 Found 337 hits with Last Name = 'mullin' and Initial = 'r'
Found 337 hits with Last Name = 'mullin' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002471
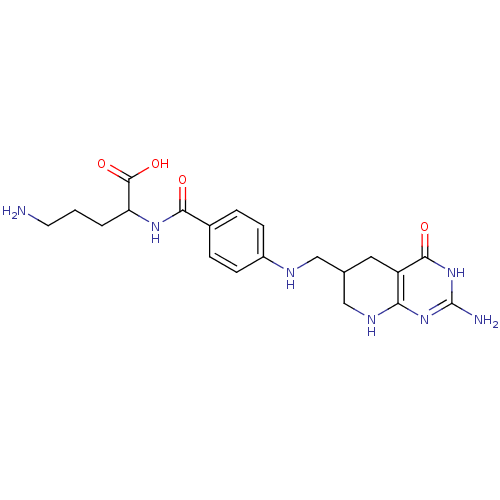
(5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C20H27N7O4/c21-7-1-2-15(19(30)31)25-17(28)12-3-5-13(6-4-12)23-9-11-8-14-16(24-10-11)26-20(22)27-18(14)29/h3-6,11,15,23H,1-2,7-10,21H2,(H,25,28)(H,30,31)(H4,22,24,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Potent inhibitor of Folyl-polyglutamate synthase obtained from porcine |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Trifunctional purine biosynthetic protein adenosine-3
(Mus musculus) | BDBM50002471

(5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...)Show SMILES NCCCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O Show InChI InChI=1S/C20H27N7O4/c21-7-1-2-15(19(30)31)25-17(28)12-3-5-13(6-4-12)23-9-11-8-14-16(24-10-11)26-20(22)27-18(14)29/h3-6,11,15,23H,1-2,7-10,21H2,(H,25,28)(H,30,31)(H4,22,24,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
potent inhibitor of GAR Tfase obtained from porcine |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002473
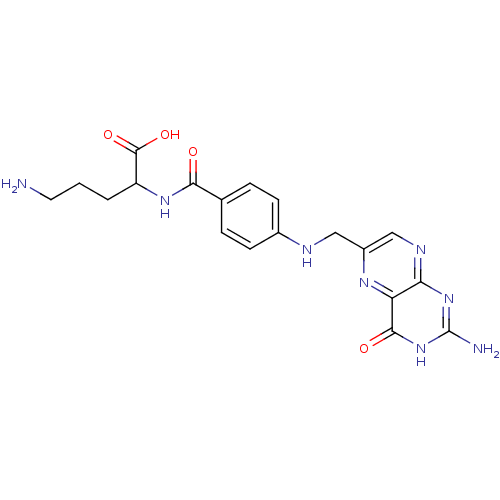
(5-Amino-2-{4-[(2-amino-4-oxo-3,4-dihydro-pteridin-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2cnc3nc(N)[nH]c(=O)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H22N8O4/c20-7-1-2-13(18(30)31)25-16(28)10-3-5-11(6-4-10)22-8-12-9-23-15-14(24-12)17(29)27-19(21)26-15/h3-6,9,13,22H,1-2,7-8,20H2,(H,25,28)(H,30,31)(H3,21,23,26,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Homo sapiens (Human)) | BDBM50002472
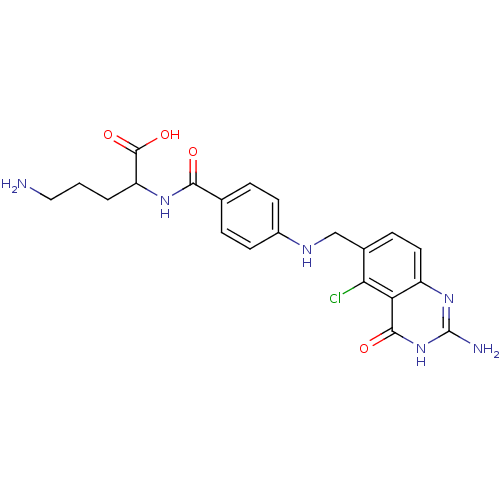
