

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609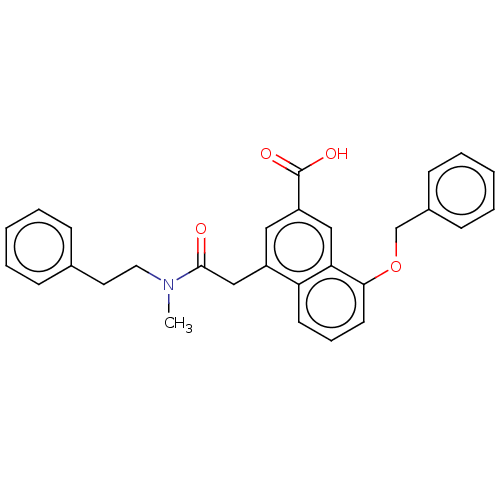 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against LTB4 receptor using guinea pig (GP) spleen cell membrane | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9665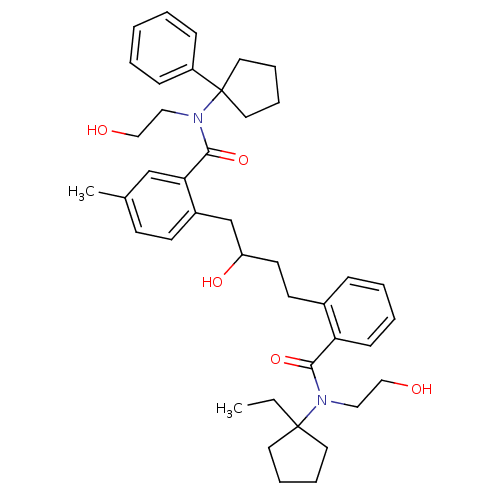 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304781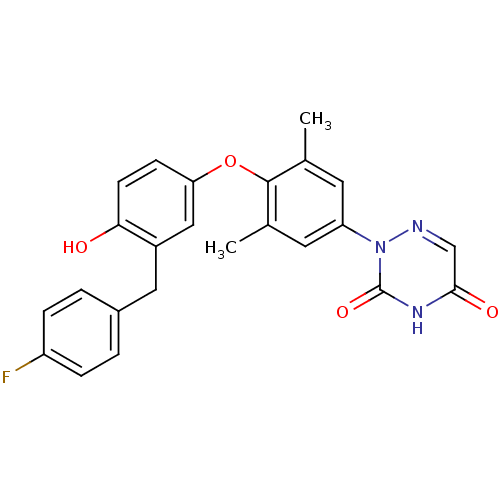 (2-(4-(3-(4-fluorobenzyl)-4-hydroxyphenoxy)-3,5-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304777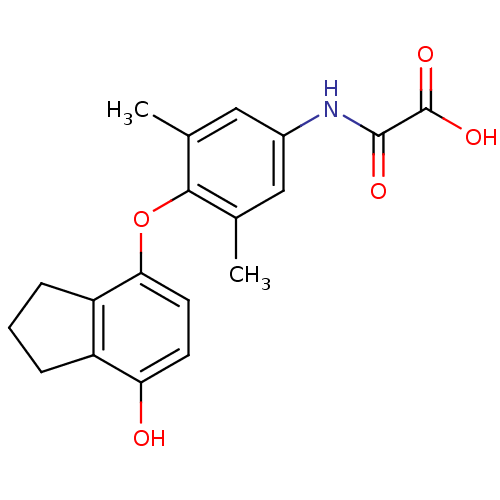 (2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304778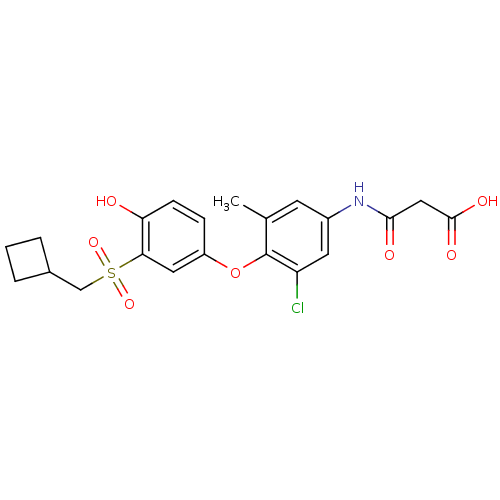 (3-(3-chloro-4-(3-(cyclobutylmethylsulfonyl)-4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304780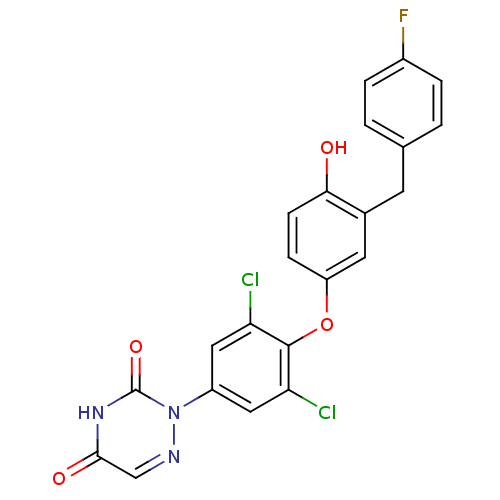 (2-(3,5-dichloro-4-(3-(4-fluorobenzyl)-4-hydroxyphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304777 (2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123049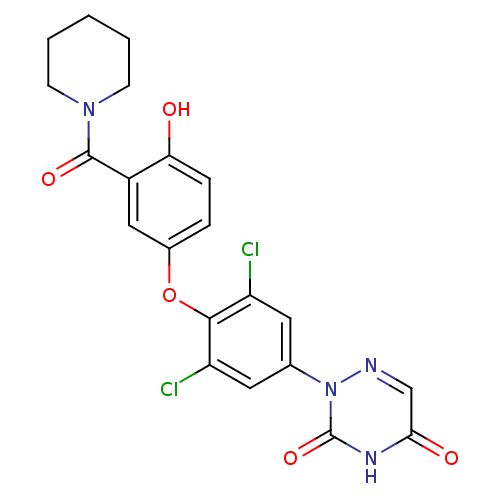 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462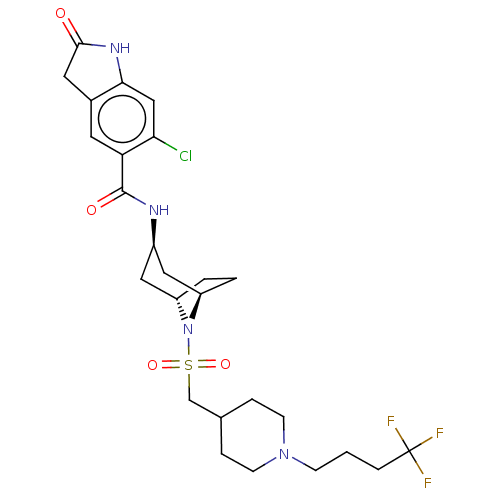 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against monkey neutrophil LTB4 receptor 2 min after an iv dose of 3 mg/kg . | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063920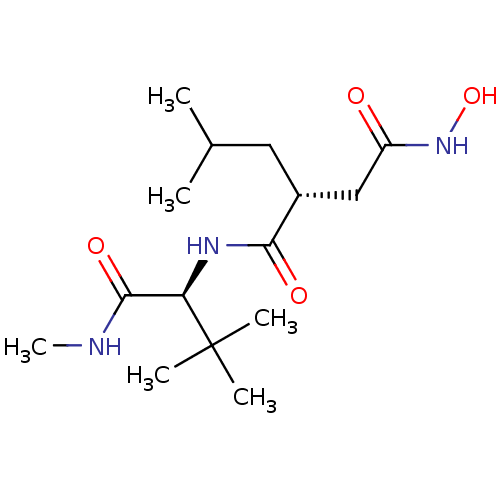 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304779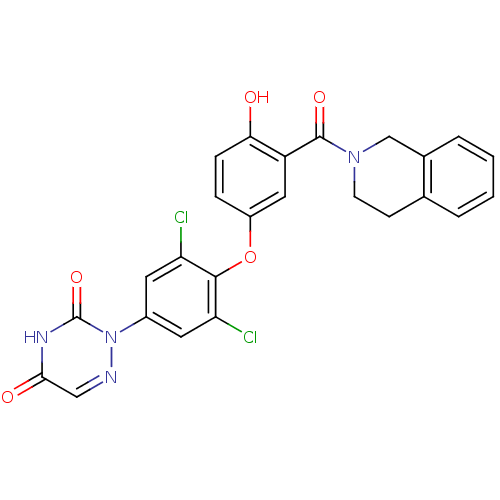 (2-(3,5-dichloro-4-(4-hydroxy-3-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9674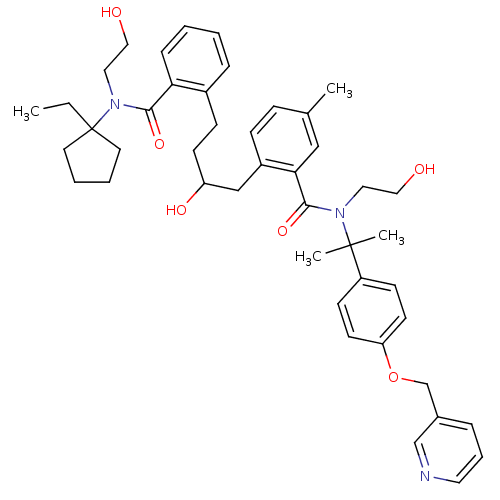 