-,33+,34-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231932

(CHEMBL253930 | N1-((2S,3R)-3-hydroxy-4-((1-(methyl...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1ccc2ccn(c2c1)S(C)(=O)=O)N(C)S(C)(=O)=O)c1ccccc1 Show InChI InChI=1S/C38H43N5O7S2/c1-26(29-13-9-6-10-14-29)40-37(45)31-21-32(23-33(22-31)42(2)51(3,47)48)38(46)41-34(19-27-11-7-5-8-12-27)36(44)25-39-24-28-15-16-30-17-18-43(35(30)20-28)52(4,49)50/h5-18,20-23,26,34,36,39,44H,19,24-25H2,1-4H3,(H,40,45)(H,41,46)/t26-,34+,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231933
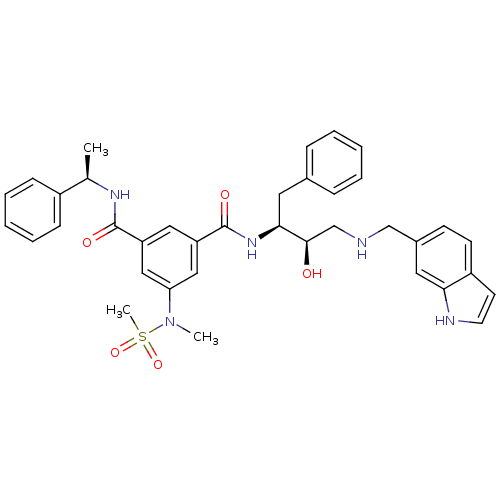
(CHEMBL253929 | N1-((2S,3R)-4-((1H-indol-6-yl)methy...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1ccc2cc[nH]c2c1)N(C)S(C)(=O)=O)c1ccccc1 Show InChI InChI=1S/C37H41N5O5S/c1-25(28-12-8-5-9-13-28)40-36(44)30-20-31(22-32(21-30)42(2)48(3,46)47)37(45)41-34(18-26-10-6-4-7-11-26)35(43)24-38-23-27-14-15-29-16-17-39-33(29)19-27/h4-17,19-22,25,34-35,38-39,43H,18,23-24H2,1-3H3,(H,40,44)(H,41,45)/t25-,34+,35-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
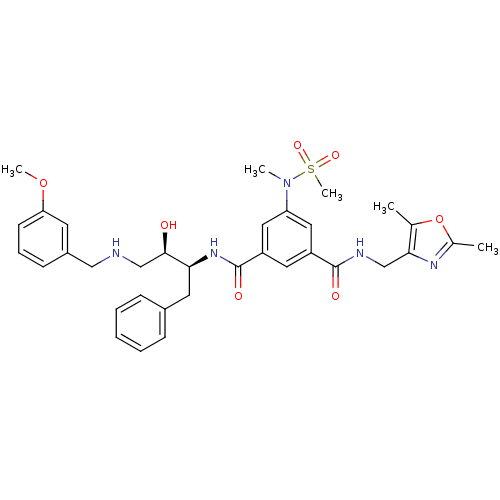
(Homo sapiens (Human)) | BDBM50231941

(CHEMBL253103 | N1-((2S,3R)-3-hydroxy-4-(isopropyla...)Show SMILES CC(C)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(cc(c1)C(=O)N[C@H](C)c1ccccc1)N(C)S(C)(=O)=O Show InChI InChI=1S/C31H40N4O5S/c1-21(2)32-20-29(36)28(16-23-12-8-6-9-13-23)34-31(38)26-17-25(18-27(19-26)35(4)41(5,39)40)30(37)33-22(3)24-14-10-7-11-15-24/h6-15,17-19,21-22,28-29,32,36H,16,20H2,1-5H3,(H,33,37)(H,34,38)/t22-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231931
(CHEMBL403483 | N1-((2S,3R)-4-(3-methoxybenzylamino...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(cc(c2)C(=O)NCc2nc(C)oc2C)N(C)S(C)(=O)=O)c1 Show InChI InChI=1S/C34H41N5O7S/c1-22-31(37-23(2)46-22)20-36-33(41)26-16-27(18-28(17-26)39(3)47(5,43)44)34(42)38-30(15-24-10-7-6-8-11-24)32(40)21-35-19-25-12-9-13-29(14-25)45-4/h6-14,16-18,30,32,35,40H,15,19-21H2,1-5H3,(H,36,41)(H,38,42)/t30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 552 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231940