(5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2ccc3nc(N)[nH]c(=O)c3c2Cl)cc1)C(O)=O Show InChI InChI=1S/C21H23ClN6O4/c22-17-12(5-8-14-16(17)19(30)28-21(24)27-14)10-25-13-6-3-11(4-7-13)18(29)26-15(20(31)32)2-1-9-23/h3-8,15,25H,1-2,9-10,23H2,(H,26,29)(H,31,32)(H3,24,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against hog liver Folyl-polyglutamate synthase |
J Med Chem 35: 2002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2X9297Q |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560806
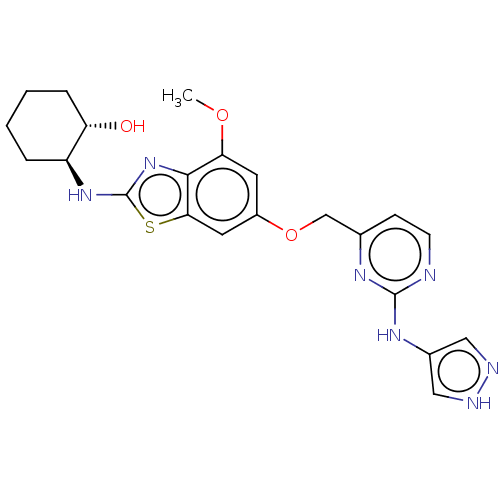
(CHEMBL4758092)Show SMILES COc1cc(OCc2ccnc(Nc3cn[nH]c3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50460035
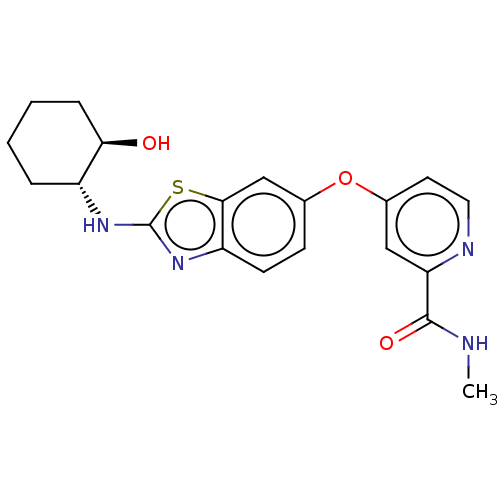
(CHEMBL4227505)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)ccn1 |r| Show InChI InChI=1S/C20H22N4O3S/c1-21-19(26)16-10-13(8-9-22-16)27-12-6-7-15-18(11-12)28-20(24-15)23-14-4-2-3-5-17(14)25/h6-11,14,17,25H,2-5H2,1H3,(H,21,26)(H,23,24)/t14-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSF1R (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256016
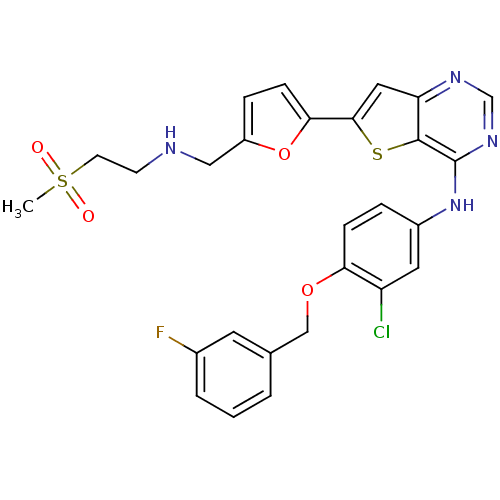
(CHEMBL475768 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-22-26(38-25)27(32-16-31-22)33-19-5-7-23(21(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560804
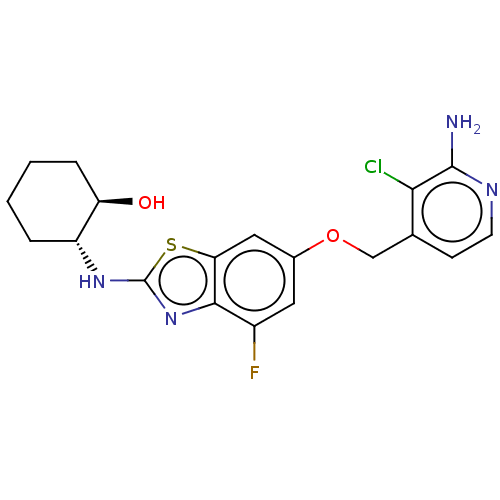
(CHEMBL4763875)Show SMILES Nc1nccc(COc2cc(F)c3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560807
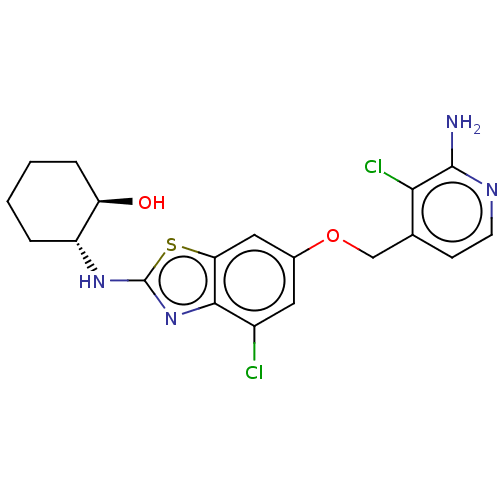
(CHEMBL4751287)Show SMILES Nc1nccc(COc2cc(Cl)c3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560786
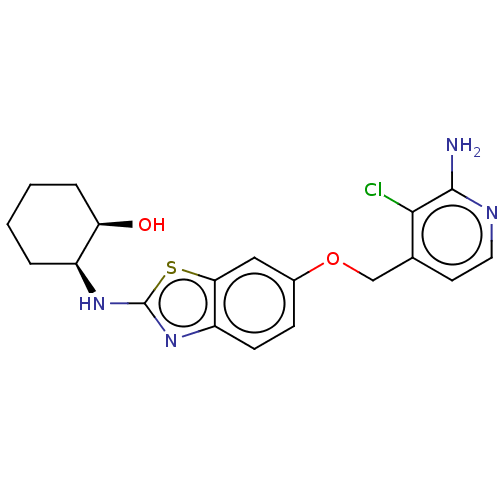
(CHEMBL4798876)Show SMILES Nc1nccc(COc2ccc3nc(N[C@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560798
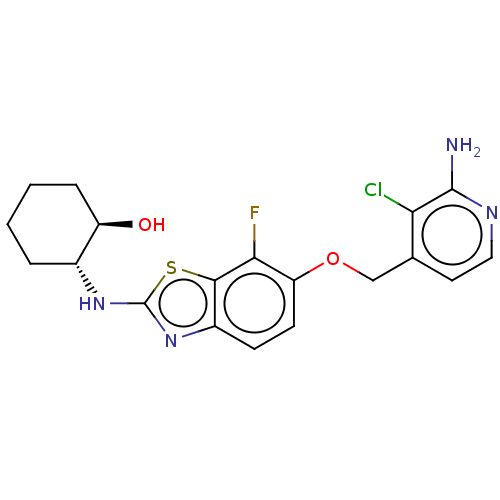
(CHEMBL4795462)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2F)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560791
