

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826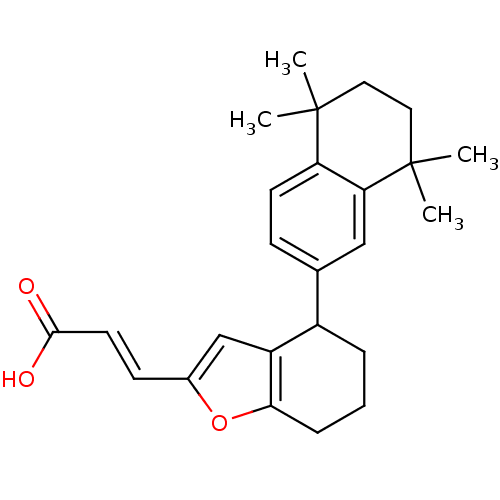 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143827 ((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143825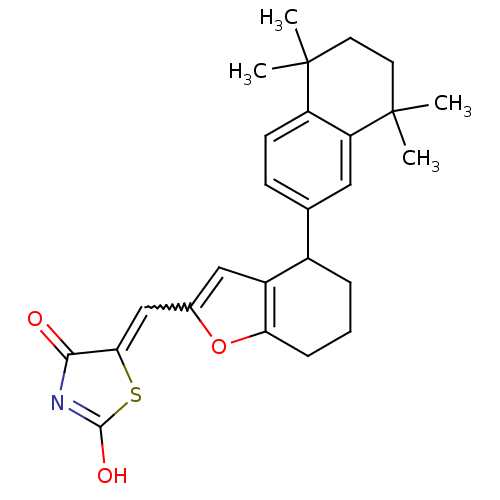 (5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143832 ((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143833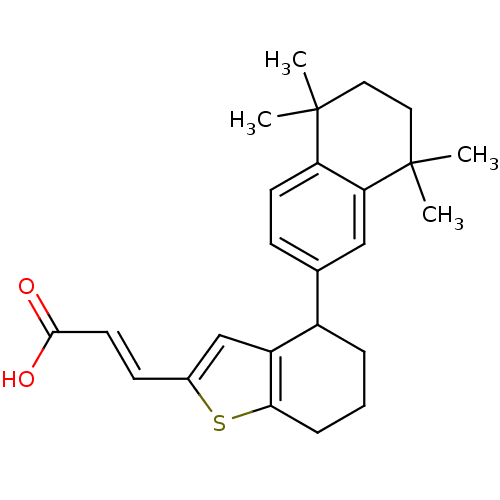 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50409928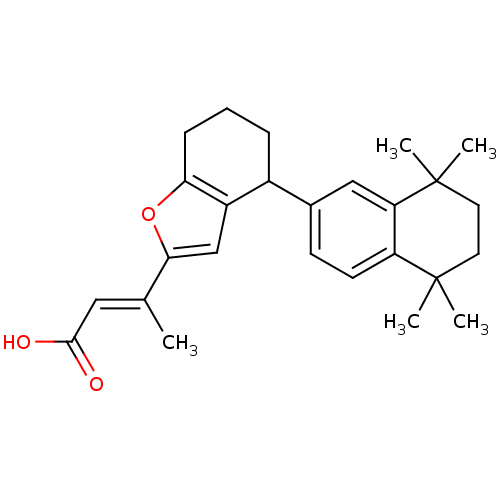 (CHEMBL2113737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50409929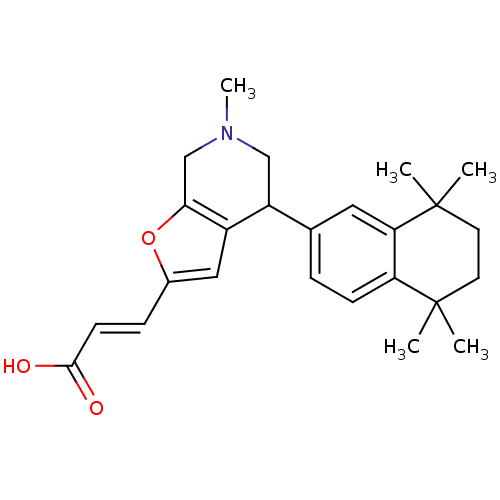 (CHEMBL2113736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143824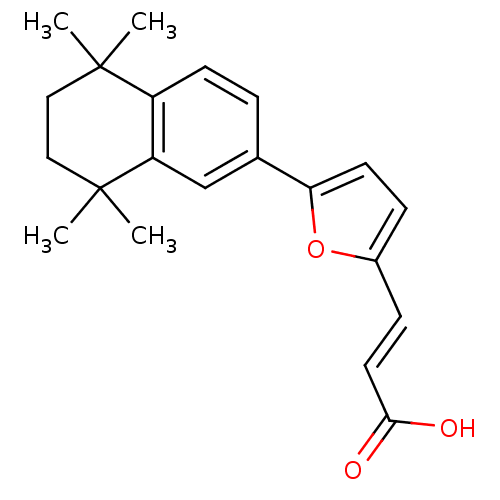 ((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143821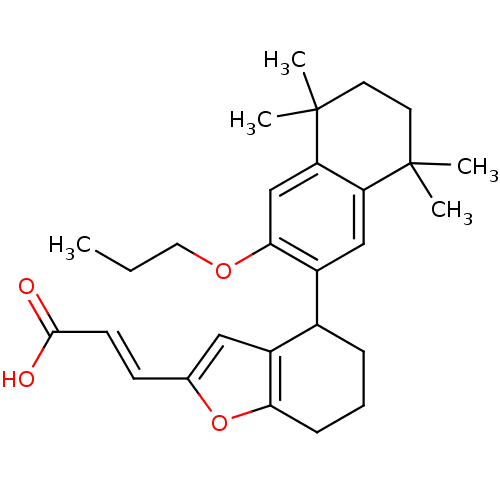 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143835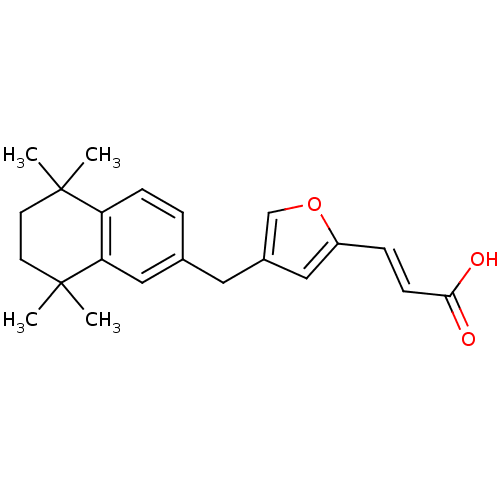 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143831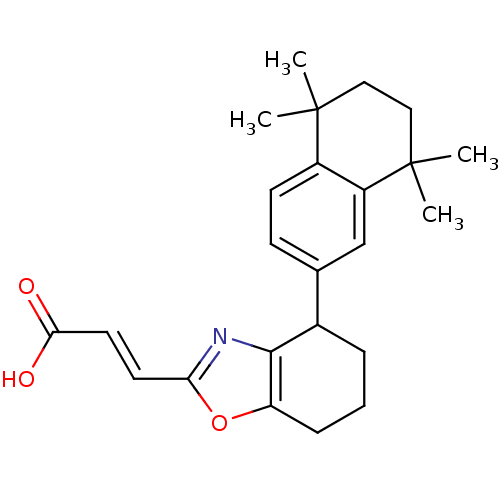 