 Found 270 hits with Last Name = 'morton' and Initial = 'pa'
Found 270 hits with Last Name = 'morton' and Initial = 'pa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382932
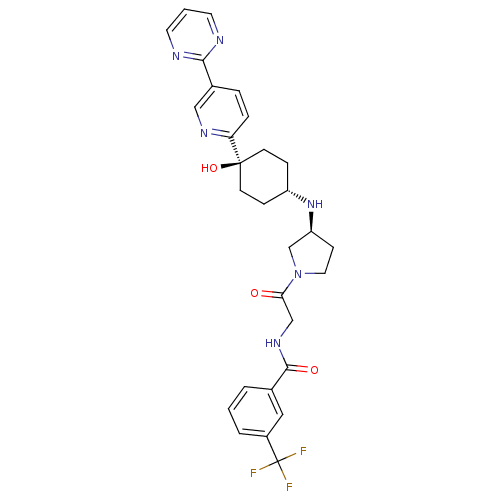
(CHEMBL2029422)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:8.8,1.0,(25.05,5.32,;26.39,4.55,;27.72,3.79,;29.05,4.55,;29.05,6.09,;27.72,6.87,;26.39,6.09,;30.38,6.86,;31.72,6.08,;31.73,4.55,;33.2,4.09,;34.1,5.35,;33.17,6.58,;35.42,6.13,;35.41,7.67,;36.76,5.37,;38.09,6.15,;39.43,5.39,;39.44,3.85,;40.76,6.17,;40.74,7.71,;42.06,8.48,;43.41,7.73,;43.42,6.18,;42.09,5.4,;44.76,5.41,;44.77,3.87,;46.09,6.19,;46,4.52,;25.06,3.78,;23.72,4.55,;22.39,3.78,;22.39,2.24,;23.74,1.47,;25.06,2.24,;21.06,1.46,;19.73,2.22,;18.4,1.45,;18.4,-.09,;19.75,-.86,;21.07,-.08,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-4-1-3-19(15-21)27(40)36-17-25(39)38-14-9-23(18-38)37-22-7-10-28(41,11-8-22)24-6-5-20(16-35-24)26-33-12-2-13-34-26/h1-6,12-13,15-16,22-23,37,41H,7-11,14,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CCR2-mediated Erk phosphorylation |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382942
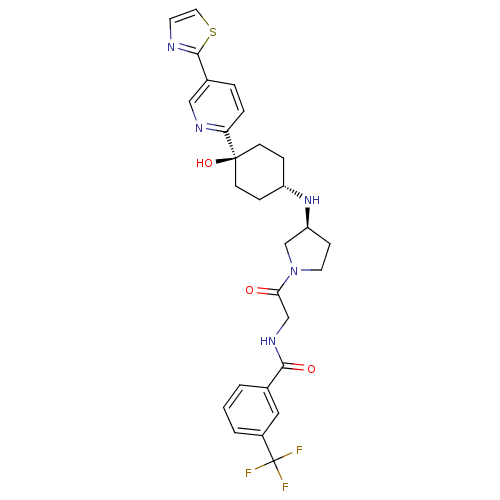
(CHEMBL2029423)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1nccs1 |r,wU:4.7,wD:8.8,1.0,(-4.43,-4.47,;-3.09,-5.24,;-1.76,-6,;-.43,-5.24,;-.43,-3.7,;-1.76,-2.92,;-3.09,-3.7,;.91,-2.94,;2.24,-3.71,;2.26,-5.24,;3.73,-5.7,;4.62,-4.44,;3.7,-3.21,;5.95,-3.67,;5.94,-2.13,;7.29,-4.43,;8.61,-3.65,;9.95,-4.41,;9.96,-5.95,;11.28,-3.63,;11.26,-2.1,;12.58,-1.32,;13.93,-2.08,;13.94,-3.62,;12.62,-4.4,;15.28,-4.38,;15.29,-5.92,;16.61,-3.6,;16.53,-5.27,;-4.42,-6.01,;-5.75,-5.24,;-7.09,-6.01,;-7.08,-7.56,;-5.74,-8.32,;-4.41,-7.55,;-8.42,-8.33,;-9.83,-7.71,;-10.85,-8.86,;-10.08,-10.19,;-8.58,-9.87,)| Show InChI InChI=1S/C28H30F3N5O3S/c29-28(30,31)20-3-1-2-18(14-20)25(38)34-16-24(37)36-12-8-22(17-36)35-21-6-9-27(39,10-7-21)23-5-4-19(15-33-23)26-32-11-13-40-26/h1-5,11,13-15,21-22,35,39H,6-10,12,16-17H2,(H,34,38)/t21-,22-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382939
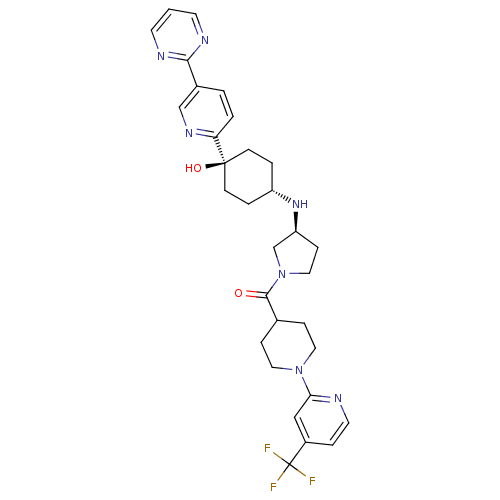
(CHEMBL2029568)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)C1CCN(CC1)c1cc(ccn1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:1.0,8.8,(-3.38,-21.11,;-2.05,-21.88,;-.72,-22.64,;.61,-21.88,;.61,-20.34,;-.72,-19.56,;-2.05,-20.34,;1.95,-19.57,;3.28,-20.35,;3.3,-21.88,;4.77,-22.34,;5.66,-21.08,;4.74,-19.85,;6.99,-20.3,;6.98,-18.76,;8.33,-21.06,;8.33,-22.6,;9.66,-23.36,;10.99,-22.58,;10.99,-21.04,;9.65,-20.27,;12.33,-23.35,;13.65,-22.57,;14.99,-23.33,;15,-24.87,;13.66,-25.65,;12.33,-24.88,;16.31,-22.55,;17.65,-23.31,;16.3,-21.01,;17.64,-21.76,;-3.38,-22.65,;-4.71,-21.88,;-6.04,-22.65,;-6.04,-24.19,;-4.7,-24.96,;-3.37,-24.19,;-7.37,-24.97,;-8.71,-24.21,;-10.04,-24.98,;-10.03,-26.52,;-8.69,-27.29,;-7.36,-26.51,)| Show InChI InChI=1S/C31H36F3N7O2/c32-31(33,34)23-6-14-35-27(18-23)40-15-7-21(8-16-40)29(42)41-17-9-25(20-41)39-24-4-10-30(43,11-5-24)26-3-2-22(19-38-26)28-36-12-1-13-37-28/h1-3,6,12-14,18-19,21,24-25,39,43H,4-5,7-11,15-17,20H2/t24-,25-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337608
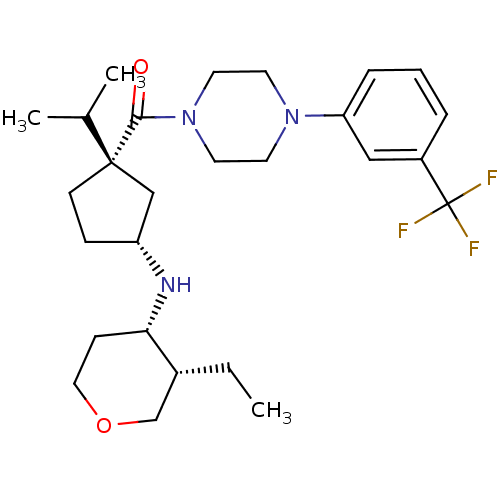
(CHEMBL1683063 | cis-((1S,3R)-3-(3-ethyl-tetrahydro...)Show SMILES CC[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H40F3N3O2/c1-4-20-18-35-15-9-24(20)31-22-8-10-26(17-22,19(2)3)25(34)33-13-11-32(12-14-33)23-7-5-6-21(16-23)27(28,29)30/h5-7,16,19-20,22,24,31H,4,8-15,17-18H2,1-3H3/t20-,22-,24+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337604