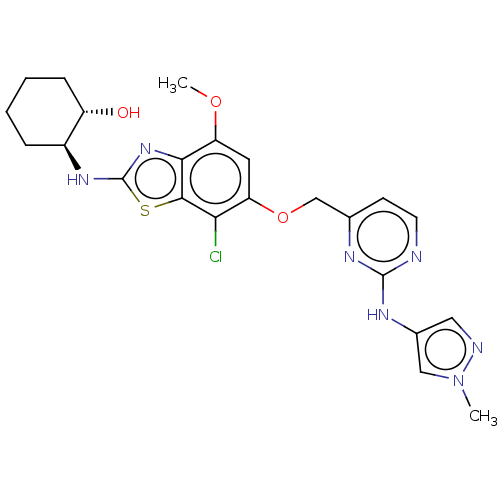
(CHEMBL4740157)Show SMILES COc1cc(OCc2ccnc(Nc3cnn(C)c3)n2)c(Cl)c2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560801
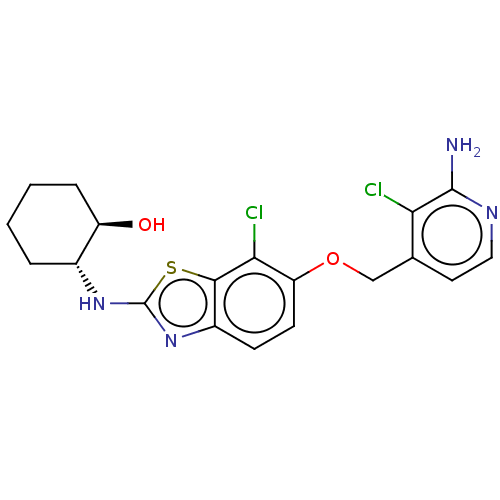
(CHEMBL4798601)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2Cl)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560788
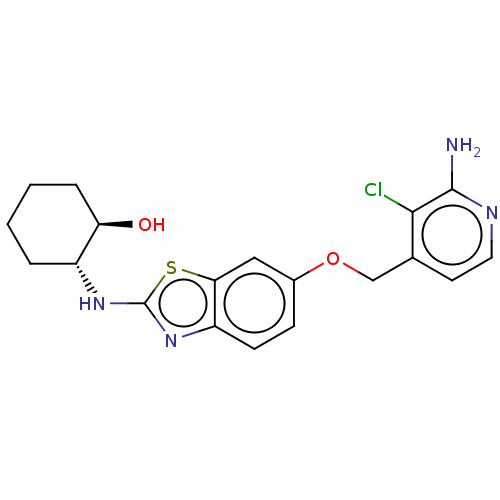
(CHEMBL4759047)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560812
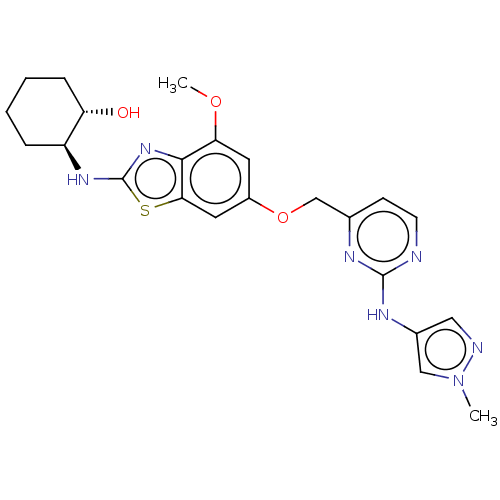
(CHEMBL4748740)Show SMILES COc1cc(OCc2ccnc(Nc3cnn(C)c3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560789
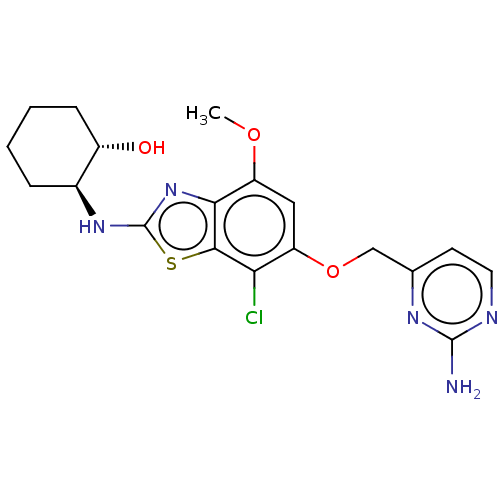
(CHEMBL4779878)Show SMILES COc1cc(OCc2ccnc(N)n2)c(Cl)c2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256032
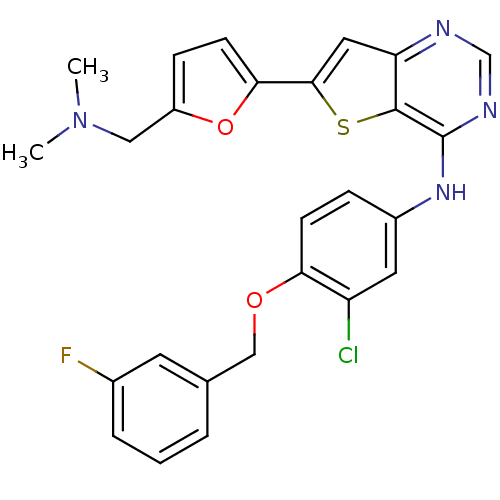
(CHEMBL475594 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN(C)Cc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H22ClFN4O2S/c1-32(2)13-19-7-9-23(34-19)24-12-21-25(35-24)26(30-15-29-21)31-18-6-8-22(20(27)11-18)33-14-16-4-3-5-17(28)10-16/h3-12,15H,13-14H2,1-2H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560790
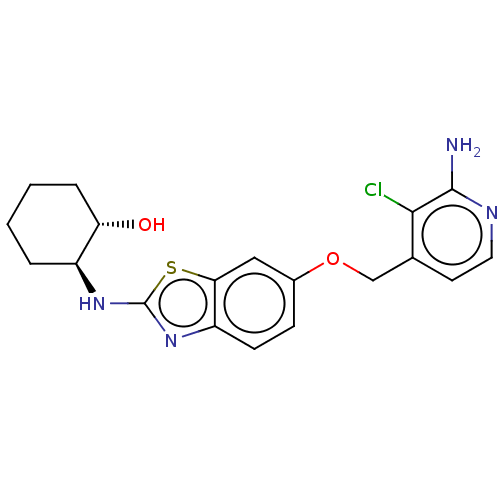
(CHEMBL4741283)Show SMILES Nc1nccc(COc2ccc3nc(N[C@H]4CCCC[C@@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560816
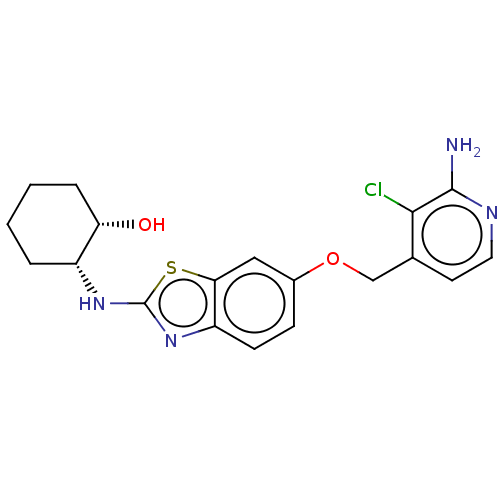
(CHEMBL4756510)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560800
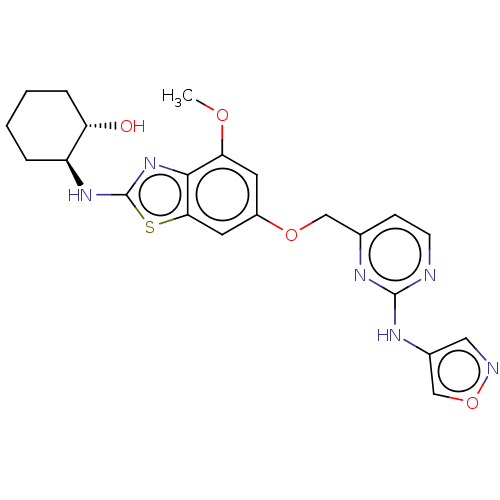
(CHEMBL4779197)Show SMILES COc1cc(OCc2ccnc(Nc3cnoc3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256034
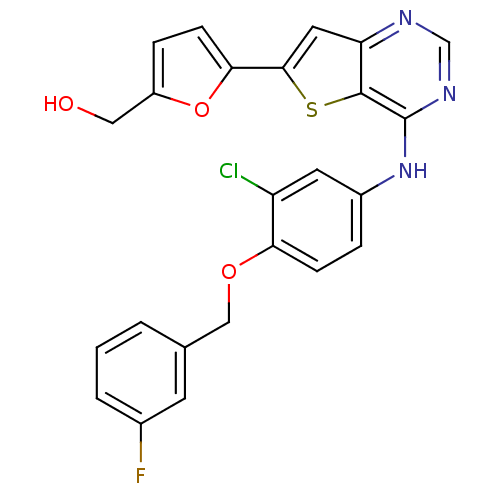
