

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558211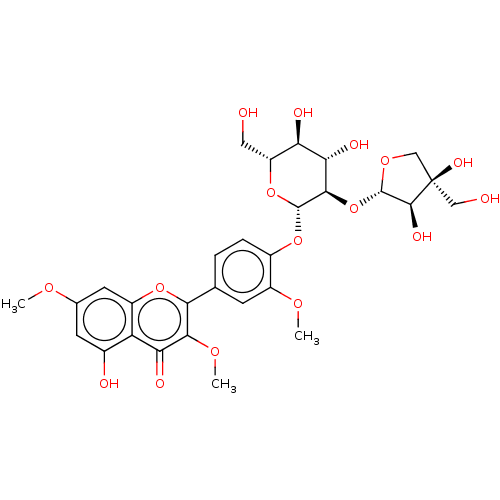 (CHEMBL2332114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558212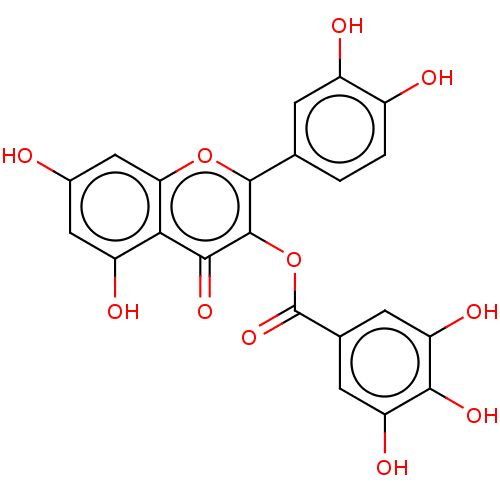 (3-O-Galloylquercetin | CHEMBL1935379) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558210 (CHEMBL4798780) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50379283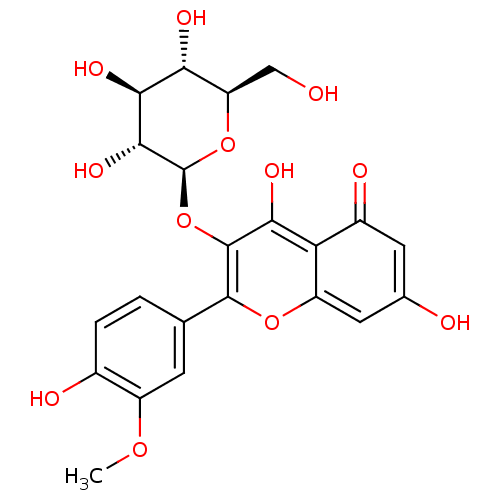 (CHEMBL234316) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558209 (CHEMBL4761282) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50056315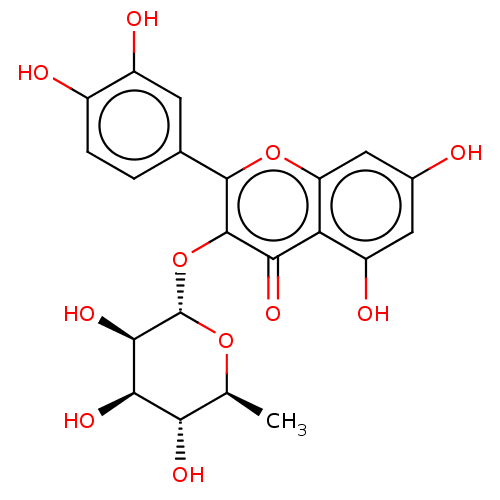 (CHEBI:17558 | Quercitrin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558213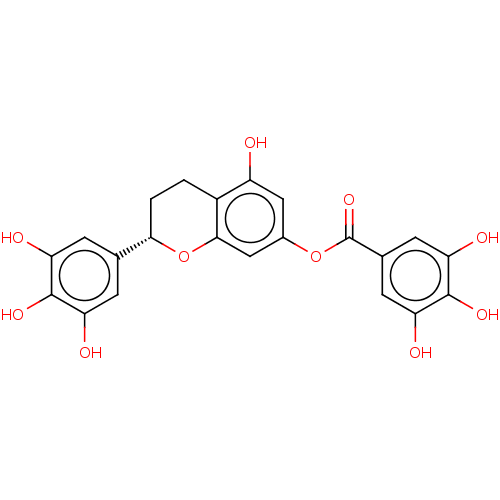 (CHEMBL4747916) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of sEH (unknown origin) using varying levels of PHOME as substrate by Dixon plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50196661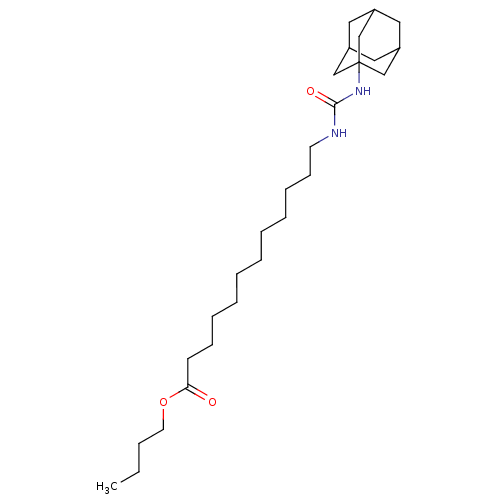 (12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558213 (CHEMBL4747916) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558212 (3-O-Galloylquercetin | CHEMBL1935379) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50056315 (CHEBI:17558 | Quercitrin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558211 (CHEMBL2332114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558209 (CHEMBL4761282) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50558210 (CHEMBL4798780) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50379283 (CHEMBL234316) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.034 BindingDB Entry DOI: 10.7270/Q2833WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447860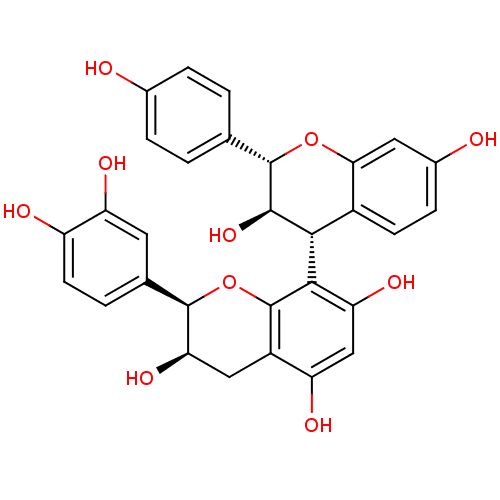 (CHEMBL3114757) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM23416 (α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447857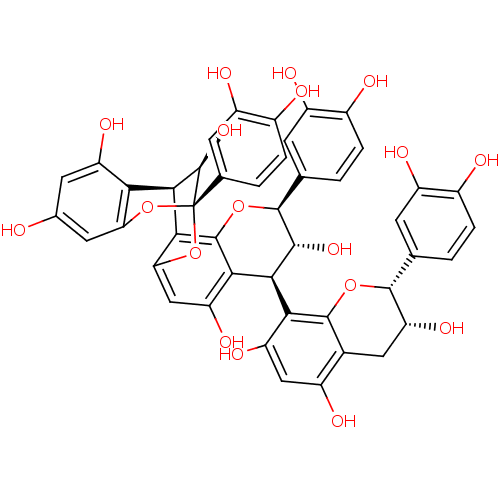 (Aescultitannin B) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447858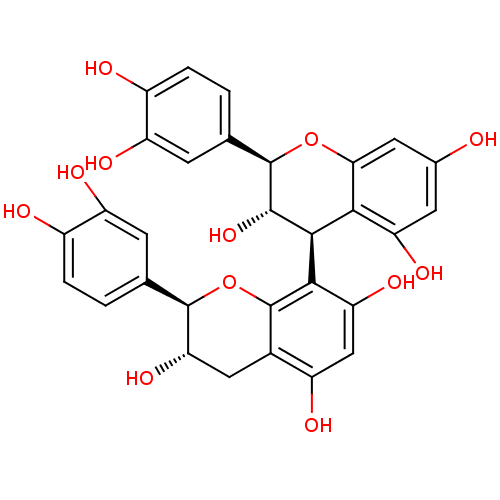 (CHEBI:75630 | CHEMBL501490) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447859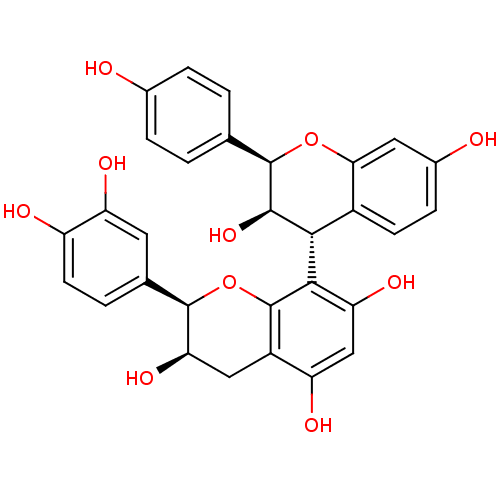 (CHEMBL3114756) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447856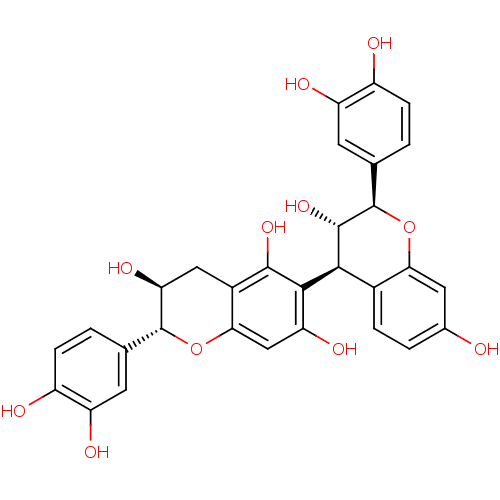 (CHEMBL3114755) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM23417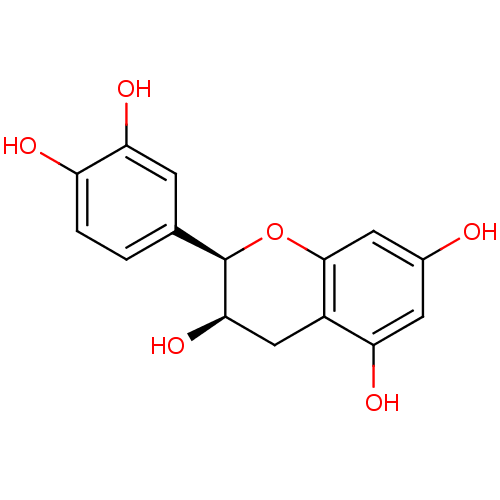 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447854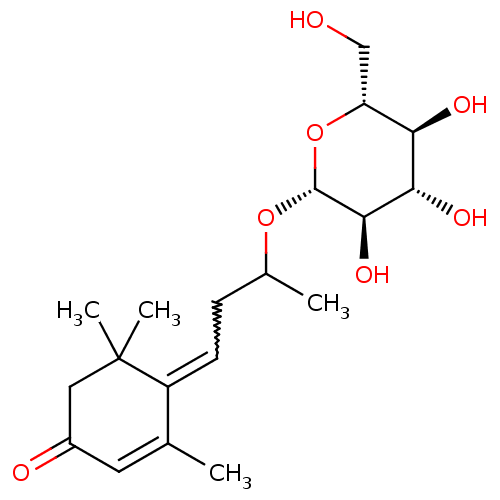 (CHEMBL3114753 | CHEMBL3114754) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447853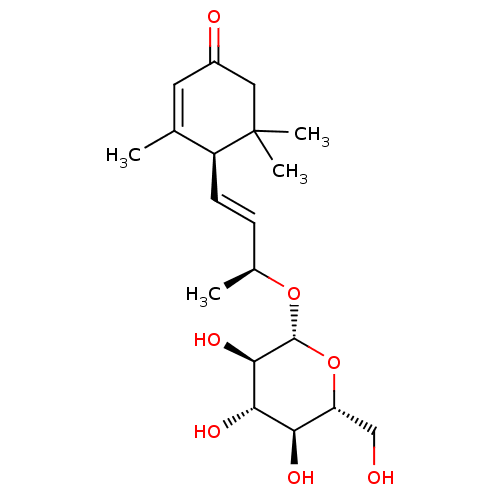 (CHEMBL1808110) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447852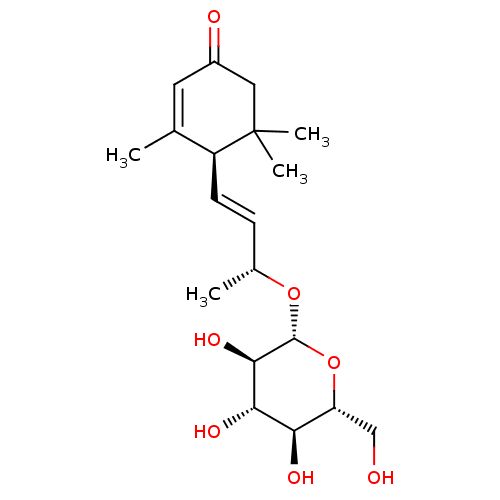 (CHEMBL3114752) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447861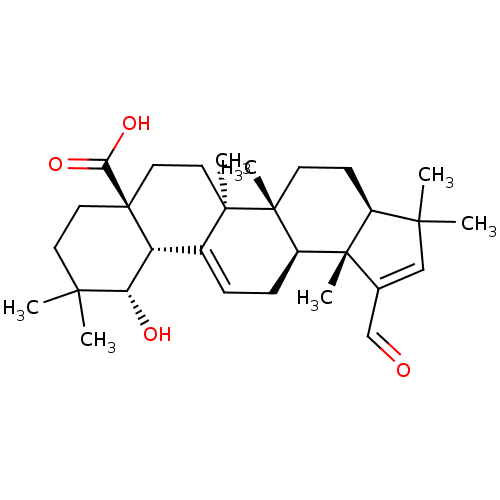 (CHEMBL3114759) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447862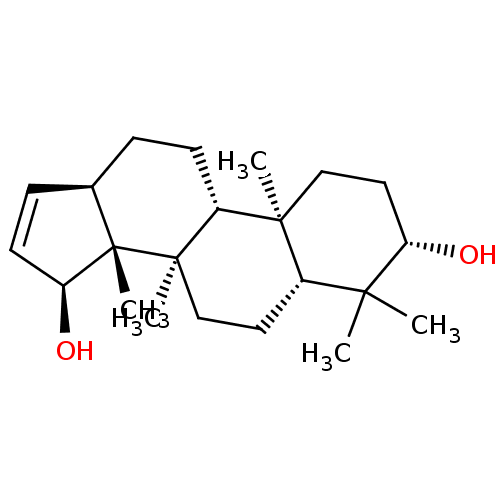 (CHEMBL3114760) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50447854 (CHEMBL3114753 | CHEMBL3114754) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using p-nitrophenyl-alpha-d-glucopyranoside as substrate incubated for 10 mins prior to substrate addition measu... | Bioorg Med Chem Lett 24: 1192-6 (2014) Article DOI: 10.1016/j.bmcl.2013.12.098 BindingDB Entry DOI: 