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063920 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3425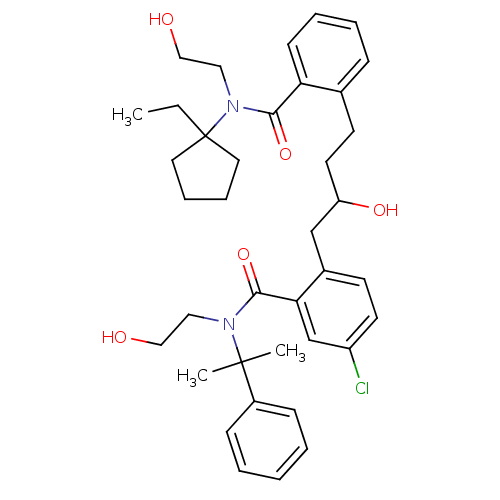 (5-chloro-2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414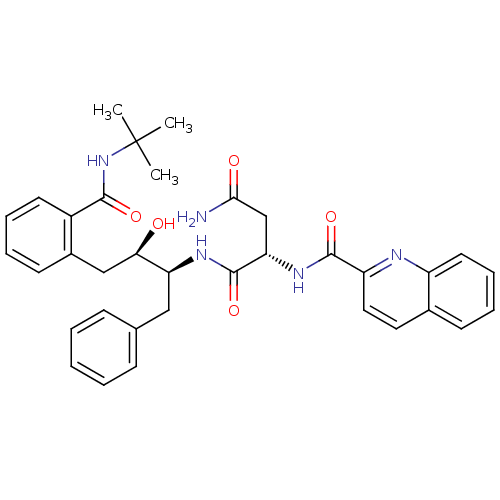 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304778 (3-(3-chloro-4-(3-(cyclobutylmethylsulfonyl)-4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304781 (2-(4-(3-(4-fluorobenzyl)-4-hydroxyphenoxy)-3,5-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9669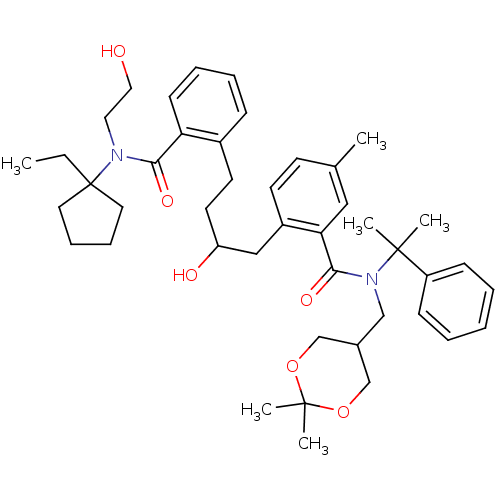 (N-(1-Ethylcyclopentyl)-2-[4-[2-[(2,2-dimethyl[1,3]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Mixed type inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed N-ter... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9673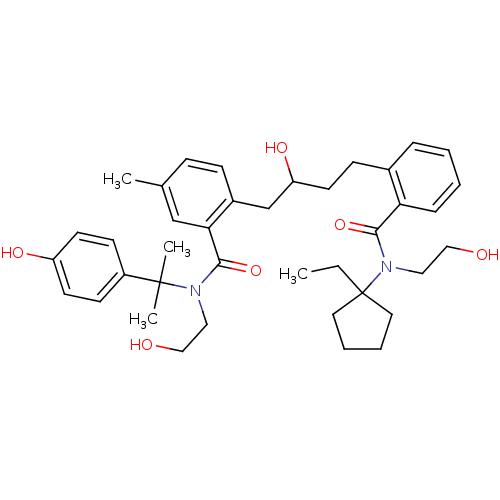 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9666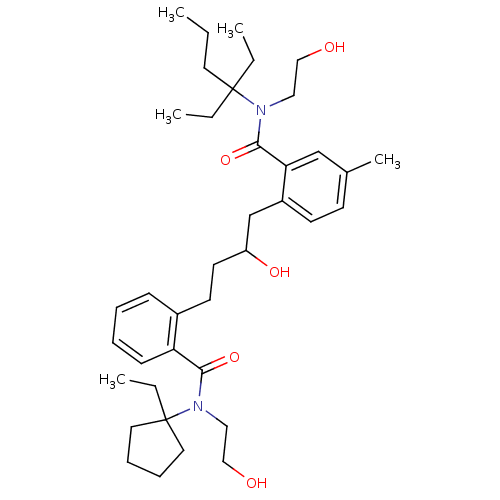 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304780 (2-(3,5-dichloro-4-(3-(4-fluorobenzyl)-4-hydroxyphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9664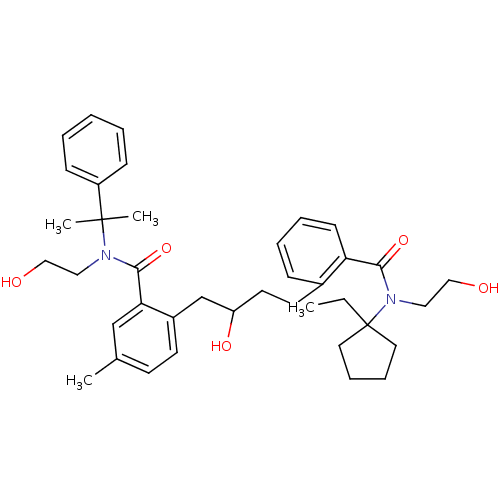 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9675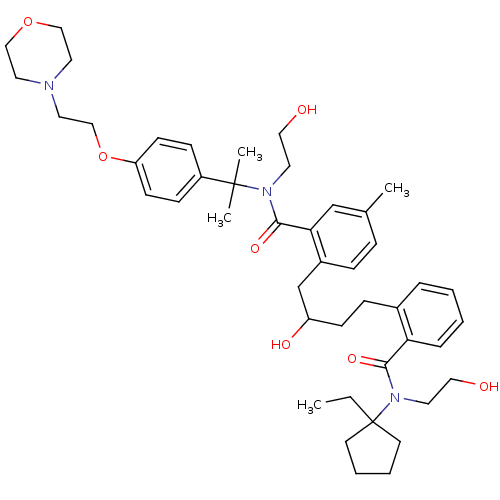 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9670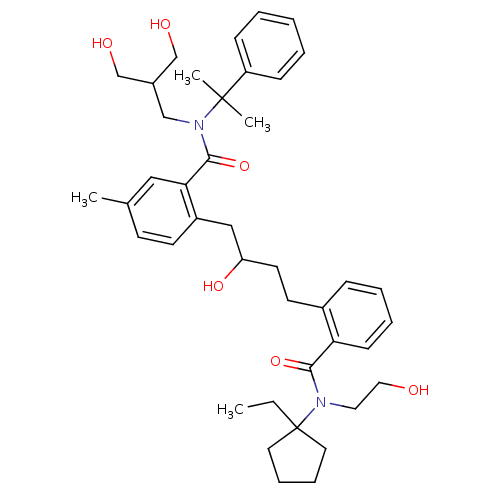 (2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9672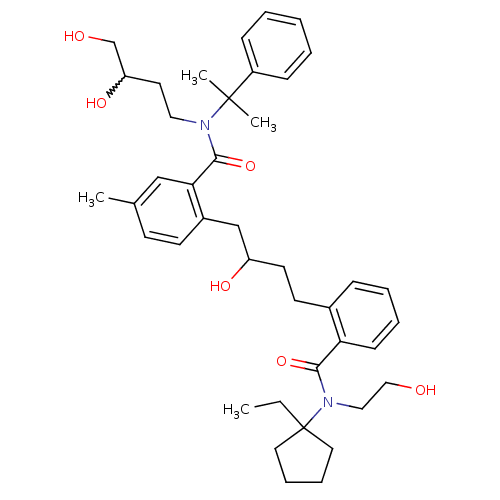 (1-[2-[N-(alpha,alpha-Dimethylbenzyl)-N-(3,4-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103099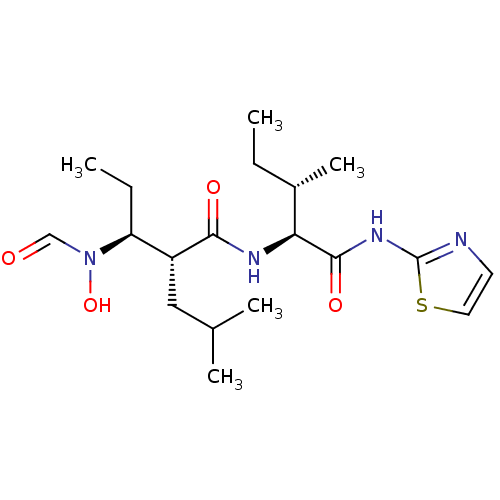 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103097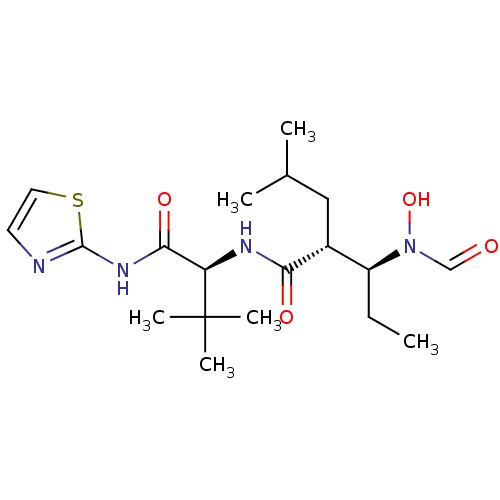 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103097 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103102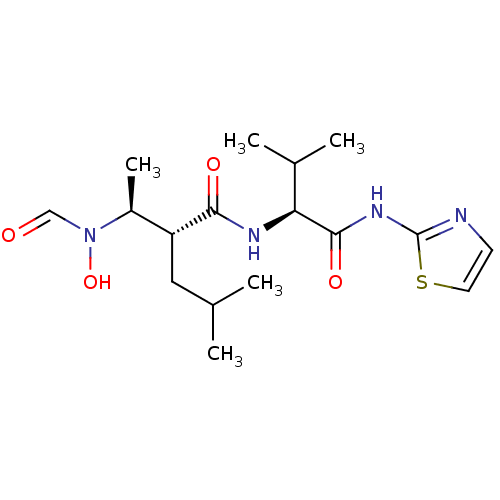 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103092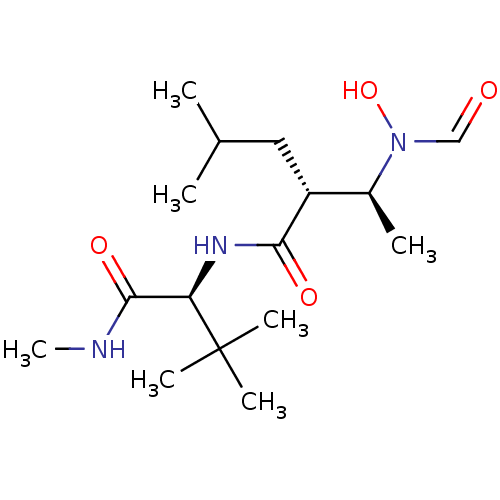 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103096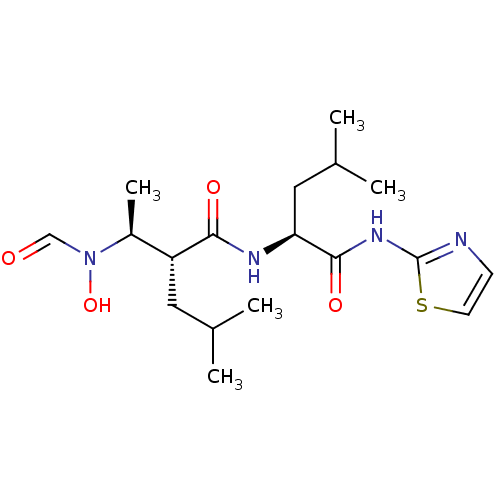 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9663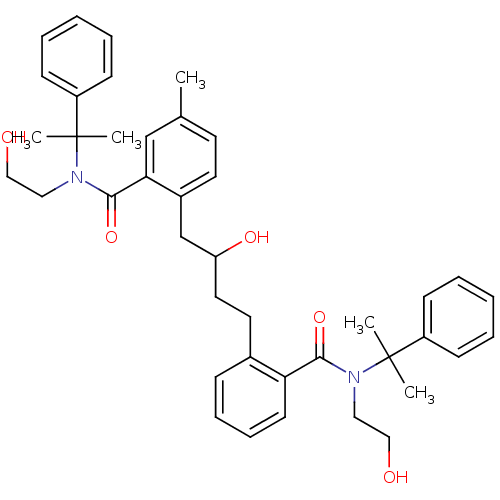 (2-(2-hydroxy-4-{2-[(2-hydroxyethyl)(2-phenylpropan...