

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50103634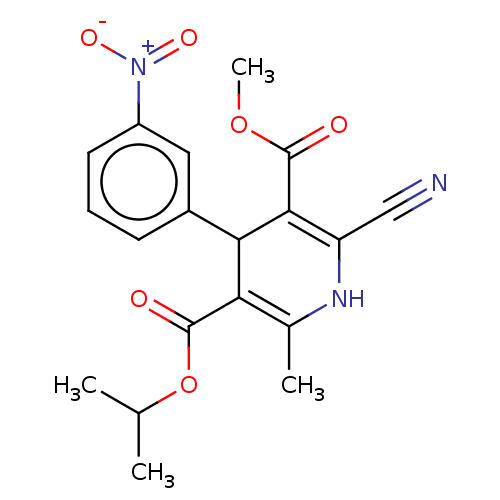 (CL-287389 | FK-235 | Nilvadipine | Nivadipine | SK...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50227967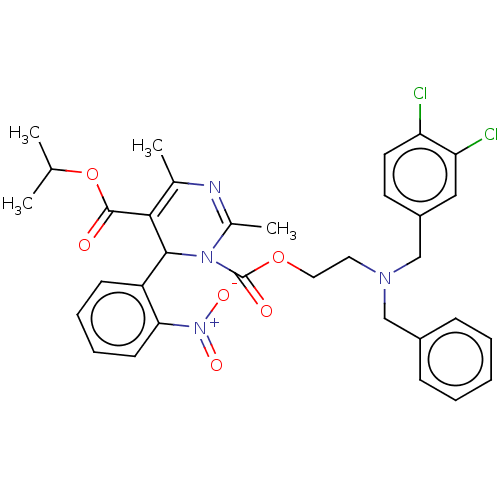 (CHEMBL78973) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]- Nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101817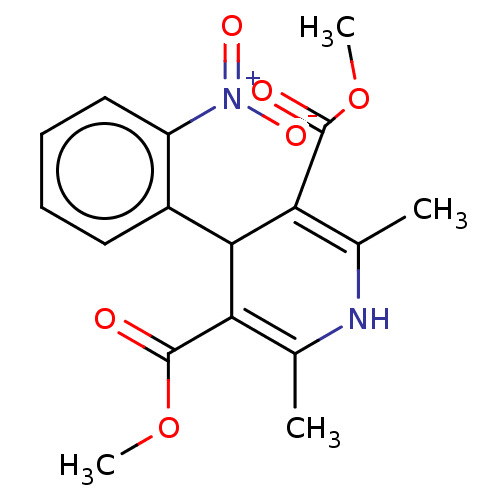 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101815 (CHEBI:7550 | Nicardipine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430986 (CHEMBL2338312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50227968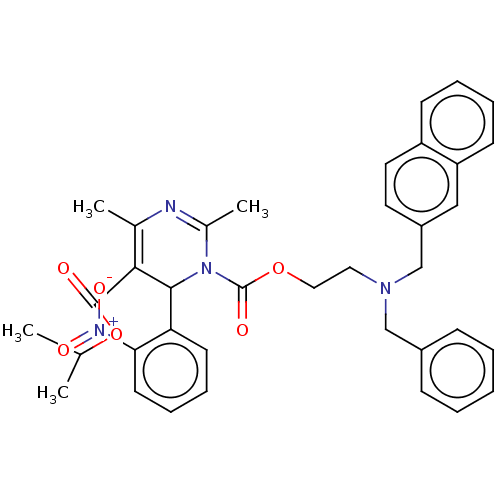 (CHEMBL310953) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]- Nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50227969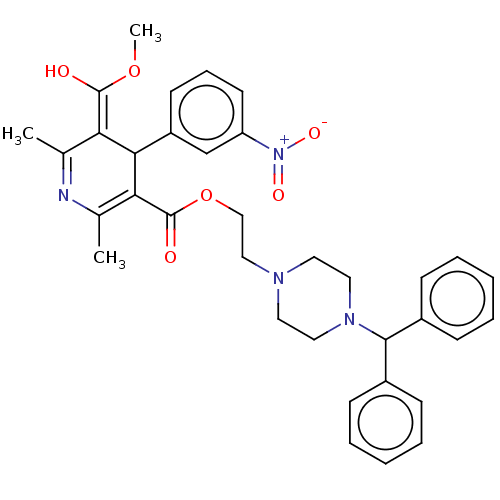 (CHEMBL312176 | CV-4093) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]- Nitrendipine binding to L-type calcium channels of rat cerebral cortex | J Med Chem 32: 2399-406 (1989) BindingDB Entry DOI: 10.7270/Q2GH9M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430985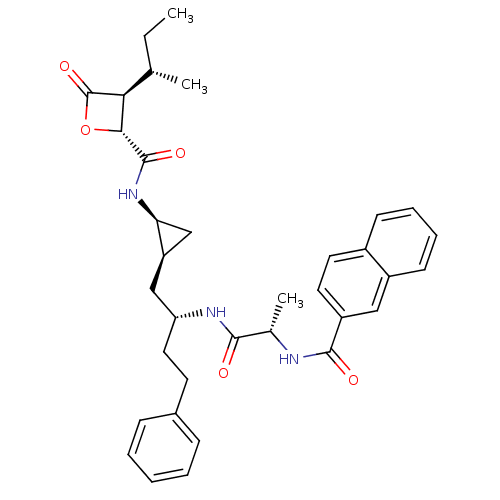 (CHEMBL2338319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431011 (CHEMBL2331625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430991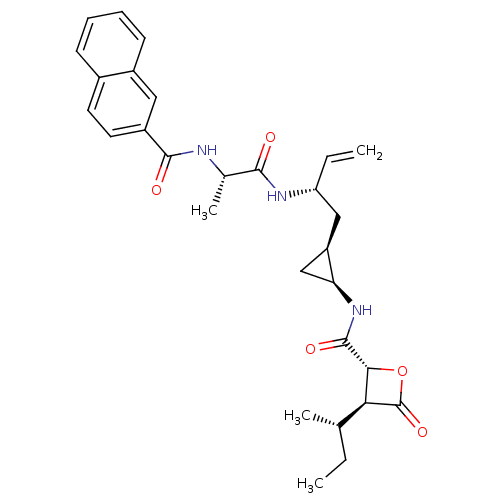 (CHEMBL2338308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431010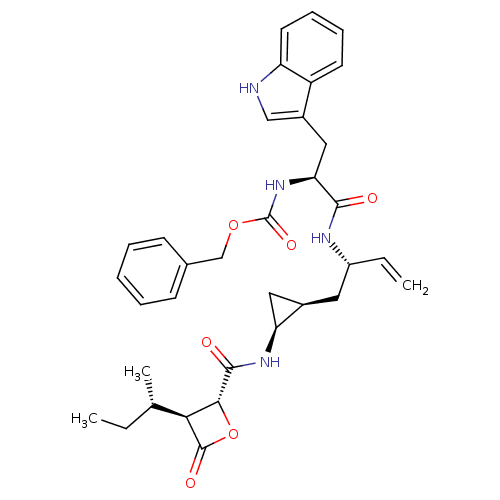 (CHEMBL2337887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431009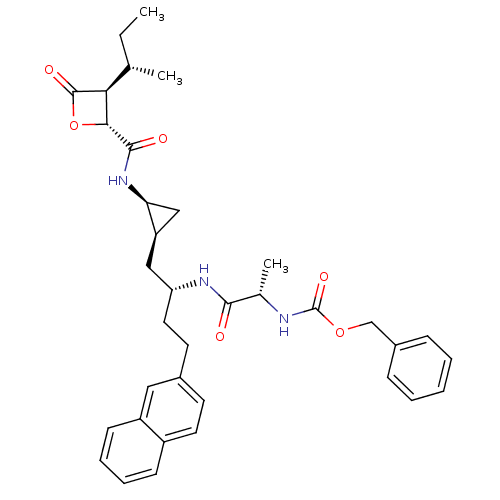 (CHEMBL2338317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430988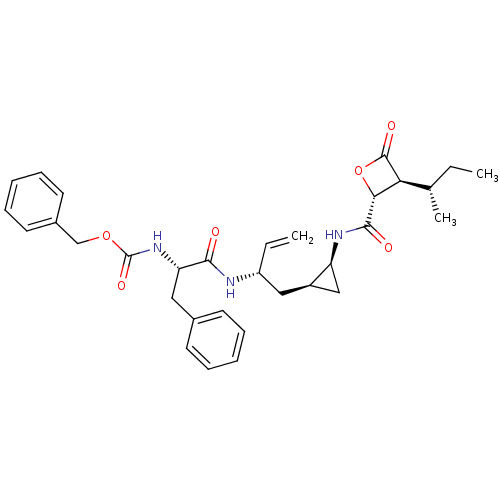 (CHEMBL2337886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431008 (CHEMBL2338318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431007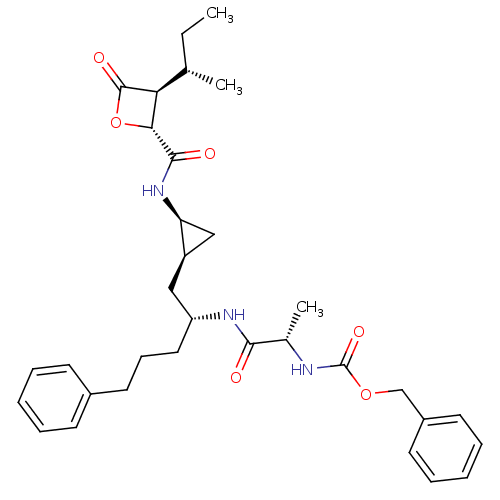 (CHEMBL2338313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431006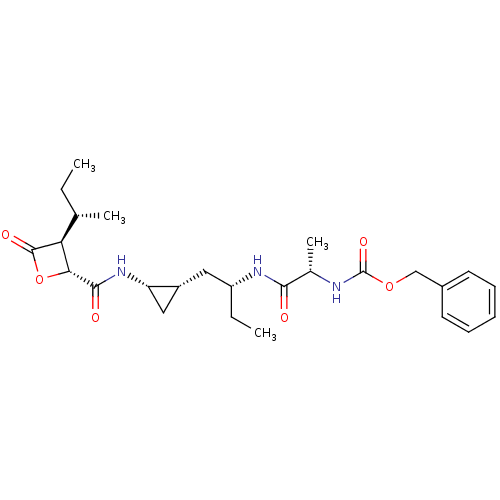 (CHEMBL2338310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431005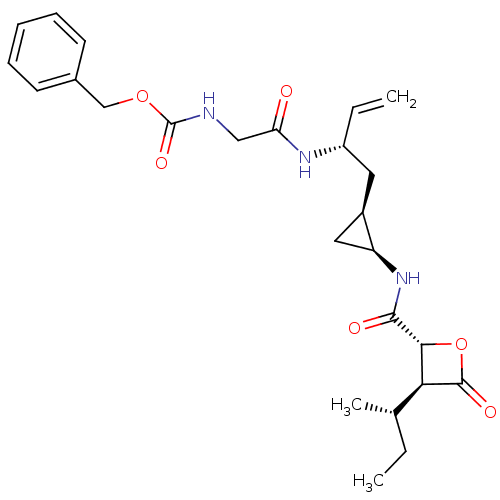 (CHEMBL2337884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431004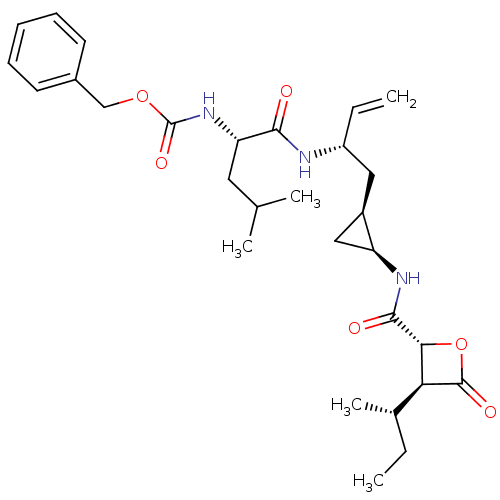 (CHEMBL2337885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430987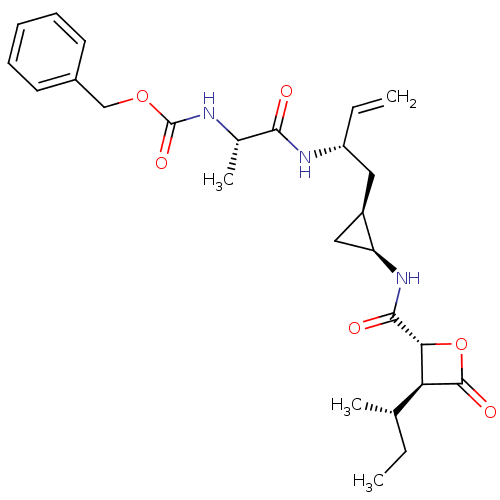 (CHEMBL2337877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431003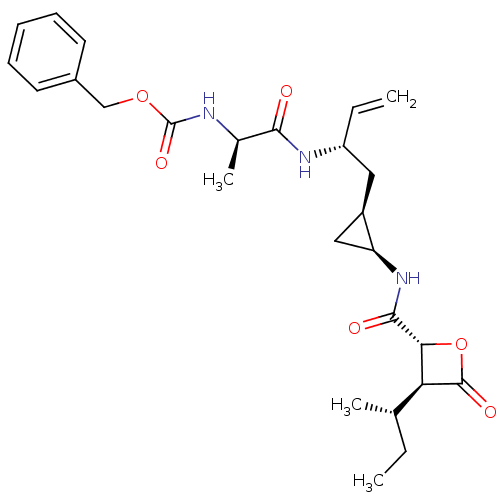 (CHEMBL2337883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430989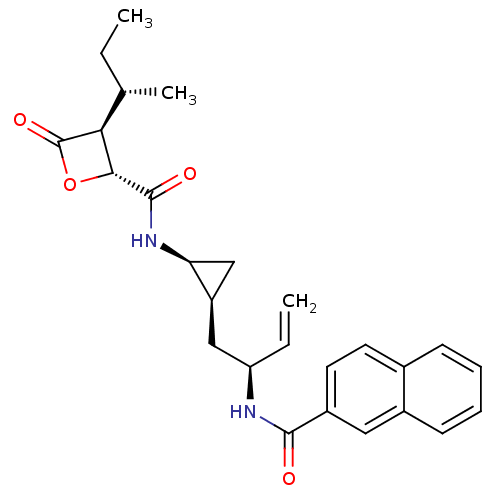 (CHEMBL2337881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431002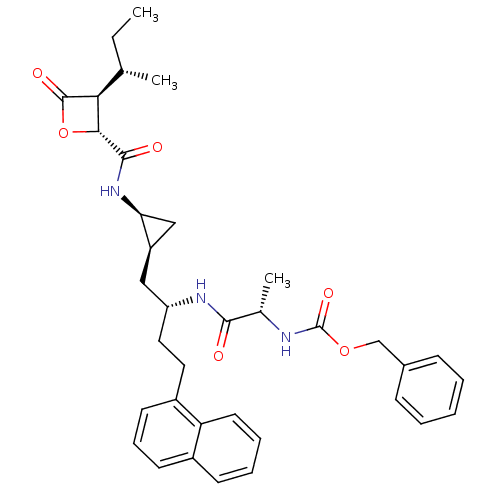 (CHEMBL2338316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431001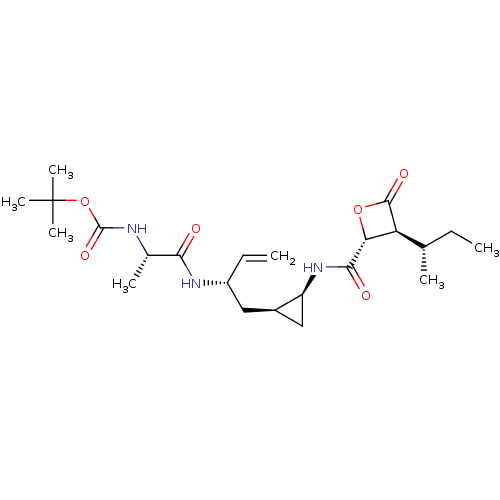 (CHEMBL2338309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50431000 (CHEMBL2338307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430999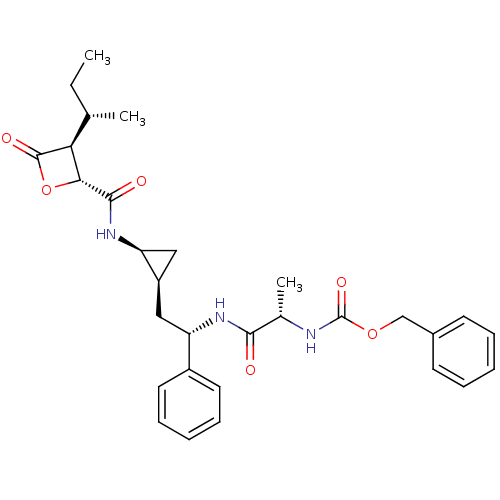 (CHEMBL2338311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430998 (CHEMBL2337880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430997 (CHEMBL2338314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430996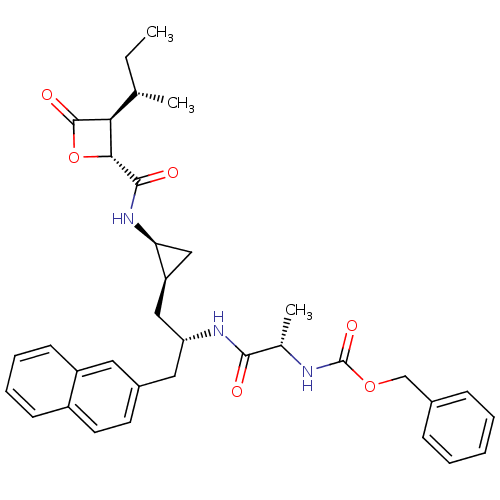 (CHEMBL2338315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430995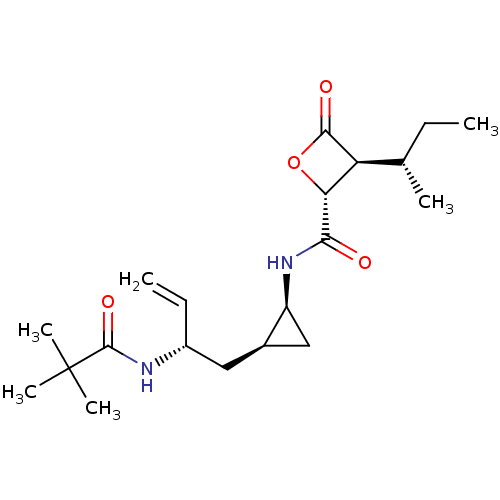 (CHEMBL2337879) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430994 (CHEMBL2337878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430993 (CHEMBL2338306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430992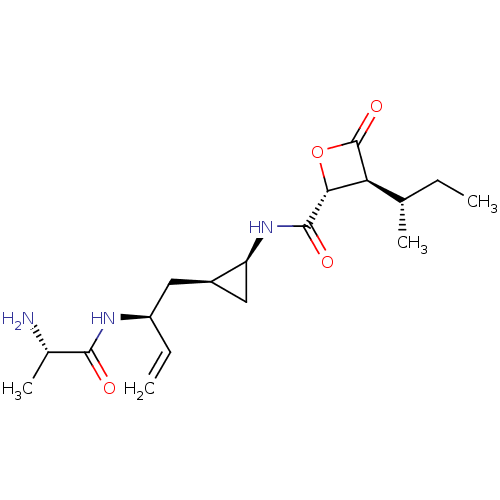 (CHEMBL2337882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50430989 (CHEMBL2337881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50430991 (CHEMBL2338308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50430991 (CHEMBL2338308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430990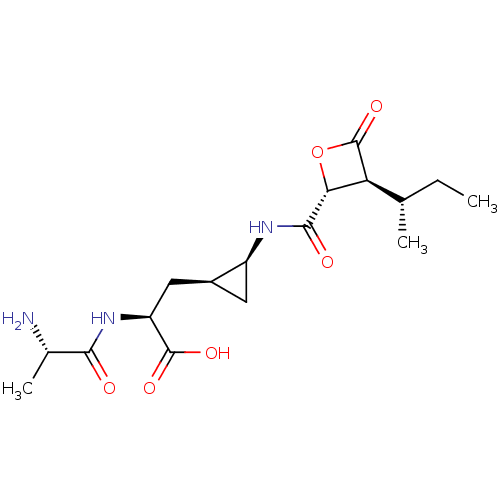 (BELACTOSIN A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50430987 (CHEMBL2337877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50430988 (CHEMBL2337886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50430989 (CHEMBL2337881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50430986 (CHEMBL2338312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50430988 (CHEMBL2337886) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50430986 (CHEMBL2338312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50430987 (CHEMBL2337877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50430985 (CHEMBL2338319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of caspase-like activity of human 20S proteasome beta 1 subunit using Ac-nLPnLD-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50430985 (CHEMBL2338319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of trypsin-like activity of human 20S proteasome beta 2 subunit using Ac-RLR-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||