(CHEMBL252276 | N1-((2S,3R)-3-hydroxy-4-((S)-1-(iso...)Show SMILES CC(C)NC(=O)[C@@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(cc(c1)C(=O)N[C@H](C)c1ccccc1)N(C)S(C)(=O)=O)C(C)C Show InChI InChI=1S/C36H49N5O6S/c1-23(2)33(36(45)38-24(3)4)37-22-32(42)31(18-26-14-10-8-11-15-26)40-35(44)29-19-28(20-30(21-29)41(6)48(7,46)47)34(43)39-25(5)27-16-12-9-13-17-27/h8-17,19-21,23-25,31-33,37,42H,18,22H2,1-7H3,(H,38,45)(H,39,43)(H,40,44)/t25-,31+,32-,33+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 564 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231934
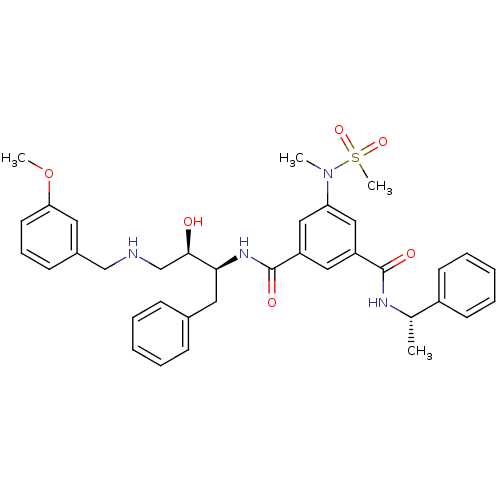
(CHEMBL403269 | N1-((2S,3R)-4-(3-methoxybenzylamino...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(cc(c2)C(=O)N[C@@H](C)c2ccccc2)N(C)S(C)(=O)=O)c1 Show InChI InChI=1S/C36H42N4O6S/c1-25(28-15-9-6-10-16-28)38-35(42)29-20-30(22-31(21-29)40(2)47(4,44)45)36(43)39-33(19-26-12-7-5-8-13-26)34(41)24-37-23-27-14-11-17-32(18-27)46-3/h5-18,20-22,25,33-34,37,41H,19,23-24H2,1-4H3,(H,38,42)(H,39,43)/t25-,33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231939
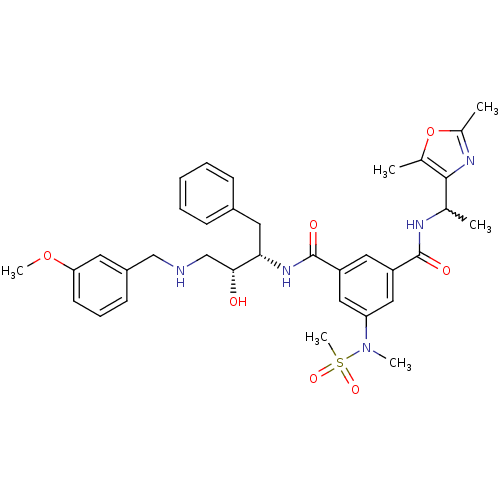
(CHEMBL404144 | N1-((2S,3R)-4-(3-methoxybenzylamino...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(cc(c2)C(=O)NC(C)c2nc(C)oc2C)N(C)S(C)(=O)=O)c1 |w:32.34| Show InChI InChI=1S/C35H43N5O7S/c1-22(33-23(2)47-24(3)38-33)37-34(42)27-17-28(19-29(18-27)40(4)48(6,44)45)35(43)39-31(16-25-11-8-7-9-12-25)32(41)21-36-20-26-13-10-14-30(15-26)46-5/h7-15,17-19,22,31-32,36,41H,16,20-21H2,1-6H3,(H,37,42)(H,39,43)/t22?,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 894 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231936

(CHEMBL253931 | N1-((2R,3S)-1-(3-methoxybenzylamino...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](CC(C)C)NC(=O)c2cc(cc(c2)C(=O)N[C@H](C)c2ccccc2)N(C)S(C)(=O)=O)c1 Show InChI InChI=1S/C33H44N4O6S/c1-22(2)15-30(31(38)21-34-20-24-11-10-14-29(16-24)43-5)36-33(40)27-17-26(18-28(19-27)37(4)44(6,41)42)32(39)35-23(3)25-12-8-7-9-13-25/h7-14,16-19,22-23,30-31,34,38H,15,20-21H2,1-6H3,(H,35,39)(H,36,40)/t23-,30+,31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 916 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231935