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50123049 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103093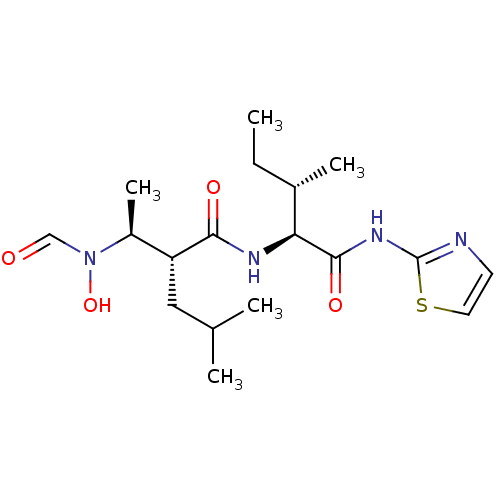 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103098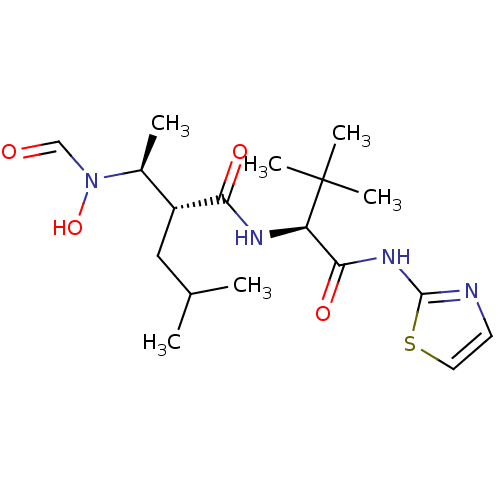 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3423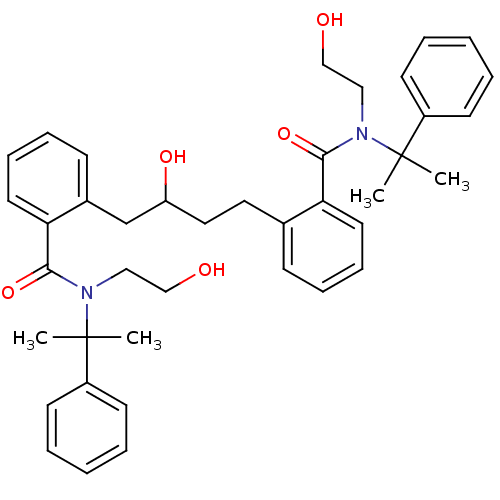 (2-(3-hydroxy-4-{2-[(2-hydroxyethyl)(2-phenylpropan...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu-(p-NO2-Phe)-Glu-Ala-Nleu-Ser was use... | J Med Chem 39: 2795-811 (1996) Article DOI: 10.1021/jm960092w BindingDB Entry DOI: 10.7270/Q2028PRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103092 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103099 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50103098 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-1 