((5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)t...)Show SMILES OCc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C24H17ClFN3O3S/c25-18-9-16(4-6-20(18)31-12-14-2-1-3-15(26)8-14)29-24-23-19(27-13-28-24)10-22(33-23)21-7-5-17(11-30)32-21/h1-10,13,30H,11-12H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256036
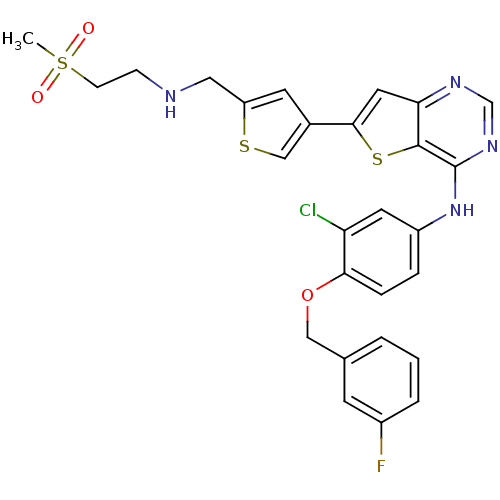
(CHEMBL474431 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1cc(cs1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O3S3/c1-39(34,35)8-7-30-13-21-10-18(15-37-21)25-12-23-26(38-25)27(32-16-31-23)33-20-5-6-24(22(28)11-20)36-14-17-3-2-4-19(29)9-17/h2-6,9-12,15-16,30H,7-8,13-14H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256035
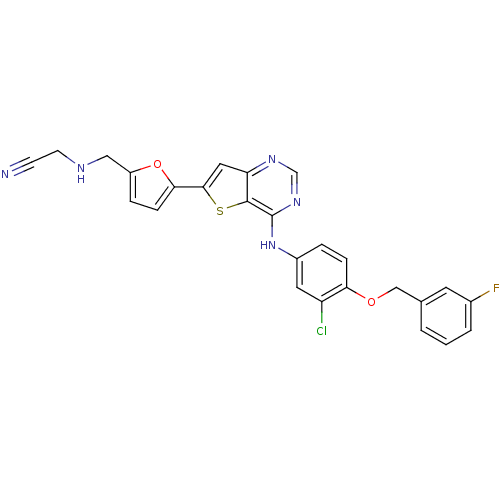
(2-((5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamin...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3ccc(CNCC#N)o3)cc2Cl)c1 Show InChI InChI=1S/C26H19ClFN5O2S/c27-20-11-18(4-6-22(20)34-14-16-2-1-3-17(28)10-16)33-26-25-21(31-15-32-26)12-24(36-25)23-7-5-19(35-23)13-30-9-8-29/h1-7,10-12,15,30H,9,13-14H2,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560813

(CHEMBL4746711)Show SMILES COc1cc(OCc2ccnc(N)c2Cl)cc2sc(N[C@@H]3CCCC[C@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560785
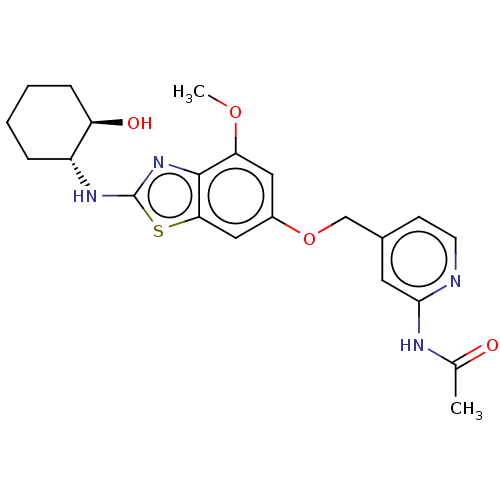
(CHEMBL4754431)Show SMILES COc1cc(OCc2ccnc(NC(C)=O)c2)cc2sc(N[C@@H]3CCCC[C@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256038
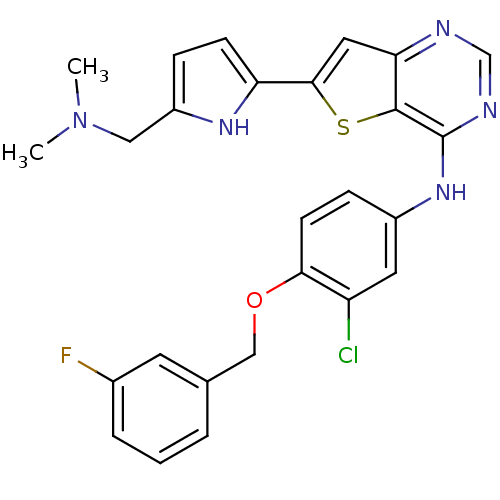
(CHEMBL473437 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN(C)Cc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H23ClFN5OS/c1-33(2)13-19-6-8-21(31-19)24-12-22-25(35-24)26(30-15-29-22)32-18-7-9-23(20(27)11-18)34-14-16-4-3-5-17(28)10-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256017
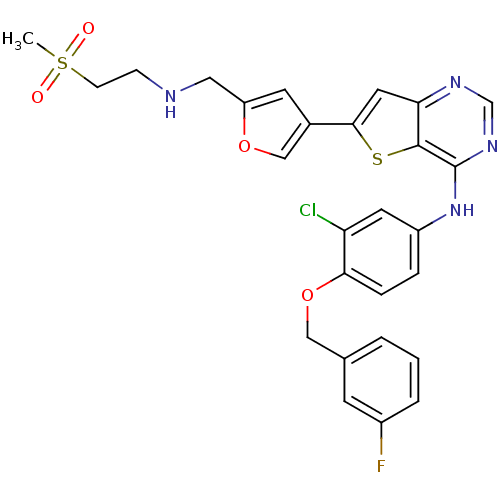