((2,5-dimethyloxazol-4-yl)methyl (R)-1-((2S,3R)-4-(...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)[C@H](CS(C)(=O)=O)NC(=O)OCc2nc(C)oc2C)c1 Show InChI InChI=1S/C29H38N4O8S/c1-19-25(31-20(2)41-19)17-40-29(36)33-26(18-42(4,37)38)28(35)32-24(14-21-9-6-5-7-10-21)27(34)16-30-15-22-11-8-12-23(13-22)39-3/h5-13,24,26-27,30,34H,14-18H2,1-4H3,(H,32,35)(H,33,36)/t24-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant memapsin 2 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231937

(CHEMBL403268 | N-{(1S,2R)-1-benzyl-2-hydroxy-3-[(3...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(cc(c2)C(=O)N[C@H](C)c2ccccc2)N(C)S(C)(=O)=O)c1 Show InChI InChI=1S/C36H42N4O6S/c1-25(28-15-9-6-10-16-28)38-35(42)29-20-30(22-31(21-29)40(2)47(4,44)45)36(43)39-33(19-26-12-7-5-8-13-26)34(41)24-37-23-27-14-11-17-32(18-27)46-3/h5-18,20-22,25,33-34,37,41H,19,23-24H2,1-4H3,(H,38,42)(H,39,43)/t25-,33+,34-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of memapsin 2 expressed in CHO cells |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231932

(CHEMBL253930 | N1-((2S,3R)-3-hydroxy-4-((1-(methyl...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1ccc2ccn(c2c1)S(C)(=O)=O)N(C)S(C)(=O)=O)c1ccccc1 Show InChI InChI=1S/C38H43N5O7S2/c1-26(29-13-9-6-10-14-29)40-37(45)31-21-32(23-33(22-31)42(2)51(3,47)48)38(46)41-34(19-27-11-7-5-8-12-27)36(44)25-39-24-28-15-16-30-17-18-43(35(30)20-28)52(4,49)50/h5-18,20-23,26,34,36,39,44H,19,24-25H2,1-4H3,(H,40,45)(H,41,46)/t26-,34+,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of memapsin 2 expressed in CHO cells |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231933

(CHEMBL253929 | N1-((2S,3R)-4-((1H-indol-6-yl)methy...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1ccc2cc[nH]c2c1)N(C)S(C)(=O)=O)c1ccccc1 Show InChI InChI=1S/C37H41N5O5S/c1-25(28-12-8-5-9-13-28)40-36(44)30-20-31(22-32(21-30)42(2)48(3,46)47)37(45)41-34(18-26-10-6-4-7-11-26)35(43)24-38-23-27-14-15-29-16-17-39-33(29)19-27/h4-17,19-22,25,34-35,38-39,43H,18,23-24H2,1-3H3,(H,40,44)(H,41,45)/t25-,34+,35-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of memapsin 2 expressed in CHO cells |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50231937

(CHEMBL403268 | N-{(1S,2R)-1-benzyl-2-hydroxy-3-[(3...)Show SMILES COc1cccc(CNC[C@@H](O)[C@H](Cc2ccccc2)NC(=O)c2cc(cc(c2)C(=O)N[C@H](C)c2ccccc2)N(C)S(C)(=O)=O)c1 Show InChI InChI=1S/C36H42N4O6S/c1-25(28-15-9-6-10-16-28)38-35(42)29-20-30(22-31(21-29)40(2)47(4,44)45)36(43)39-33(19-26-12-7-5-8-13-26)34(41)24-37-23-27-14-11-17-32(18-27)46-3/h5-18,20-22,25,33-34,37,41H,19,23-24H2,1-4H3,(H,38,42)(H,39,43)/t25-,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 696 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231941

(CHEMBL253103 | N1-((2S,3R)-3-hydroxy-4-(isopropyla...)Show SMILES CC(C)NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(cc(c1)C(=O)N[C@H](C)c1ccccc1)N(C)S(C)(=O)=O Show InChI InChI=1S/C31H40N4O5S/c1-21(2)32-20-29(36)28(16-23-12-8-6-9-13-23)34-31(38)26-17-25(18-27(19-26)35(4)41(5,39)40)30(37)33-22(3)24-14-10-7-11-15-24/h6-15,17-19,21-22,28-29,32,36H,16,20H2,1-5H3,(H,33,37)(H,34,38)/t22-,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of memapsin 2 expressed in CHO cells |
Bioorg Med Chem Lett 18: 1031-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.028
BindingDB Entry DOI: 10.7270/Q2513XZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data