(CHEMBL1683059 | Cis-((1S,3R)-1-isopropyl-3-(3-meth...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)N[C@H]1CCOC[C@H]1C)C(=O)N1CCN(CC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H38F3N3O2/c1-18(2)25(9-7-21(16-25)30-23-8-14-34-17-19(23)3)24(33)32-12-10-31(11-13-32)22-6-4-5-20(15-22)26(27,28)29/h4-6,15,18-19,21,23,30H,7-14,16-17H2,1-3H3/t19-,21-,23+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382933
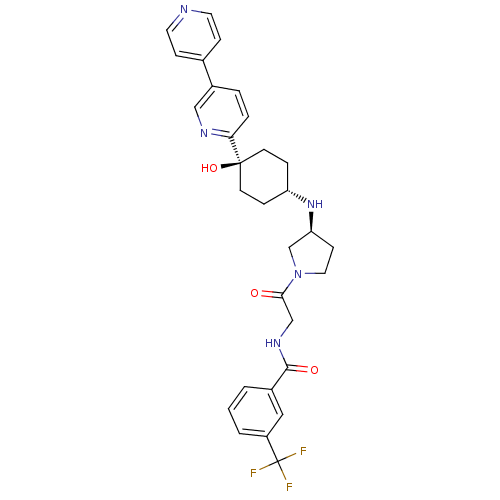
(CHEMBL2029419)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ccncc1 |r,wU:4.7,wD:8.8,1.0,(-3.3,-44.06,;-1.96,-44.83,;-.63,-45.59,;.7,-44.83,;.7,-43.29,;-.63,-42.51,;-1.96,-43.29,;2.03,-42.53,;3.37,-43.3,;3.38,-44.83,;4.85,-45.29,;5.75,-44.03,;4.82,-42.8,;7.07,-43.25,;7.06,-41.71,;8.41,-44.01,;9.74,-43.23,;11.08,-44,;11.09,-45.54,;12.41,-43.22,;12.39,-41.69,;13.71,-40.9,;15.06,-41.66,;15.07,-43.21,;13.74,-43.98,;16.41,-43.97,;16.42,-45.51,;17.74,-43.19,;17.65,-44.86,;-3.29,-45.6,;-4.63,-44.83,;-5.96,-45.6,;-5.96,-47.14,;-4.61,-47.91,;-3.29,-47.14,;-7.29,-47.92,;-8.62,-47.16,;-9.95,-47.93,;-9.95,-49.47,;-8.6,-50.24,;-7.28,-49.46,)| Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-3-1-2-21(16-23)28(40)36-18-27(39)38-15-10-25(19-38)37-24-6-11-29(41,12-7-24)26-5-4-22(17-35-26)20-8-13-34-14-9-20/h1-5,8-9,13-14,16-17,24-25,37,41H,6-7,10-12,15,18-19H2,(H,36,40)/t24-,25-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Rattus norvegicus) | BDBM50382932

(CHEMBL2029422)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:8.8,1.0,(25.05,5.32,;26.39,4.55,;27.72,3.79,;29.05,4.55,;29.05,6.09,;27.72,6.87,;26.39,6.09,;30.38,6.86,;31.72,6.08,;31.73,4.55,;33.2,4.09,;34.1,5.35,;33.17,6.58,;35.42,6.13,;35.41,7.67,;36.76,5.37,;38.09,6.15,;39.43,5.39,;39.44,3.85,;40.76,6.17,;40.74,7.71,;42.06,8.48,;43.41,7.73,;43.42,6.18,;42.09,5.4,;44.76,5.41,;44.77,3.87,;46.09,6.19,;46,4.52,;25.06,3.78,;23.72,4.55,;22.39,3.78,;22.39,2.24,;23.74,1.47,;25.06,2.24,;21.06,1.46,;19.73,2.22,;18.4,1.45,;18.4,-.09,;19.75,-.86,;21.07,-.08,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-4-1-3-19(15-21)27(40)36-17-25(39)38-14-9-23(18-38)37-22-7-10-28(41,11-8-22)24-6-5-20(16-35-24)26-33-12-2-13-34-26/h1-6,12-13,15-16,22-23,37,41H,7-11,14,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CCR2 |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Mus musculus) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to mouse CCR2 |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338145
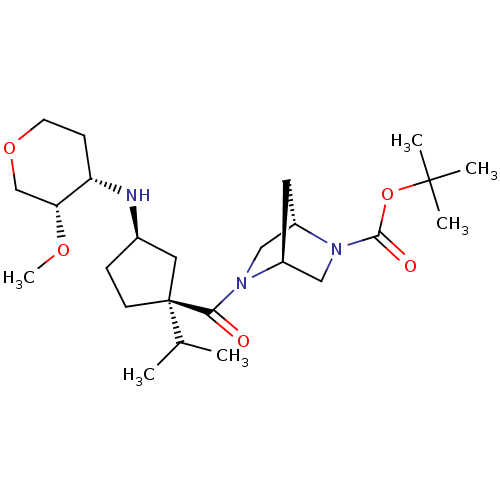
((1S,4S)-tert-butyl 5-((1S,3R)-1-isopropyl-3-((3S,4...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1C[C@@H]2C[C@H]1CN2C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C25H43N3O5/c1-16(2)25(9-7-17(12-25)26-20-8-10-32-15-21(20)31-6)22(29)27-13-19-11-18(27)14-28(19)23(30)33-24(3,4)5/h16-21,26H,7-15H2,1-6H3/t17-,18+,19+,20+,21-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 1 hrs by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Rattus norvegicus) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to rat CCR2 |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated for 30 mins by human whole cell binding assay |
Bioorg Med Chem Lett 21: 2626-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.034
BindingDB Entry DOI: 10.7270/Q2CJ8DT9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337604

(CHEMBL1683059 | Cis-((1S,3R)-1-isopropyl-3-(3-meth...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)N[C@H]1CCOC[C@H]1C)C(=O)N1CCN(CC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H38F3N3O2/c1-18(2)25(9-7-21(16-25)30-23-8-14-34-17-19(23)3)24(33)32-12-10-31(11-13-32)22-6-4-5-20(15-22)26(27,28)29/h4-6,15,18-19,21,23,30H,7-14,16-17H2,1-3H3/t19-,21-,23+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338141
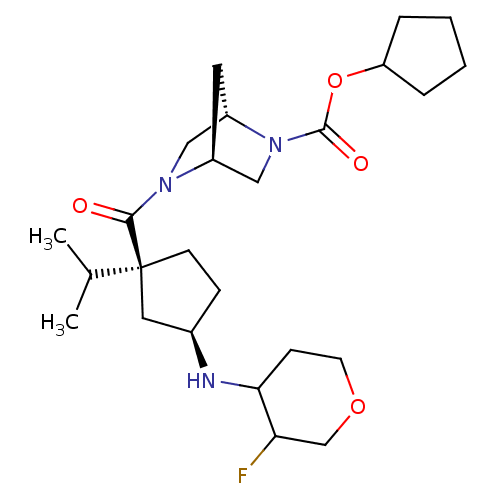
((1S,4S)-cyclopentyl 5-((1S,3R)-3-(3-fluorotetrahyd...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1F)C(=O)N1C[C@@H]2C[C@H]1CN2C(=O)OC1CCCC1 |r| Show InChI InChI=1S/C25H40FN3O4/c1-16(2)25(9-7-17(12-25)27-22-8-10-32-15-21(22)26)23(30)28-13-19-11-18(28)14-29(19)24(31)33-20-5-3-4-6-20/h16-22,27H,3-15H2,1-2H3/t17-,18+,19+,21?,22?,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 30 mins by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338135
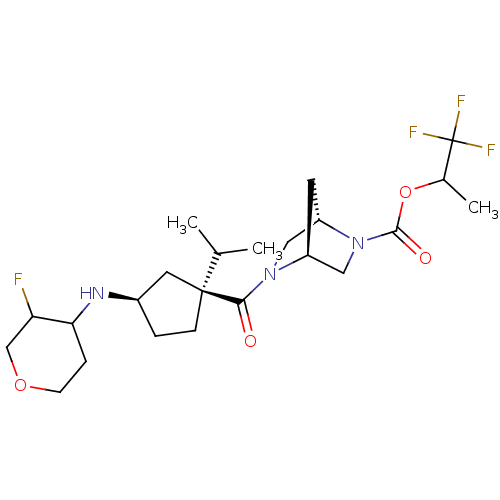
((1S,4S)-1,1,1-trifluoropropan-2-yl 5-((1S,3R)-3-(3...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1F)C(=O)N1C[C@@H]2C[C@H]1CN2C(=O)OC(C)C(F)(F)F |r| Show InChI InChI=1S/C23H35F4N3O4/c1-13(2)22(6-4-15(9-22)28-19-5-7-33-12-18(19)24)20(31)29-10-17-8-16(29)11-30(17)21(32)34-14(3)23(25,26)27/h13-19,28H,4-12H2,1-3H3/t14?,15-,16+,17+,18?,19?,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 30 mins by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337634
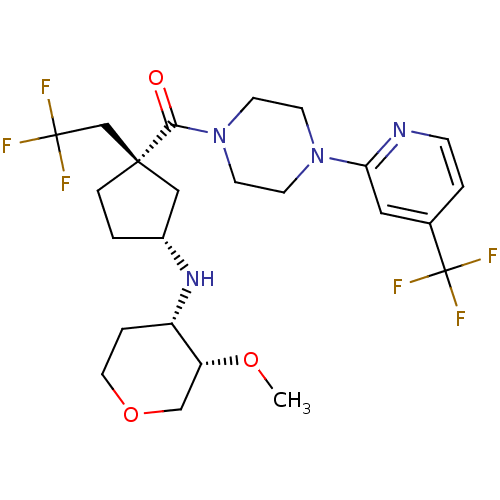
(((1S,3R)-3-((3S,4S)-3-methoxy-tetrahydro-2H-pyran-...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@@](CC(F)(F)F)(C1)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C24H32F6N4O3/c1-36-19-14-37-11-4-18(19)32-17-2-5-22(13-17,15-23(25,26)27)21(35)34-9-7-33(8-10-34)20-12-16(3-6-31-20)24(28,29)30/h3,6,12,17-19,32H,2,4-5,7-11,13-15H2,1H3/t17-,18+,19-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of MCP-Alexa 488 from CCR2 in human whole blood after 5 mins by flow cytometry |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 assessed as inhibition of MCP1-induced chemotaxis by cell based assay |
Bioorg Med Chem Lett 21: 2626-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.034
BindingDB Entry DOI: 10.7270/Q2CJ8DT9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382939

(CHEMBL2029568)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)C1CCN(CC1)c1cc(ccn1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:1.0,8.8,(-3.38,-21.11,;-2.05,-21.88,;-.72,-22.64,;.61,-21.88,;.61,-20.34,;-.72,-19.56,;-2.05,-20.34,;1.95,-19.57,;3.28,-20.35,;3.3,-21.88,;4.77,-22.34,;5.66,-21.08,;4.74,-19.85,;6.99,-20.3,;6.98,-18.76,;8.33,-21.06,;8.33,-22.6,;9.66,-23.36,;10.99,-22.58,;10.99,-21.04,;9.65,-20.27,;12.33,-23.35,;13.65,-22.57,;14.99,-23.33,;15,-24.87,;13.66,-25.65,;12.33,-24.88,;16.31,-22.55,;17.65,-23.31,;16.3,-21.01,;17.64,-21.76,;-3.38,-22.65,;-4.71,-21.88,;-6.04,-22.65,;-6.04,-24.19,;-4.7,-24.96,;-3.37,-24.19,;-7.37,-24.97,;-8.71,-24.21,;-10.04,-24.98,;-10.03,-26.52,;-8.69,-27.29,;-7.36,-26.51,)| Show InChI InChI=1S/C31H36F3N7O2/c32-31(33,34)23-6-14-35-27(18-23)40-15-7-21(8-16-40)29(42)41-17-9-25(20-41)39-24-4-10-30(43,11-5-24)26-3-2-22(19-38-26)28-36-12-1-13-37-28/h1-3,6,12-14,18-19,21,24-25,39,43H,4-5,7-11,15-17,20H2/t24-,25-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382935
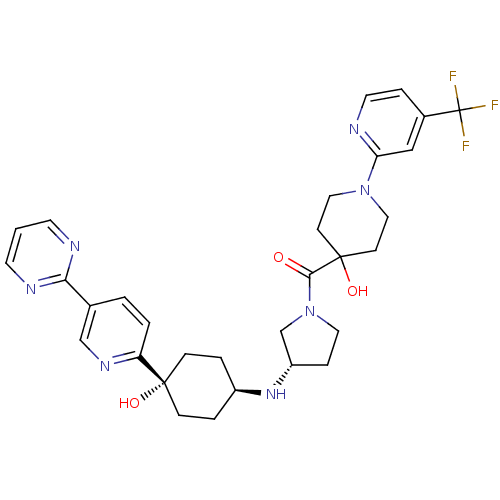
(CHEMBL2029572)Show SMILES OC1(CCN(CC1)c1cc(ccn1)C(F)(F)F)C(=O)N1CC[C@@H](C1)N[C@H]1CC[C@](O)(CC1)c1ccc(cn1)-c1ncccn1 |r,wU:25.27,wD:28.31,22.26,(7.23,-39.74,;7.24,-41.29,;7.24,-42.83,;8.57,-43.59,;9.9,-42.81,;9.9,-41.27,;8.56,-40.5,;11.24,-43.58,;12.56,-42.8,;13.9,-43.56,;13.91,-45.1,;12.57,-45.88,;11.24,-45.11,;15.22,-42.78,;16.56,-43.54,;15.21,-41.24,;16.55,-41.99,;5.9,-40.53,;5.89,-38.99,;4.57,-41.31,;3.68,-42.57,;2.21,-42.11,;2.19,-40.58,;3.65,-40.08,;.86,-39.8,;-.48,-40.57,;-.48,-42.11,;-1.81,-42.87,;-3.13,-42.11,;-4.47,-41.34,;-3.13,-40.57,;-1.81,-39.79,;-4.47,-42.88,;-5.8,-42.11,;-7.13,-42.88,;-7.13,-44.42,;-5.79,-45.19,;-4.46,-44.42,;-8.46,-45.2,;-9.79,-44.44,;-11.13,-45.21,;-11.12,-46.75,;-9.78,-47.52,;-8.45,-46.74,)| Show InChI InChI=1S/C31H36F3N7O3/c32-31(33,34)22-6-14-35-26(18-22)40-16-10-30(44,11-17-40)28(42)41-15-7-24(20-41)39-23-4-8-29(43,9-5-23)25-3-2-21(19-38-25)27-36-12-1-13-37-27/h1-3,6,12-14,18-19,23-24,39,43-44H,4-5,7-11,15-17,20H2/t23-,24-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382932

(CHEMBL2029422)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:8.8,1.0,(25.05,5.32,;26.39,4.55,;27.72,3.79,;29.05,4.55,;29.05,6.09,;27.72,6.87,;26.39,6.09,;30.38,6.86,;31.72,6.08,;31.73,4.55,;33.2,4.09,;34.1,5.35,;33.17,6.58,;35.42,6.13,;35.41,7.67,;36.76,5.37,;38.09,6.15,;39.43,5.39,;39.44,3.85,;40.76,6.17,;40.74,7.71,;42.06,8.48,;43.41,7.73,;43.42,6.18,;42.09,5.4,;44.76,5.41,;44.77,3.87,;46.09,6.19,;46,4.52,;25.06,3.78,;23.72,4.55,;22.39,3.78,;22.39,2.24,;23.74,1.47,;25.06,2.24,;21.06,1.46,;19.73,2.22,;18.4,1.45,;18.4,-.09,;19.75,-.86,;21.07,-.08,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-4-1-3-19(15-21)27(40)36-17-25(39)38-14-9-23(18-38)37-22-7-10-28(41,11-8-22)24-6-5-20(16-35-24)26-33-12-2-13-34-26/h1-6,12-13,15-16,22-23,37,41H,7-11,14,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CCR2-mediated calcium mobilization |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337634

(((1S,3R)-3-((3S,4S)-3-methoxy-tetrahydro-2H-pyran-...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@@](CC(F)(F)F)(C1)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C24H32F6N4O3/c1-36-19-14-37-11-4-18(19)32-17-2-5-22(13-17,15-23(25,26)27)21(35)34-9-7-33(8-10-34)20-12-16(3-6-31-20)24(28,29)30/h3,6,12,17-19,32H,2,4-5,7-11,13-15H2,1H3/t17-,18+,19-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382941
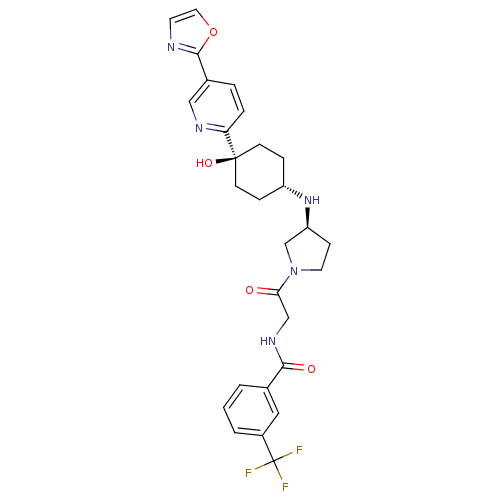
(CHEMBL2029424)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncco1 |r,wU:4.7,wD:8.8,1.0,(24.38,-2.82,;25.71,-3.59,;27.04,-4.36,;28.37,-3.59,;28.37,-2.05,;27.04,-1.28,;25.71,-2.05,;29.71,-1.29,;31.04,-2.06,;31.06,-3.6,;32.53,-4.05,;33.42,-2.8,;32.5,-1.56,;34.75,-2.02,;34.74,-.48,;36.09,-2.78,;37.42,-2,;38.75,-2.76,;38.77,-4.3,;40.08,-1.98,;40.06,-.45,;41.38,.33,;42.73,-.43,;42.74,-1.97,;41.42,-2.75,;44.08,-2.73,;44.09,-4.27,;45.41,-1.95,;45.33,-3.62,;24.38,-4.37,;23.05,-3.59,;21.72,-4.37,;21.72,-5.91,;23.06,-6.68,;24.39,-5.9,;20.39,-6.68,;18.97,-6.07,;17.95,-7.21,;18.72,-8.54,;20.23,-8.22,)| Show InChI InChI=1S/C28H30F3N5O4/c29-28(30,31)20-3-1-2-18(14-20)25(38)34-16-24(37)36-12-8-22(17-36)35-21-6-9-27(39,10-7-21)23-5-4-19(15-33-23)26-32-11-13-40-26/h1-5,11,13-15,21-22,35,39H,6-10,12,16-17H2,(H,34,38)/t21-,22-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338129

((1S,4S)-1,1,1-trifluoropropan-2-yl 5-((1S,3R)-1-is...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1)C(=O)N1C[C@@H]2C[C@H]1CN2C(=O)OC(C)C(F)(F)F |r| Show InChI InChI=1S/C23H36F3N3O4/c1-14(2)22(7-4-17(11-22)27-16-5-8-32-9-6-16)20(30)28-12-19-10-18(28)13-29(19)21(31)33-15(3)23(24,25)26/h14-19,27H,4-13H2,1-3H3/t15?,17-,18+,19+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 30 mins by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382940
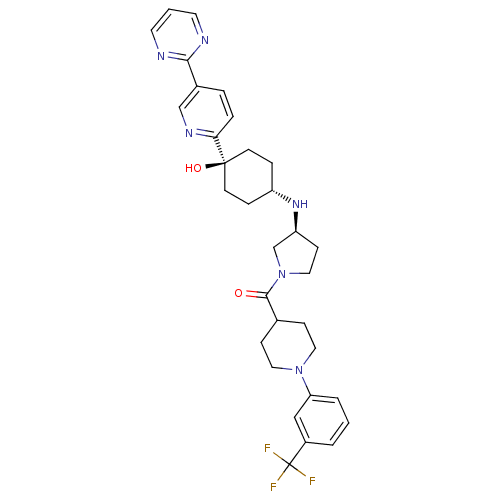
(CHEMBL2029566)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)C1CCN(CC1)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:1.0,8.8,(-3.21,-12.83,;-1.87,-13.6,;-.55,-14.36,;.78,-13.6,;.78,-12.06,;-.55,-11.28,;-1.87,-12.06,;2.12,-11.29,;3.45,-12.06,;3.47,-13.6,;4.94,-14.06,;5.83,-12.8,;4.91,-11.57,;7.16,-12.02,;7.15,-10.48,;8.5,-12.78,;8.5,-14.31,;9.83,-15.07,;11.16,-14.3,;11.16,-12.76,;9.82,-11.99,;12.5,-15.06,;12.5,-16.6,;13.83,-17.37,;15.17,-16.59,;15.16,-15.04,;13.82,-14.28,;16.48,-14.26,;17.82,-15.02,;16.47,-12.72,;17.81,-13.48,;-3.21,-14.37,;-4.54,-13.6,;-5.87,-14.37,;-5.87,-15.91,;-4.53,-16.68,;-3.2,-15.9,;-7.2,-16.68,;-8.53,-15.92,;-9.87,-16.69,;-9.86,-18.24,;-8.52,-19,;-7.19,-18.23,)| Show InChI InChI=1S/C32H37F3N6O2/c33-32(34,35)24-3-1-4-27(19-24)40-16-9-22(10-17-40)30(42)41-18-11-26(21-41)39-25-7-12-31(43,13-8-25)28-6-5-23(20-38-28)29-36-14-2-15-37-29/h1-6,14-15,19-20,22,25-26,39,43H,7-13,16-18,21H2/t25-,26-,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50363953

(CHEMBL1951778 | CHEMBL1963131)Show SMILES COc1ccc(cn1)[C@]1(O)CC[C@@H](CC1)N1CC[C@H](C1)NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F |r,wU:12.16,wD:18.22,8.9,(-10.53,-32.06,;-9.74,-33.38,;-8.2,-33.35,;-7.45,-32,;-5.92,-31.97,;-5.12,-33.28,;-5.86,-34.64,;-7.4,-34.67,;-3.58,-33.25,;-4.36,-31.9,;-2.77,-34.56,;-1.24,-34.53,;-.49,-33.18,;-1.29,-31.87,;-2.83,-31.89,;1.05,-33.15,;1.97,-34.38,;3.43,-33.87,;3.4,-32.33,;1.92,-31.89,;4.72,-31.55,;6.06,-32.3,;6.08,-33.84,;7.39,-31.51,;8.73,-32.27,;10.05,-31.48,;10.04,-29.94,;11.4,-32.23,;11.41,-33.77,;12.75,-34.53,;14.08,-33.74,;14.05,-32.19,;12.71,-31.45,;15.37,-31.4,;16.72,-32.15,;15.35,-29.86,;16.7,-30.62,)| Show InChI InChI=1S/C26H31F3N4O4/c1-37-23-6-5-19(14-30-23)25(36)10-7-21(8-11-25)33-12-9-20(16-33)32-22(34)15-31-24(35)17-3-2-4-18(13-17)26(27,28)29/h2-6,13-14,20-21,36H,7-12,15-16H2,1H3,(H,31,35)(H,32,34)/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382943

(CHEMBL2029421)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1cnccn1 |r,wU:4.7,wD:8.8,1.0,(-4.25,4.39,;-2.91,3.62,;-1.58,2.85,;-.25,3.62,;-.25,5.16,;-1.58,5.93,;-2.91,5.16,;1.09,5.92,;2.42,5.15,;2.43,3.61,;3.9,3.16,;4.8,4.41,;3.88,5.65,;6.13,5.19,;6.11,6.73,;7.46,4.43,;8.79,5.21,;10.13,4.45,;10.14,2.91,;11.46,5.23,;11.44,6.76,;12.76,7.55,;14.11,6.78,;14.12,5.24,;12.79,4.46,;15.46,4.48,;15.47,2.94,;16.79,5.26,;16.71,3.59,;-4.24,2.84,;-5.57,3.62,;-6.91,2.84,;-6.91,1.3,;-5.56,.53,;-4.23,1.31,;-8.24,.53,;-9.57,1.29,;-10.9,.52,;-10.9,-1.02,;-9.55,-1.79,;-8.23,-1.01,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-3-1-2-19(14-21)27(40)36-17-26(39)38-13-8-23(18-38)37-22-6-9-28(41,10-7-22)25-5-4-20(15-35-25)24-16-33-11-12-34-24/h1-5,11-12,14-16,22-23,37,41H,6-10,13,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of MCP-Alexa 488 from CCR2 in human whole blood after 5 mins by flow cytometry |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382932

(CHEMBL2029422)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:8.8,1.0,(25.05,5.32,;26.39,4.55,;27.72,3.79,;29.05,4.55,;29.05,6.09,;27.72,6.87,;26.39,6.09,;30.38,6.86,;31.72,6.08,;31.73,4.55,;33.2,4.09,;34.1,5.35,;33.17,6.58,;35.42,6.13,;35.41,7.67,;36.76,5.37,;38.09,6.15,;39.43,5.39,;39.44,3.85,;40.76,6.17,;40.74,7.71,;42.06,8.48,;43.41,7.73,;43.42,6.18,;42.09,5.4,;44.76,5.41,;44.77,3.87,;46.09,6.19,;46,4.52,;25.06,3.78,;23.72,4.55,;22.39,3.78,;22.39,2.24,;23.74,1.47,;25.06,2.24,;21.06,1.46,;19.73,2.22,;18.4,1.45,;18.4,-.09,;19.75,-.86,;21.07,-.08,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-4-1-3-19(15-21)27(40)36-17-25(39)38-14-9-23(18-38)37-22-7-10-28(41,11-8-22)24-6-5-20(16-35-24)26-33-12-2-13-34-26/h1-6,12-13,15-16,22-23,37,41H,7-11,14,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 by whole blood assay |
Bioorg Med Chem Lett 21: 2626-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.034
BindingDB Entry DOI: 10.7270/Q2CJ8DT9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338125

((1S,4S)-tert-butyl 5-((1S,3R)-3-(3-fluorotetrahydr...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1F)C(=O)N1C[C@@H]2C[C@H]1CN2C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C24H40FN3O4/c1-15(2)24(8-6-16(11-24)26-20-7-9-31-14-19(20)25)21(29)27-12-18-10-17(27)13-28(18)22(30)32-23(3,4)5/h15-20,26H,6-14H2,1-5H3/t16-,17+,18+,19?,20?,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 1 hrs by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338142
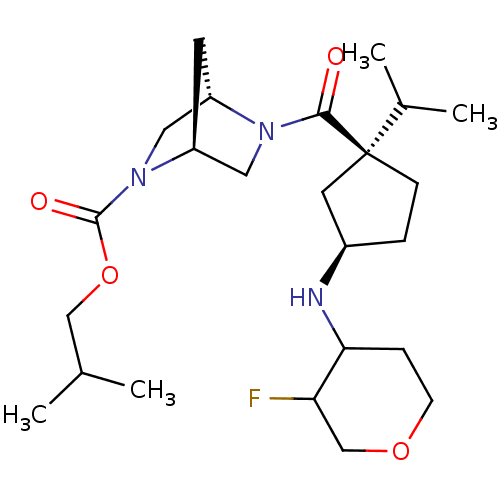
((1S,4S)-isobutyl 5-((1S,3R)-3-(3-fluorotetrahydro-...)Show SMILES CC(C)COC(=O)N1C[C@@H]2C[C@H]1CN2C(=O)[C@]1(CC[C@H](C1)NC1CCOCC1F)C(C)C |r| Show InChI InChI=1S/C24H40FN3O4/c1-15(2)13-32-23(30)28-12-18-9-19(28)11-27(18)22(29)24(16(3)4)7-5-17(10-24)26-21-6-8-31-14-20(21)25/h15-21,26H,5-14H2,1-4H3/t17-,18+,19+,20?,21?,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 30 mins by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382938
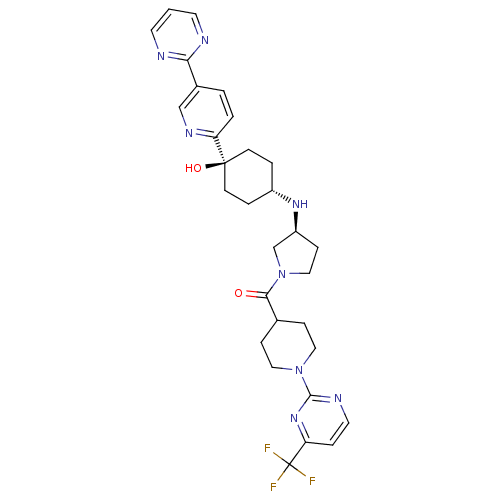
(CHEMBL2029569)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)C1CCN(CC1)c1nccc(n1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:1.0,8.8,(26.25,-19.16,;27.59,-19.93,;28.92,-20.7,;30.25,-19.93,;30.25,-18.39,;28.92,-17.62,;27.59,-18.39,;31.58,-17.63,;32.91,-18.4,;32.93,-19.94,;34.4,-20.4,;35.29,-19.14,;34.37,-17.9,;36.62,-18.36,;36.61,-16.82,;37.96,-19.12,;37.96,-20.65,;39.29,-21.41,;40.63,-20.64,;40.62,-19.1,;39.28,-18.33,;41.96,-21.4,;41.96,-22.94,;43.3,-23.7,;44.63,-22.93,;44.62,-21.38,;43.28,-20.62,;45.95,-20.6,;47.29,-21.36,;45.94,-19.06,;47.27,-19.82,;26.26,-20.71,;24.92,-19.94,;23.59,-20.71,;23.59,-22.25,;24.94,-23.02,;26.26,-22.24,;22.26,-23.02,;20.93,-22.26,;19.6,-23.03,;19.6,-24.57,;20.95,-25.34,;22.27,-24.56,)| Show InChI InChI=1S/C30H35F3N8O2/c31-30(32,33)25-6-14-36-28(39-25)40-15-7-20(8-16-40)27(42)41-17-9-23(19-41)38-22-4-10-29(43,11-5-22)24-3-2-21(18-37-24)26-34-12-1-13-35-26/h1-3,6,12-14,18,20,22-23,38,43H,4-5,7-11,15-17,19H2/t22-,23-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of labeled MIP-1beta from human CCR5 receptor |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382934
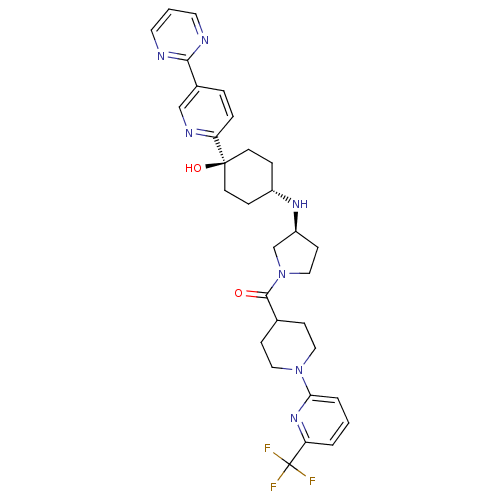
(CHEMBL2029567)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)C1CCN(CC1)c1cccc(n1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:1.0,8.8,(26.16,-10.9,;27.49,-11.67,;28.82,-12.43,;30.15,-11.67,;30.15,-10.13,;28.82,-9.35,;27.49,-10.13,;31.49,-9.36,;32.82,-10.14,;32.84,-11.67,;34.31,-12.13,;35.2,-10.87,;34.28,-9.64,;36.53,-10.09,;36.52,-8.55,;37.87,-10.85,;37.87,-12.38,;39.2,-13.14,;40.53,-12.37,;40.53,-10.83,;39.19,-10.06,;41.87,-13.14,;41.87,-14.67,;43.2,-15.44,;44.54,-14.66,;44.53,-13.11,;43.19,-12.36,;45.85,-12.33,;47.19,-13.1,;45.84,-10.79,;47.18,-11.55,;26.16,-12.44,;24.83,-11.67,;23.5,-12.44,;23.5,-13.98,;24.84,-14.75,;26.17,-13.97,;22.17,-14.75,;20.83,-13.99,;19.5,-14.77,;19.51,-16.31,;20.85,-17.07,;22.18,-16.3,)| Show InChI InChI=1S/C31H36F3N7O2/c32-31(33,34)26-3-1-4-27(39-26)40-16-9-21(10-17-40)29(42)41-18-11-24(20-41)38-23-7-12-30(43,13-8-23)25-6-5-22(19-37-25)28-35-14-2-15-36-28/h1-6,14-15,19,21,23-24,38,43H,7-13,16-18,20H2/t23-,24-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50346294
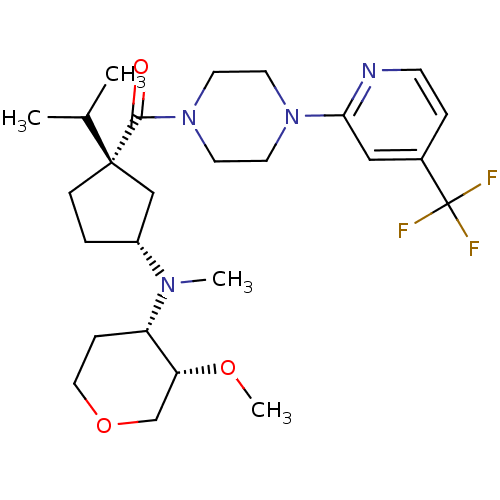
(CHEMBL1782377 | cis-((1S,3R)-1-isopropyl-3-(((3S,4...)Show SMILES CO[C@@H]1COCC[C@@H]1N(C)[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C26H39F3N4O3/c1-18(2)25(8-5-20(16-25)31(3)21-7-14-36-17-22(21)35-4)24(34)33-12-10-32(11-13-33)23-15-19(6-9-30-23)26(27,28)29/h6,9,15,18,20-22H,5,7-8,10-14,16-17H2,1-4H3/t20-,21+,22-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated for 30 mins by human whole cell binding assay |
Bioorg Med Chem Lett 21: 2626-30 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.034
BindingDB Entry DOI: 10.7270/Q2CJ8DT9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337608

(CHEMBL1683063 | cis-((1S,3R)-3-(3-ethyl-tetrahydro...)Show SMILES CC[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H40F3N3O2/c1-4-20-18-35-15-9-24(20)31-22-8-10-26(17-22,19(2)3)25(34)33-13-11-32(12-14-33)23-7-5-6-21(16-23)27(28,29)30/h5-7,16,19-20,22,24,31H,4,8-15,17-18H2,1-3H3/t20-,22-,24+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50363953

(CHEMBL1951778 | CHEMBL1963131)Show SMILES COc1ccc(cn1)[C@]1(O)CC[C@@H](CC1)N1CC[C@H](C1)NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F |r,wU:12.16,wD:18.22,8.9,(-10.53,-32.06,;-9.74,-33.38,;-8.2,-33.35,;-7.45,-32,;-5.92,-31.97,;-5.12,-33.28,;-5.86,-34.64,;-7.4,-34.67,;-3.58,-33.25,;-4.36,-31.9,;-2.77,-34.56,;-1.24,-34.53,;-.49,-33.18,;-1.29,-31.87,;-2.83,-31.89,;1.05,-33.15,;1.97,-34.38,;3.43,-33.87,;3.4,-32.33,;1.92,-31.89,;4.72,-31.55,;6.06,-32.3,;6.08,-33.84,;7.39,-31.51,;8.73,-32.27,;10.05,-31.48,;10.04,-29.94,;11.4,-32.23,;11.41,-33.77,;12.75,-34.53,;14.08,-33.74,;14.05,-32.19,;12.71,-31.45,;15.37,-31.4,;16.72,-32.15,;15.35,-29.86,;16.7,-30.62,)| Show InChI InChI=1S/C26H31F3N4O4/c1-37-23-6-5-19(14-30-23)25(36)10-7-21(8-11-25)33-12-9-20(16-33)32-22(34)15-31-24(35)17-3-2-4-18(13-17)26(27,28)29/h2-6,13-14,20-21,36H,7-12,15-16H2,1H3,(H,31,35)(H,32,34)/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50338146

((1S,4S)-tert-butyl 5-((1S,3R)-1-isopropyl-3-(tetra...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)NC1CCOCC1)C(=O)N1C[C@@H]2C[C@H]1CN2C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C24H41N3O4/c1-16(2)24(9-6-18(13-24)25-17-7-10-30-11-8-17)21(28)26-14-20-12-19(26)15-27(20)22(29)31-23(3,4)5/h16-20,25H,6-15H2,1-5H3/t18-,19+,20+,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 preincubated 1 hrs by human whole cell binding assay |
Bioorg Med Chem Lett 21: 1827-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.052
BindingDB Entry DOI: 10.7270/Q2GM87M5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382932

(CHEMBL2029422)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:8.8,1.0,(25.05,5.32,;26.39,4.55,;27.72,3.79,;29.05,4.55,;29.05,6.09,;27.72,6.87,;26.39,6.09,;30.38,6.86,;31.72,6.08,;31.73,4.55,;33.2,4.09,;34.1,5.35,;33.17,6.58,;35.42,6.13,;35.41,7.67,;36.76,5.37,;38.09,6.15,;39.43,5.39,;39.44,3.85,;40.76,6.17,;40.74,7.71,;42.06,8.48,;43.41,7.73,;43.42,6.18,;42.09,5.4,;44.76,5.41,;44.77,3.87,;46.09,6.19,;46,4.52,;25.06,3.78,;23.72,4.55,;22.39,3.78,;22.39,2.24,;23.74,1.47,;25.06,2.24,;21.06,1.46,;19.73,2.22,;18.4,1.45,;18.4,-.09,;19.75,-.86,;21.07,-.08,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-4-1-3-19(15-21)27(40)36-17-25(39)38-14-9-23(18-38)37-22-7-10-28(41,11-8-22)24-6-5-20(16-35-24)26-33-12-2-13-34-26/h1-6,12-13,15-16,22-23,37,41H,7-11,14,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50337619

(((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C25H37F3N4O3/c1-17(2)24(7-4-19(15-24)30-20-6-13-35-16-21(20)34-3)23(33)32-11-9-31(10-12-32)22-14-18(5-8-29-22)25(26,27)28/h5,8,14,17,19-21,30H,4,6-7,9-13,15-16H2,1-3H3/t19-,20+,21-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of labeled MIP-1beta from human CCR5 receptor |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382944
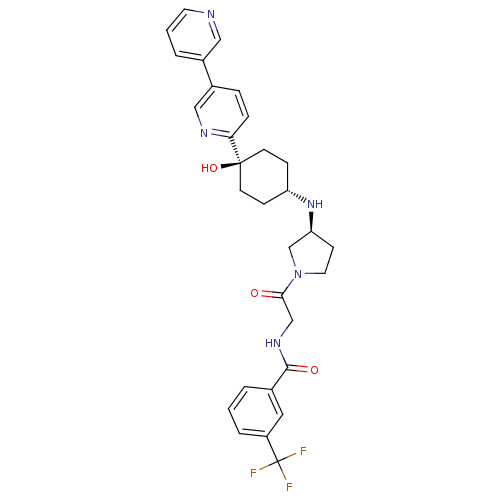
(CHEMBL2029418)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1cccnc1 |r,wU:4.7,wD:8.8,1.0,(49.25,-32.12,;50.59,-32.89,;51.92,-33.65,;53.24,-32.89,;53.24,-31.35,;51.92,-30.57,;50.59,-31.35,;54.58,-30.59,;55.91,-31.36,;55.93,-32.89,;57.4,-33.35,;58.29,-32.09,;57.37,-30.86,;59.62,-31.32,;59.61,-29.78,;60.96,-32.08,;62.29,-31.3,;63.63,-32.06,;63.64,-33.6,;64.96,-31.28,;64.94,-29.75,;66.26,-28.97,;67.61,-29.73,;67.62,-31.27,;66.29,-32.05,;68.96,-32.03,;68.97,-33.57,;70.28,-31.25,;70.2,-32.92,;49.25,-33.66,;47.92,-32.89,;46.59,-33.66,;46.59,-35.21,;47.93,-35.97,;49.26,-35.2,;45.26,-35.98,;43.93,-35.22,;42.6,-35.99,;42.6,-37.53,;43.94,-38.3,;45.27,-37.52,)| Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-5-1-3-20(15-23)28(40)36-18-27(39)38-14-10-25(19-38)37-24-8-11-29(41,12-9-24)26-7-6-22(17-35-26)21-4-2-13-34-16-21/h1-7,13,15-17,24-25,37,41H,8-12,14,18-19H2,(H,36,40)/t24-,25-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 receptor in human PBMC assessed as inhibition of MCP1-mediated leukocyte chemotaxis after 30 mins by microscopy |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382938

(CHEMBL2029569)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)C1CCN(CC1)c1nccc(n1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:1.0,8.8,(26.25,-19.16,;27.59,-19.93,;28.92,-20.7,;30.25,-19.93,;30.25,-18.39,;28.92,-17.62,;27.59,-18.39,;31.58,-17.63,;32.91,-18.4,;32.93,-19.94,;34.4,-20.4,;35.29,-19.14,;34.37,-17.9,;36.62,-18.36,;36.61,-16.82,;37.96,-19.12,;37.96,-20.65,;39.29,-21.41,;40.63,-20.64,;40.62,-19.1,;39.28,-18.33,;41.96,-21.4,;41.96,-22.94,;43.3,-23.7,;44.63,-22.93,;44.62,-21.38,;43.28,-20.62,;45.95,-20.6,;47.29,-21.36,;45.94,-19.06,;47.27,-19.82,;26.26,-20.71,;24.92,-19.94,;23.59,-20.71,;23.59,-22.25,;24.94,-23.02,;26.26,-22.24,;22.26,-23.02,;20.93,-22.26,;19.6,-23.03,;19.6,-24.57,;20.95,-25.34,;22.27,-24.56,)| Show InChI InChI=1S/C30H35F3N8O2/c31-30(32,33)25-6-14-36-28(39-25)40-15-7-20(8-16-40)27(42)41-17-9-23(19-41)38-22-4-10-29(43,11-5-22)24-3-2-21(18-37-24)26-34-12-1-13-35-26/h1-3,6,12-14,18,20,22-23,38,43H,4-5,7-11,15-17,19H2/t22-,23-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382941

(CHEMBL2029424)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncco1 |r,wU:4.7,wD:8.8,1.0,(24.38,-2.82,;25.71,-3.59,;27.04,-4.36,;28.37,-3.59,;28.37,-2.05,;27.04,-1.28,;25.71,-2.05,;29.71,-1.29,;31.04,-2.06,;31.06,-3.6,;32.53,-4.05,;33.42,-2.8,;32.5,-1.56,;34.75,-2.02,;34.74,-.48,;36.09,-2.78,;37.42,-2,;38.75,-2.76,;38.77,-4.3,;40.08,-1.98,;40.06,-.45,;41.38,.33,;42.73,-.43,;42.74,-1.97,;41.42,-2.75,;44.08,-2.73,;44.09,-4.27,;45.41,-1.95,;45.33,-3.62,;24.38,-4.37,;23.05,-3.59,;21.72,-4.37,;21.72,-5.91,;23.06,-6.68,;24.39,-5.9,;20.39,-6.68,;18.97,-6.07,;17.95,-7.21,;18.72,-8.54,;20.23,-8.22,)| Show InChI InChI=1S/C28H30F3N5O4/c29-28(30,31)20-3-1-2-18(14-20)25(38)34-16-24(37)36-12-8-22(17-36)35-21-6-9-27(39,10-7-21)23-5-4-19(15-33-23)26-32-11-13-40-26/h1-5,11,13-15,21-22,35,39H,6-10,12,16-17H2,(H,34,38)/t21-,22-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337612
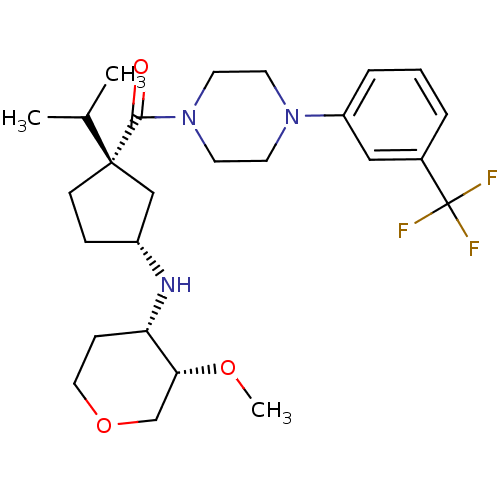
(CHEMBL1683067 | cis-((1S,3R)-1-isopropyl-3-((3S,4S...)Show SMILES CO[C@@H]1COCC[C@@H]1N[C@@H]1CC[C@](C1)(C(C)C)C(=O)N1CCN(CC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H38F3N3O3/c1-18(2)25(9-7-20(16-25)30-22-8-14-35-17-23(22)34-3)24(33)32-12-10-31(11-13-32)21-6-4-5-19(15-21)26(27,28)29/h4-6,15,18,20,22-23,30H,7-14,16-17H2,1-3H3/t20-,22+,23-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382933

(CHEMBL2029419)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ccncc1 |r,wU:4.7,wD:8.8,1.0,(-3.3,-44.06,;-1.96,-44.83,;-.63,-45.59,;.7,-44.83,;.7,-43.29,;-.63,-42.51,;-1.96,-43.29,;2.03,-42.53,;3.37,-43.3,;3.38,-44.83,;4.85,-45.29,;5.75,-44.03,;4.82,-42.8,;7.07,-43.25,;7.06,-41.71,;8.41,-44.01,;9.74,-43.23,;11.08,-44,;11.09,-45.54,;12.41,-43.22,;12.39,-41.69,;13.71,-40.9,;15.06,-41.66,;15.07,-43.21,;13.74,-43.98,;16.41,-43.97,;16.42,-45.51,;17.74,-43.19,;17.65,-44.86,;-3.29,-45.6,;-4.63,-44.83,;-5.96,-45.6,;-5.96,-47.14,;-4.61,-47.91,;-3.29,-47.14,;-7.29,-47.92,;-8.62,-47.16,;-9.95,-47.93,;-9.95,-49.47,;-8.6,-50.24,;-7.28,-49.46,)| Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-3-1-2-21(16-23)28(40)36-18-27(39)38-15-10-25(19-38)37-24-6-11-29(41,12-7-24)26-5-4-22(17-35-26)20-8-13-34-14-9-20/h1-5,8-9,13-14,16-17,24-25,37,41H,6-7,10-12,15,18-19H2,(H,36,40)/t24-,25-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50337605

(CHEMBL1683060 | Cis-((1S,3R)-1-isopropyl-3-(3-meth...)Show SMILES CC(C)[C@@]1(CC[C@H](C1)N[C@@H]1CCOC[C@@H]1C)C(=O)N1CCN(CC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H38F3N3O2/c1-18(2)25(9-7-21(16-25)30-23-8-14-34-17-19(23)3)24(33)32-12-10-31(11-13-32)22-6-4-5-20(15-22)26(27,28)29/h4-6,15,18-19,21,23,30H,7-14,16-17H2,1-3H3/t19-,21+,23+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins |
Bioorg Med Chem Lett 21: 1442-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.015
BindingDB Entry DOI: 10.7270/Q22J6C4C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382943

(CHEMBL2029421)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1cnccn1 |r,wU:4.7,wD:8.8,1.0,(-4.25,4.39,;-2.91,3.62,;-1.58,2.85,;-.25,3.62,;-.25,5.16,;-1.58,5.93,;-2.91,5.16,;1.09,5.92,;2.42,5.15,;2.43,3.61,;3.9,3.16,;4.8,4.41,;3.88,5.65,;6.13,5.19,;6.11,6.73,;7.46,4.43,;8.79,5.21,;10.13,4.45,;10.14,2.91,;11.46,5.23,;11.44,6.76,;12.76,7.55,;14.11,6.78,;14.12,5.24,;12.79,4.46,;15.46,4.48,;15.47,2.94,;16.79,5.26,;16.71,3.59,;-4.24,2.84,;-5.57,3.62,;-6.91,2.84,;-6.91,1.3,;-5.56,.53,;-4.23,1.31,;-8.24,.53,;-9.57,1.29,;-10.9,.52,;-10.9,-1.02,;-9.55,-1.79,;-8.23,-1.01,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-3-1-2-19(14-21)27(40)36-17-26(39)38-13-8-23(18-38)37-22-6-9-28(41,10-7-22)25-5-4-20(15-35-25)24-16-33-11-12-34-24/h1-5,11-12,14-16,22-23,37,41H,6-10,13,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382942

(CHEMBL2029423)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1nccs1 |r,wU:4.7,wD:8.8,1.0,(-4.43,-4.47,;-3.09,-5.24,;-1.76,-6,;-.43,-5.24,;-.43,-3.7,;-1.76,-2.92,;-3.09,-3.7,;.91,-2.94,;2.24,-3.71,;2.26,-5.24,;3.73,-5.7,;4.62,-4.44,;3.7,-3.21,;5.95,-3.67,;5.94,-2.13,;7.29,-4.43,;8.61,-3.65,;9.95,-4.41,;9.96,-5.95,;11.28,-3.63,;11.26,-2.1,;12.58,-1.32,;13.93,-2.08,;13.94,-3.62,;12.62,-4.4,;15.28,-4.38,;15.29,-5.92,;16.61,-3.6,;16.53,-5.27,;-4.42,-6.01,;-5.75,-5.24,;-7.09,-6.01,;-7.08,-7.56,;-5.74,-8.32,;-4.41,-7.55,;-8.42,-8.33,;-9.83,-7.71,;-10.85,-8.86,;-10.08,-10.19,;-8.58,-9.87,)| Show InChI InChI=1S/C28H30F3N5O3S/c29-28(30,31)20-3-1-2-18(14-20)25(38)34-16-24(37)36-12-8-22(17-36)35-21-6-9-27(39,10-7-21)23-5-4-19(15-33-23)26-32-11-13-40-26/h1-5,11,13-15,21-22,35,39H,6-10,12,16-17H2,(H,34,38)/t21-,22-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 in PBMC after 30 mins by gamma counting |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data