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103102 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103097 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103095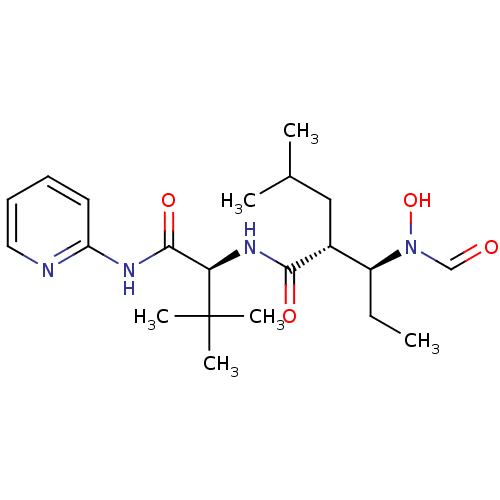 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103093 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50103101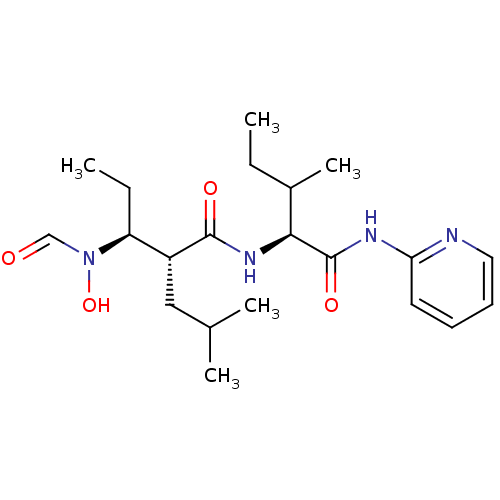 ((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitionof Tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50103100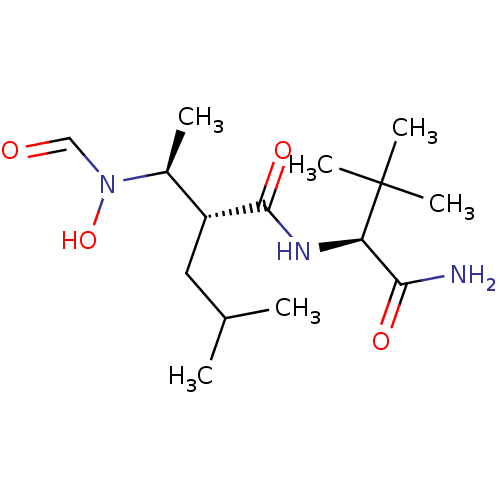 ((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-9 | Bioorg Med Chem Lett 11: 2147-51 (2001) BindingDB Entry DOI: 10.7270/Q2QZ298V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5434 total ) | Next | Last >> |