10.7270/Q2TM7CMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379791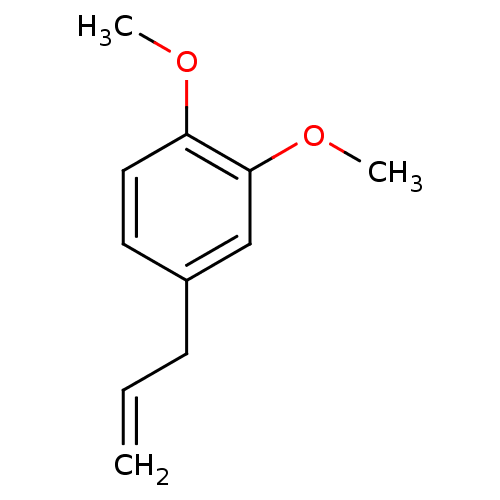 (METHYLEUGENOL | Methyl eugenol (1)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379792 (CHEMBL2011538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379793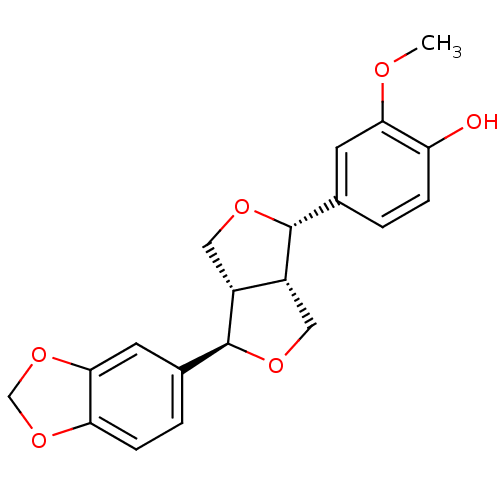 (PLUVIATILOL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379794 (PINORESINOL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379795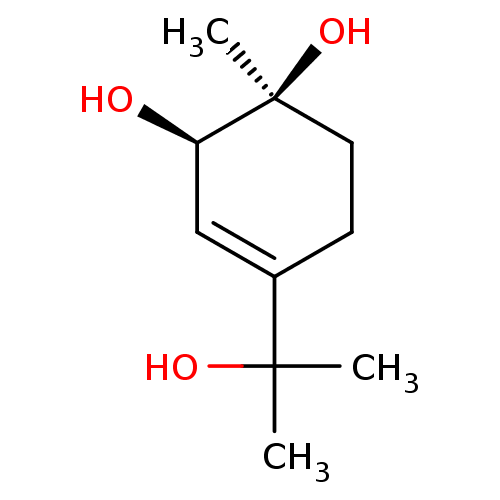 (CHEMBL2011541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379791 (METHYLEUGENOL | Methyl eugenol (1)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379796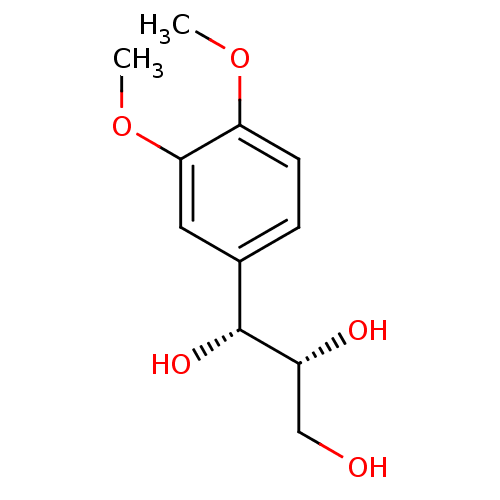 (CHEMBL2011543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379797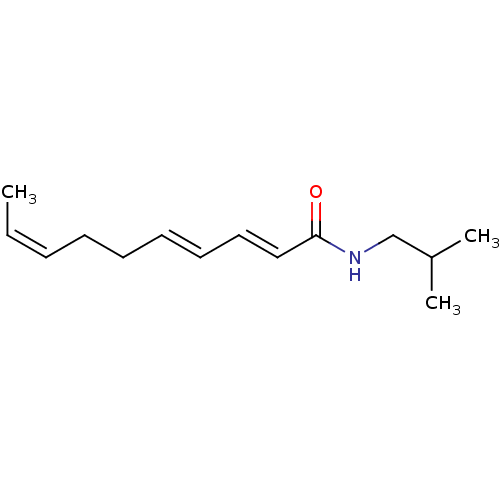 (CHEMBL2011544) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50371235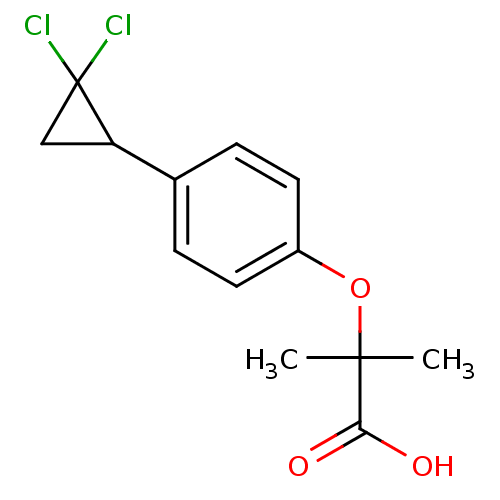 (CIPROFIBRATE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379792 (CHEMBL2011538) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379793 (PLUVIATILOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.87E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379794 (PINORESINOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.86E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379798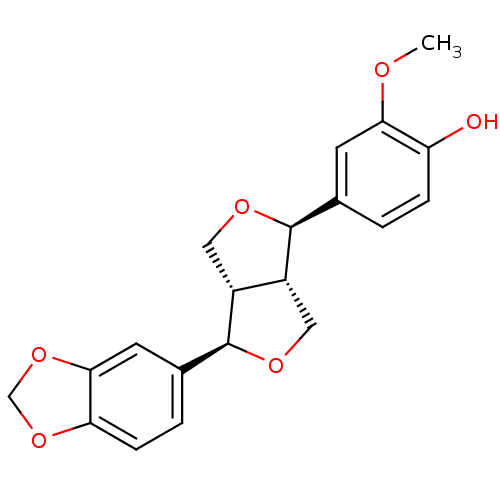 (PIPERITOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50379798 (PIPERITOL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.32E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379795 (CHEMBL2011541) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379796 (CHEMBL2011543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50379797 (CHEMBL2011544) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085045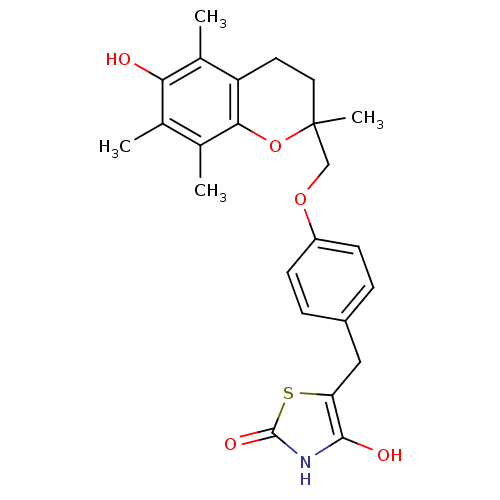 (5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 730 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50379792 (CHEMBL2011538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50379793 (PLUVIATILOL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.31E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50379794 (PINORESINOL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.95E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |