 Found 41 hits with Last Name = 'mueller' and Initial = 'wt'
Found 41 hits with Last Name = 'mueller' and Initial = 'wt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 by steady state kinetic assay |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50265079
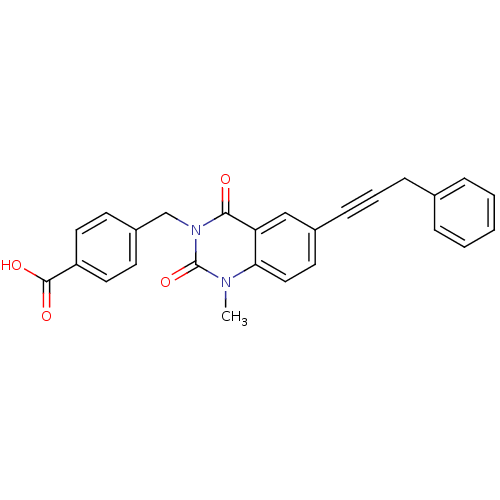
((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351
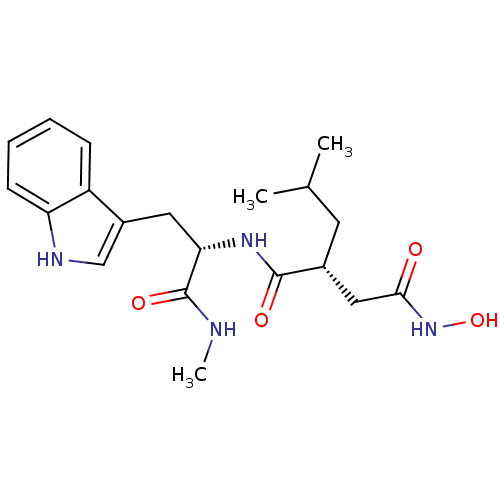
((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP17 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrilysin
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13-mediated type 2 collagen cleavage |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060738
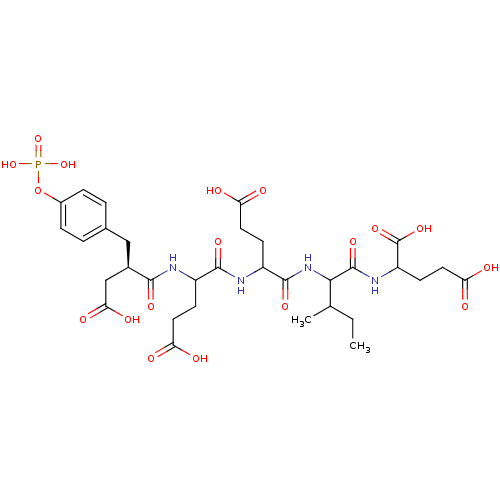
(2-[2-(4-Carboxy-2-{4-carboxy-2-[(R)-3-carboxy-2-(4...)Show SMILES CCC(C)C(NC(=O)C(CCC(O)=O)NC(=O)C(CCC(O)=O)NC(=O)[C@@H](CC(O)=O)Cc1ccc(OP(O)(O)=O)cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C32H45N4O18P/c1-3-16(2)27(31(48)35-22(32(49)50)10-13-25(41)42)36-30(47)21(9-12-24(39)40)34-29(46)20(8-11-23(37)38)33-28(45)18(15-26(43)44)14-17-4-6-19(7-5-17)54-55(51,52)53/h4-7,16,18,20-22,27H,3,8-15H2,1-2H3,(H,33,45)(H,34,46)(H,35,48)(H,36,47)(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H,49,50)(H2,51,52,53)/t16?,18-,20?,21?,22?,27?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060735
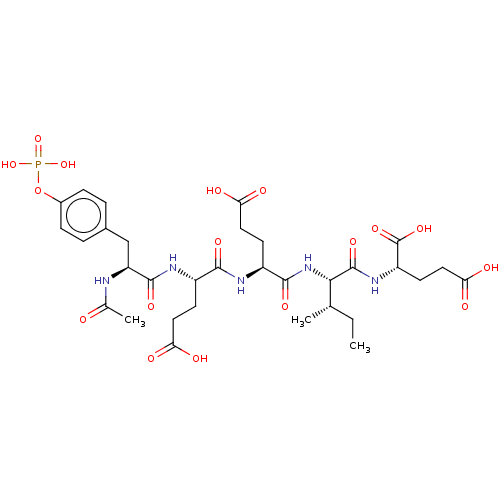
(2-[2-(2-{2-[(S)-2-Acetylamino-3-(4-phosphonooxy-ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C32H46N5O17P/c1-4-16(2)27(31(48)36-22(32(49)50)11-14-26(43)44)37-29(46)21(10-13-25(41)42)34-28(45)20(9-12-24(39)40)35-30(47)23(33-17(3)38)15-18-5-7-19(8-6-18)54-55(51,52)53/h5-8,16,20-23,27H,4,9-15H2,1-3H3,(H,33,38)(H,34,45)(H,35,47)(H,36,48)(H,37,46)(H,39,40)(H,41,42)(H,43,44)(H,49,50)(H2,51,52,53)/t16-,20-,21-,22-,23-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060736
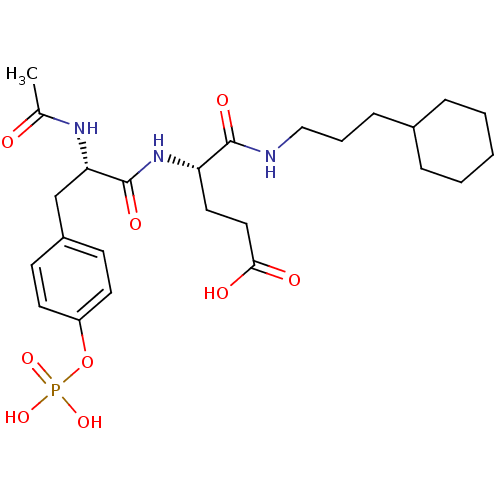
((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@@H](CCC(O)=O)C(=O)NCCCC1CCCCC1 Show InChI InChI=1S/C25H38N3O9P/c1-17(29)27-22(16-19-9-11-20(12-10-19)37-38(34,35)36)25(33)28-21(13-14-23(30)31)24(32)26-15-5-8-18-6-3-2-4-7-18/h9-12,18,21-22H,2-8,13-16H2,1H3,(H,26,32)(H,27,29)(H,28,33)(H,30,31)(H2,34,35,36)/t21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060734
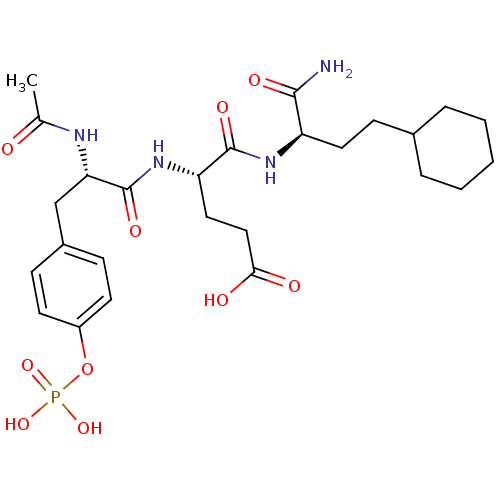
((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](CCC1CCCCC1)C(N)=O Show InChI InChI=1S/C26H39N4O10P/c1-16(31)28-22(15-18-7-10-19(11-8-18)40-41(37,38)39)26(36)30-21(13-14-23(32)33)25(35)29-20(24(27)34)12-9-17-5-3-2-4-6-17/h7-8,10-11,17,20-22H,2-6,9,12-15H2,1H3,(H2,27,34)(H,28,31)(H,29,35)(H,30,36)(H,32,33)(H2,37,38,39)/t20-,21+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060737
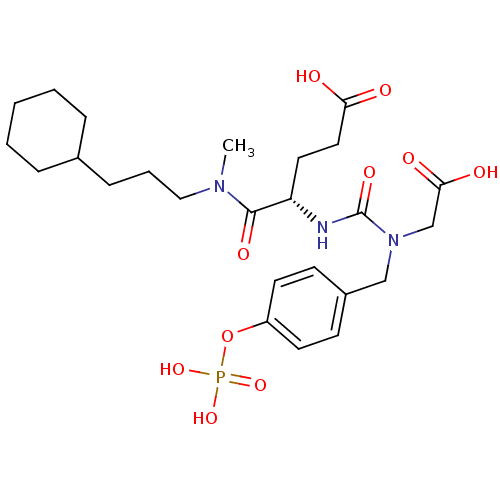
((S)-4-[3-Carboxymethyl-3-(4-phosphonooxy-benzyl)-u...)Show SMILES CN(CCCC1CCCCC1)C(=O)[C@H](CCC(O)=O)NC(=O)N(CC(O)=O)Cc1ccc(OP(O)(O)=O)cc1 Show InChI InChI=1S/C25H38N3O10P/c1-27(15-5-8-18-6-3-2-4-7-18)24(33)21(13-14-22(29)30)26-25(34)28(17-23(31)32)16-19-9-11-20(12-10-19)38-39(35,36)37/h9-12,18,21H,2-8,13-17H2,1H3,(H,26,34)(H,29,30)(H,31,32)(H2,35,36,37)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060733
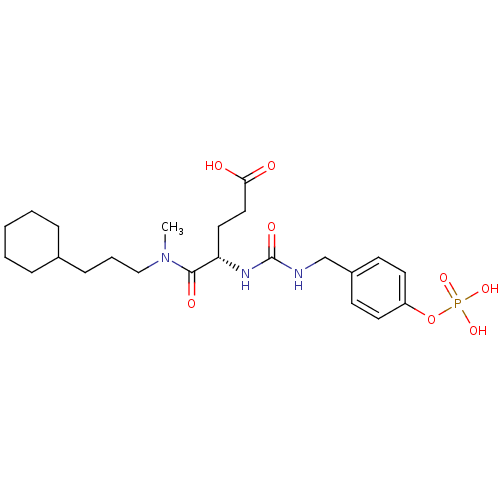
((S)-4-[(3-Cyclohexyl-propyl)-methyl-carbamoyl]-4-[...)Show SMILES CN(CCCC1CCCCC1)C(=O)[C@H](CCC(O)=O)NC(=O)NCc1ccc(OP(O)(O)=O)cc1 Show InChI InChI=1S/C23H36N3O8P/c1-26(15-5-8-17-6-3-2-4-7-17)22(29)20(13-14-21(27)28)25-23(30)24-16-18-9-11-19(12-10-18)34-35(31,32)33/h9-12,17,20H,2-8,13-16H2,1H3,(H,27,28)(H2,24,25,30)(H2,31,32,33)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50060732
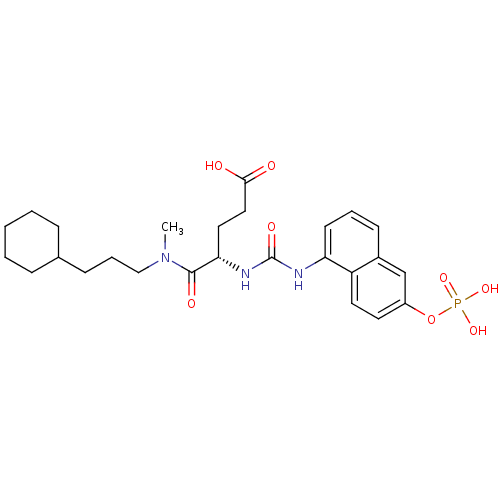
((S)-4-[(3-Cyclohexyl-propyl)-methyl-carbamoyl]-4-[...)Show SMILES CN(CCCC1CCCCC1)C(=O)[C@H](CCC(O)=O)NC(=O)Nc1cccc2cc(OP(O)(O)=O)ccc12 Show InChI InChI=1S/C26H36N3O8P/c1-29(16-6-9-18-7-3-2-4-8-18)25(32)23(14-15-24(30)31)28-26(33)27-22-11-5-10-19-17-20(12-13-21(19)22)37-38(34,35)36/h5,10-13,17-18,23H,2-4,6-9,14-16H2,1H3,(H,30,31)(H2,27,28,33)(H2,34,35,36)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-phosphopeptide binding to Src SH2 domain. |
J Med Chem 40: 3719-25 (1997)
Article DOI: 10.1021/jm970402q
BindingDB Entry DOI: 10.7270/Q2D21WQK |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 assessed as cleavage of aggrecan substrate |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 assessed as cleavage of aggrecan substrate |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP17 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50265079

((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-17
(Homo sapiens (Human)) | BDBM50234334

(BENZYL 6-BENZYL-5,7-DIOXO-6,7-DIHYDRO-5H-[1,3]THIA...)Show SMILES O=C(OCc1ccccc1)c1cn2c(cc(=O)n(Cc3ccccc3)c2=O)s1 Show InChI InChI=1S/C21H16N2O4S/c24-18-11-19-23(21(26)22(18)12-15-7-3-1-4-8-15)13-17(28-19)20(25)27-14-16-9-5-2-6-10-16/h1-11,13H,12,14H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP17 catalytic domain |
J Biol Chem 282: 27781-91 (2007)
Article DOI: 10.1074/jbc.M703286200
BindingDB Entry DOI: 10.7270/Q2Z89C58 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data