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143823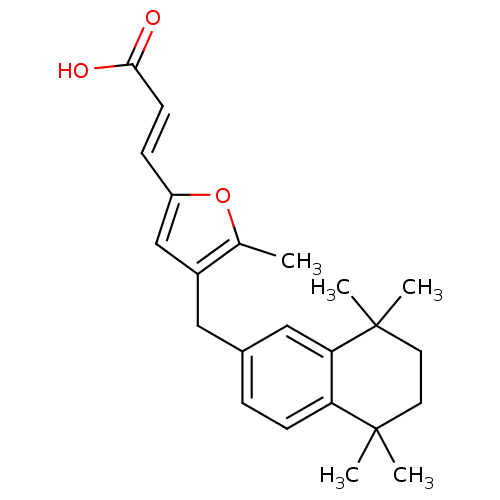 ((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143828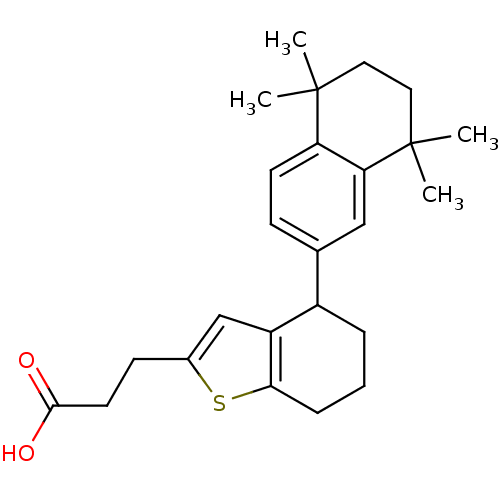 (3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143831 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143830 (4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143825 (5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143823 ((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143827 ((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143824 ((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143833 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143828 (3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50409928 (CHEMBL2113737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50409929 (CHEMBL2113736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143835 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143832 ((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143821 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143834 (5-[1-[5-(3-Bromo-phenyl)-furan-2-yl]-meth-(Z)-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143830 (4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143836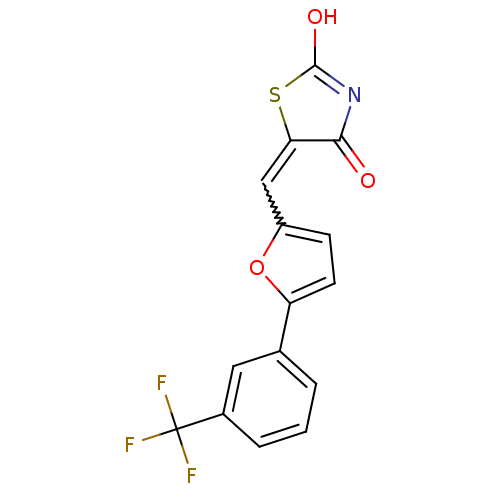 (5-[1-[5-(3-Trifluoromethyl-phenyl)-furan-2-yl]-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7751 ((12Z)-12-{[(4-{[2-(2-hydroxyethoxy)ethyl]sulfamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human PPAR delta receptor | Bioorg Med Chem Lett 13: 1517-21 (2003) BindingDB Entry DOI: 10.7270/Q2VQ322G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50127222 (CHEMBL38508 | GW-0742 | {4-[2-(3-Fluoro-4-trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human PPAR delta receptor | Bioorg Med Chem Lett 13: 1517-21 (2003) BindingDB Entry DOI: 10.7270/Q2VQ322G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7753 (N-methyl-4-({[(12Z)-11-oxo-3-thia-5,10-diazatricyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7692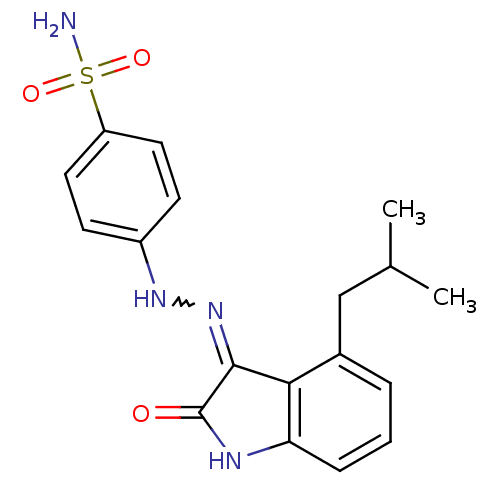 (4-[N -(4-Isobutyl-2-oxo-1,2-dihydro-indol-3-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7693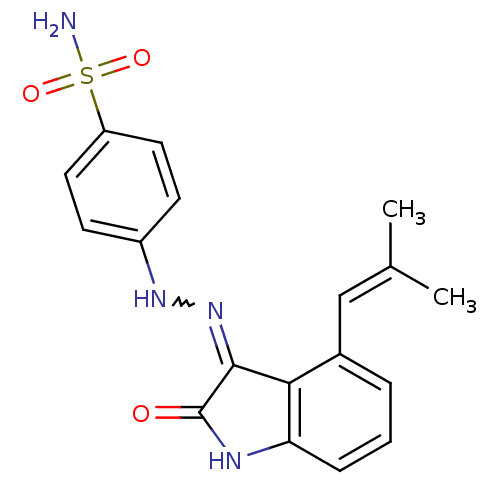 (4-{2-[(3Z)-4-(2-methylprop-1-en-1-yl)-2-oxo-2,3-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7746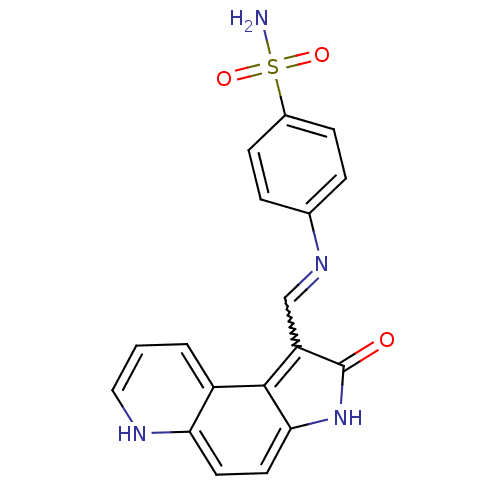 (4-({[(3Z)-4-oxo-5,10-diazatricyclo[7.4.0.0^{2,6}]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7745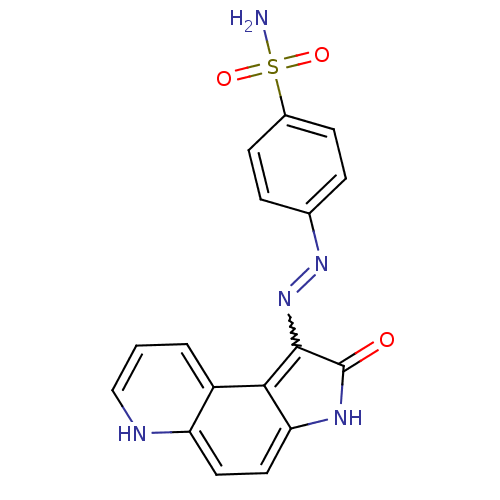 (4-[N -2-Oxo-2,3-dihydropyrrolo[3,2-f]quinolin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7727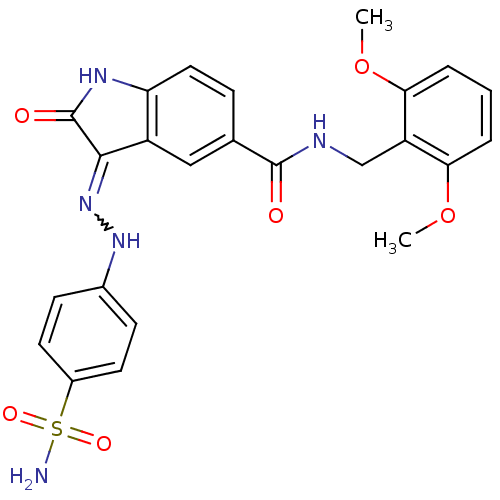 ((3Z)-N-[(2,6-dimethoxyphenyl)methyl]-2-oxo-3-[2-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7719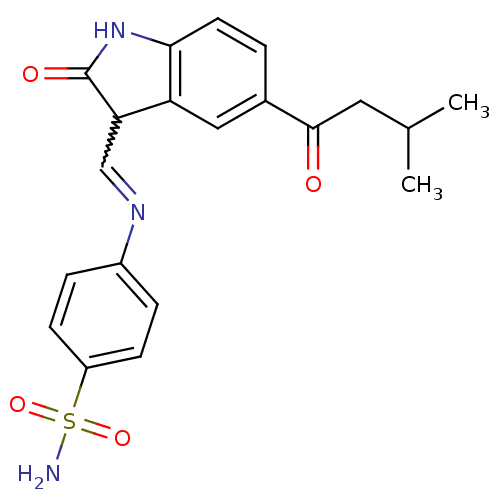 (4-({[(3Z)-5-(3-methylbutanoyl)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7688 (4-({1-[(3Z)-5-(1,3-oxazol-5-yl)-2-oxo-2,3-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7717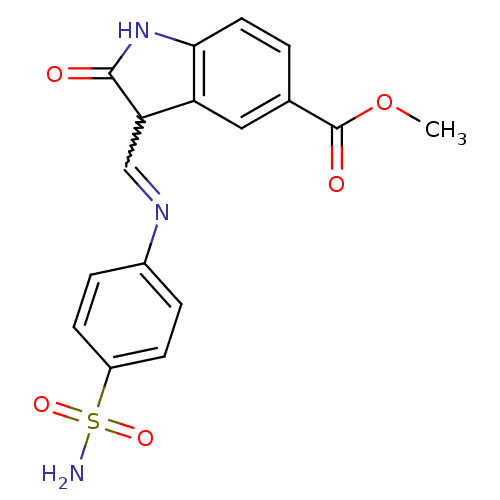 (Oxindole-Based Inhibitor 53 | methyl (3Z)-2-oxo-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7725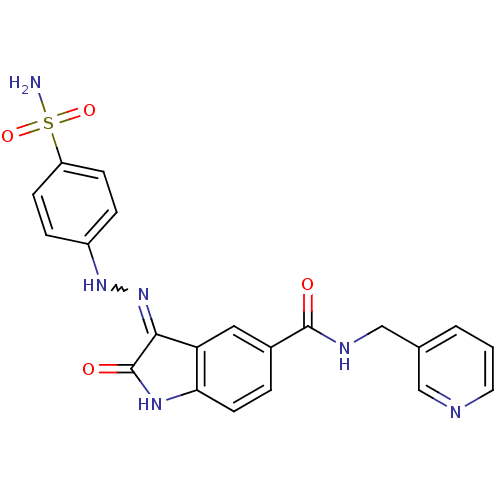 ((3Z)-2-oxo-N-(pyridin-3-ylmethyl)-3-[2-(4-sulfamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7741 (4-[N -(1-Chloro-7-oxo-6,7-dihydro-3H-pyrrolo[3,2-e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7686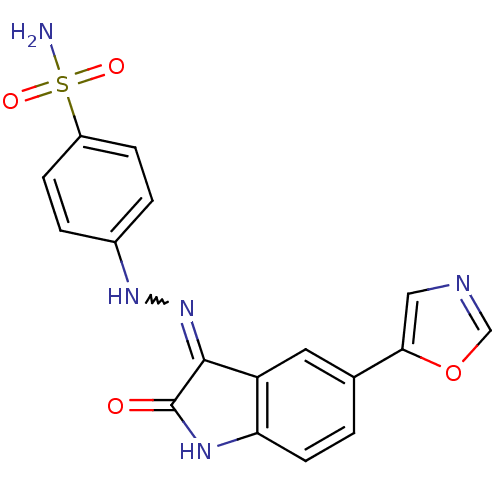 (4-[1-(5-Oxazol-5-yl-2-oxo-1,2-dihydro-indol-3-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7691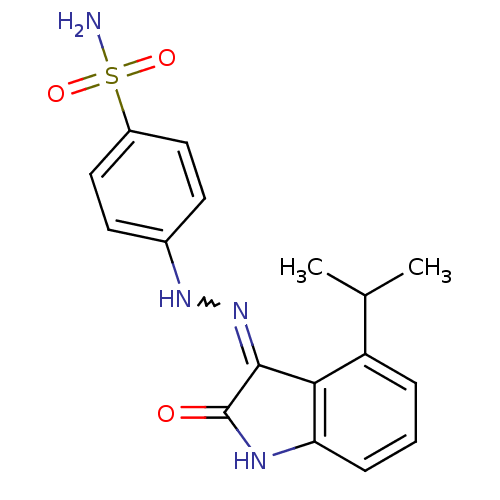 (4-[N¢-(4-Isopropyl-2-oxo-1,2-dihydro-indol-3-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7687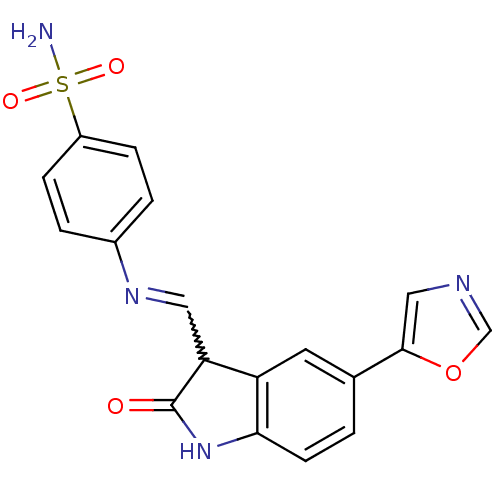 (4-({[(3Z)-5-(1,3-oxazol-5-yl)-2-oxo-2,3-dihydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A... | J Med Chem 44: 4339-58 (2001) Article DOI: 10.1021/jm010117d BindingDB Entry DOI: 10.7270/Q2ST7N10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 391 total ) | Next | Last >> |