(CHEMBL475769 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1cc(co1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)8-7-30-13-21-10-18(15-36-21)25-12-23-26(38-25)27(32-16-31-23)33-20-5-6-24(22(28)11-20)37-14-17-3-2-4-19(29)9-17/h2-6,9-12,15-16,30H,7-8,13-14H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560793

(CHEMBL4788945) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256033
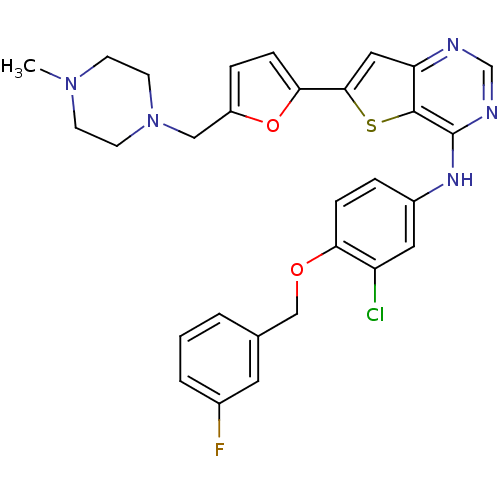
(CHEMBL475761 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN1CCN(Cc2ccc(o2)-c2cc3ncnc(Nc4ccc(OCc5cccc(F)c5)c(Cl)c4)c3s2)CC1 Show InChI InChI=1S/C29H27ClFN5O2S/c1-35-9-11-36(12-10-35)16-22-6-8-26(38-22)27-15-24-28(39-27)29(33-18-32-24)34-21-5-7-25(23(30)14-21)37-17-19-3-2-4-20(31)13-19/h2-8,13-15,18H,9-12,16-17H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560797

(CHEMBL4763289)Show SMILES COc1cc(OCc2ccnc(Nc3cn(C)nn3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256039
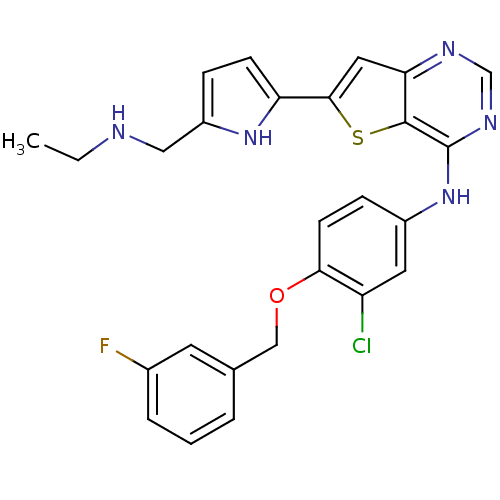
(CHEMBL473438 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CCNCc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H23ClFN5OS/c1-2-29-13-19-6-8-21(32-19)24-12-22-25(35-24)26(31-15-30-22)33-18-7-9-23(20(27)11-18)34-14-16-4-3-5-17(28)10-16/h3-12,15,29,32H,2,13-14H2,1H3,(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50256040
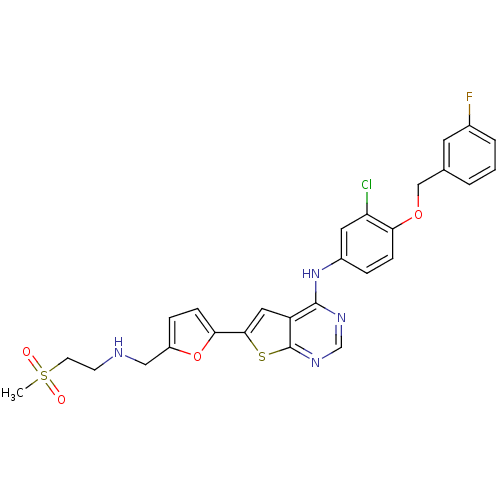
(CHEMBL480349 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2c(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-21-26(31-16-32-27(21)38-25)33-19-5-7-23(22(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM109086

(US10793535, Cmpd ID 727 | US8604016, 670 | US99382...)Show SMILES FC(F)(F)Oc1cccc(CC(=O)Nc2ccc(CCCCc3nnc(NC(=O)Cc4ccccn4)s3)nn2)c1 Show InChI InChI=1S/C26H24F3N7O3S/c27-26(28,29)39-20-9-5-6-17(14-20)15-22(37)31-21-12-11-18(33-34-21)7-1-2-10-24-35-36-25(40-24)32-23(38)16-19-8-3-4-13-30-19/h3-6,8-9,11-14H,1-2,7,10,15-16H2,(H,31,34,37)(H,32,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01398
BindingDB Entry DOI: 10.7270/Q25D8WGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM404825
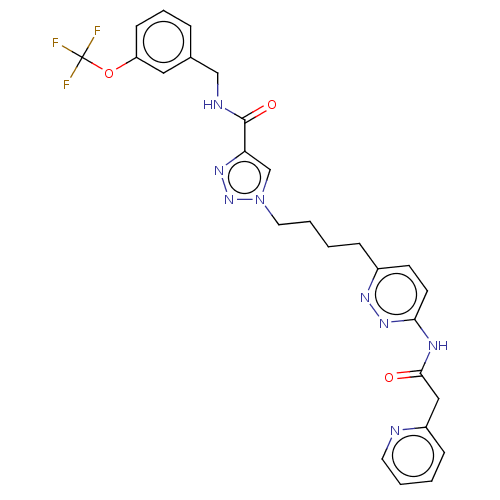
(1-(4-{6-[2-(pyridin-2- yl)acetamido]pyridazin-3-yl...)Show SMILES FC(F)(F)Oc1cccc(CNC(=O)c2cn(CCCCc3ccc(NC(=O)Cc4ccccn4)nn3)nn2)c1 Show InChI InChI=1S/C26H25F3N8O3/c27-26(28,29)40-21-9-5-6-18(14-21)16-31-25(39)22-17-37(36-34-22)13-4-2-7-19-10-11-23(35-33-19)32-24(38)15-20-8-1-3-12-30-20/h1,3,5-6,8-12,14,17H,2,4,7,13,15-16H2,(H,31,39)(H,32,35,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01398
BindingDB Entry DOI: 10.7270/Q25D8WGC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256020
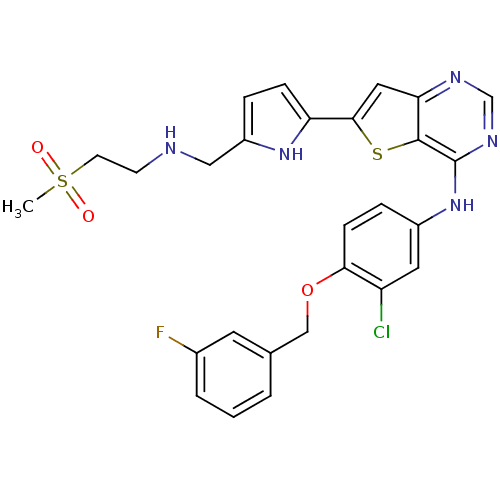
(CHEMBL475446 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H25ClFN5O3S2/c1-39(35,36)10-9-30-14-20-5-7-22(33-20)25-13-23-26(38-25)27(32-16-31-23)34-19-6-8-24(21(28)12-19)37-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30,33H,9-10,14-15H2,1H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560792
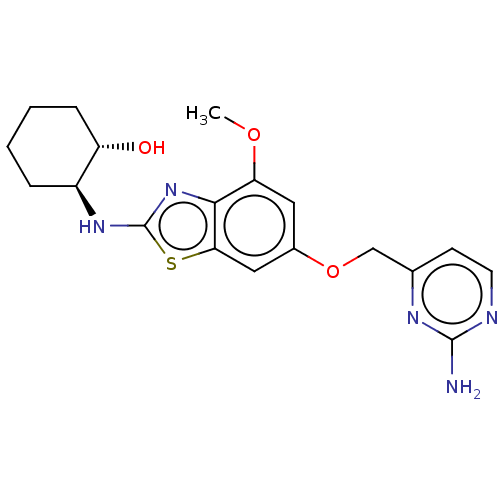
(CHEMBL4762843)Show SMILES COc1cc(OCc2ccnc(N)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM27973
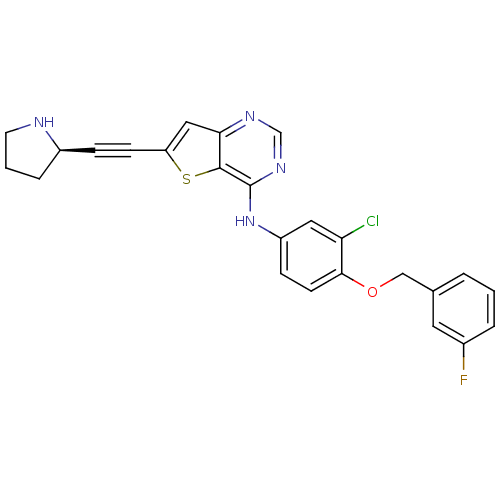
(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Mus musculus (Mouse)) | BDBM50560812

(CHEMBL4748740)Show SMILES COc1cc(OCc2ccnc(Nc3cnn(C)c3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSF1R in mouse NFS-60 cells assessed as reduction in CSF-induced cell proliferation after 72 hrs by CellTiter-Glo assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Mus musculus (Mouse)) | BDBM50560806

(CHEMBL4758092)Show SMILES COc1cc(OCc2ccnc(Nc3cn[nH]c3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSF1R in mouse NFS-60 cells assessed as reduction in CSF-induced cell proliferation after 72 hrs by CellTiter-Glo assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256018

(CHEMBL473427 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3cccs3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3OS2/c24-17-10-16(6-7-19(17)29-12-14-3-1-4-15(25)9-14)28-23-22-18(26-13-27-23)11-21(31-22)20-5-2-8-30-20/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256041
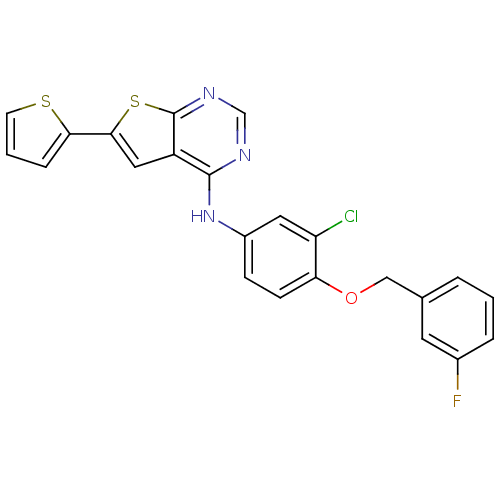
(CHEMBL506414 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4sc(cc34)-c3cccs3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3OS2/c24-18-10-16(6-7-19(18)29-12-14-3-1-4-15(25)9-14)28-22-17-11-21(20-5-2-8-30-20)31-23(17)27-13-26-22/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560809
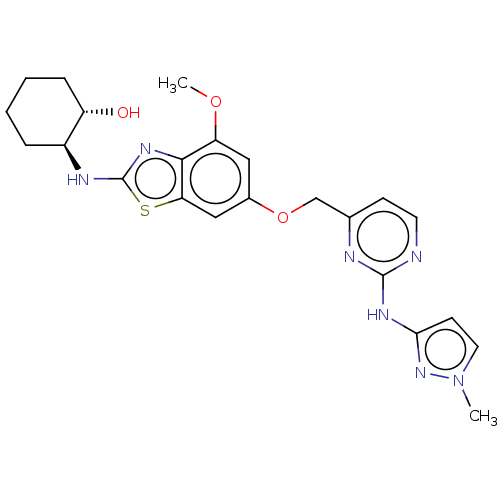
(CHEMBL4787609)Show SMILES COc1cc(OCc2ccnc(Nc3ccn(C)n3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256015

(CHEMBL479800 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3ccco3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3O2S/c24-17-10-16(6-7-19(17)30-12-14-3-1-4-15(25)9-14)28-23-22-18(26-13-27-23)11-21(31-22)20-5-2-8-29-20/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM404848

(N-methyl-1-{4-[6-(2-{4-[3- (trifluoromethoxy)pheny...)Show SMILES CNC(=O)c1cn(CCCCc2ccc(NC(=O)Cc3cc(ccn3)-c3cccc(OC(F)(F)F)c3)nn2)nn1 Show InChI InChI=1S/C26H25F3N8O3/c1-30-25(39)22-16-37(36-34-22)12-3-2-6-19-8-9-23(35-33-19)32-24(38)15-20-13-18(10-11-31-20)17-5-4-7-21(14-17)40-26(27,28)29/h4-5,7-11,13-14,16H,2-3,6,12,15H2,1H3,(H,30,39)(H,32,35,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01398
BindingDB Entry DOI: 10.7270/Q25D8WGC |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM404911
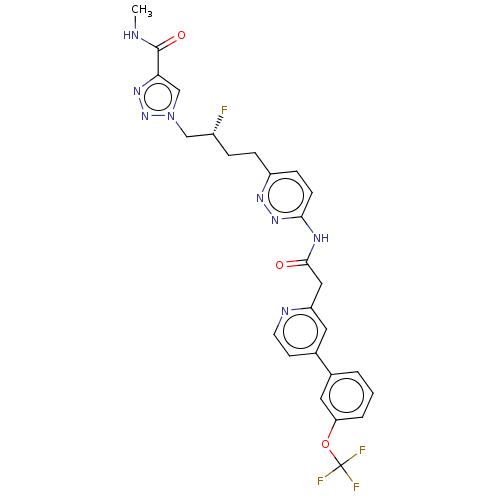
((R)-1-(2-fluoro-4-(6-(2-(4-(3- (trifluoromethoxy)p...)Show SMILES CNC(=O)c1cn(C[C@H](F)CCc2ccc(NC(=O)Cc3cc(ccn3)-c3cccc(OC(F)(F)F)c3)nn2)nn1 |r| Show InChI InChI=1S/C26H24F4N8O3/c1-31-25(40)22-15-38(37-35-22)14-18(27)5-6-19-7-8-23(36-34-19)33-24(39)13-20-11-17(9-10-32-20)16-3-2-4-21(12-16)41-26(28,29)30/h2-4,7-12,15,18H,5-6,13-14H2,1H3,(H,31,40)(H,33,36,39)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01398
BindingDB Entry DOI: 10.7270/Q25D8WGC |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM27974
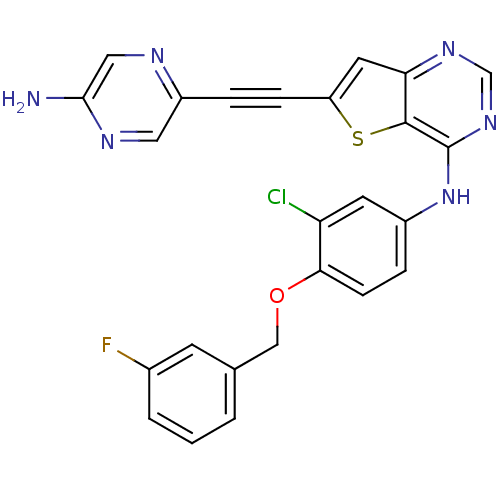
(5-{2-[4-({3-chloro-4-[(3-fluorophenyl)methoxy]phen...)Show SMILES Nc1cnc(cn1)C#Cc1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C25H16ClFN6OS/c26-20-9-17(5-7-22(20)34-13-15-2-1-3-16(27)8-15)33-25-24-21(31-14-32-25)10-19(35-24)6-4-18-11-30-23(28)12-29-18/h1-3,5,7-12,14H,13H2,(H2,28,30)(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5459

(6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...)Show SMILES CS(=O)(=O)CCNCCCCOc1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C28H30ClFN4O4S/c1-39(35,36)14-12-31-11-2-3-13-37-23-8-9-26-24(17-23)28(33-19-32-26)34-22-7-10-27(25(29)16-22)38-18-20-5-4-6-21(30)15-20/h4-10,15-17,19,31H,2-3,11-14,18H2,1H3,(H,32,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline
| Assay Description
Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... |
Bioorg Med Chem Lett 13: 637-40 (2003)
Article DOI: 10.1016/s0960-894x(02)01047-8
BindingDB Entry DOI: 10.7270/Q2D21VS2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data