 Found 303 hits with Last Name = 'mullins' and Initial = 'p'
Found 303 hits with Last Name = 'mullins' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50343062
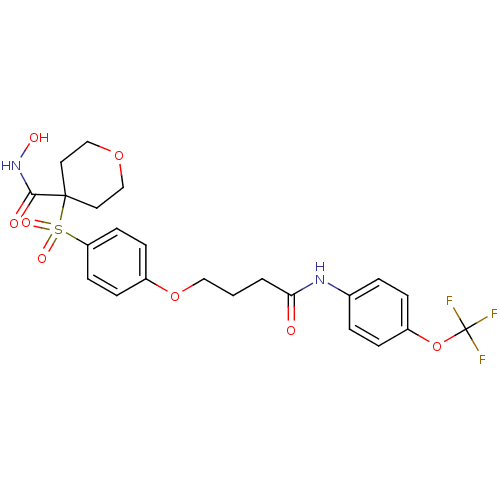
(CHEMBL1770709 | N-hydroxy-4-(4-(4-oxo-4-(4-(triflu...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H25F3N2O8S/c24-23(25,26)36-18-5-3-16(4-6-18)27-20(29)2-1-13-35-17-7-9-19(10-8-17)37(32,33)22(21(30)28-31)11-14-34-15-12-22/h3-10,31H,1-2,11-15H2,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343059

(4-(4-(4-(4-(dimethylamino)phenyl)-4-oxobutoxy)phen...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C24H30N2O7S/c1-26(2)19-7-5-18(6-8-19)22(27)4-3-15-33-20-9-11-21(12-10-20)34(30,31)24(23(28)25-29)13-16-32-17-14-24/h5-12,29H,3-4,13-17H2,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343061

(CHEMBL1770157 | N-hydroxy-4-(4-(4-(4-methoxyphenyl...)Show SMILES COc1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O8S/c1-31-18-6-4-17(5-7-18)24-21(26)3-2-14-33-19-8-10-20(11-9-19)34(29,30)23(22(27)25-28)12-15-32-16-13-23/h4-11,28H,2-3,12-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50078668

(3-(4-Phenylsulfanyl-benzenesulfonyl)-cyclohexaneth...)Show InChI InChI=1S/C18H20O2S3/c19-23(20,18-8-4-5-14(21)13-18)17-11-9-16(10-12-17)22-15-6-2-1-3-7-15/h1-3,6-7,9-12,14,18,21H,4-5,8,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343051
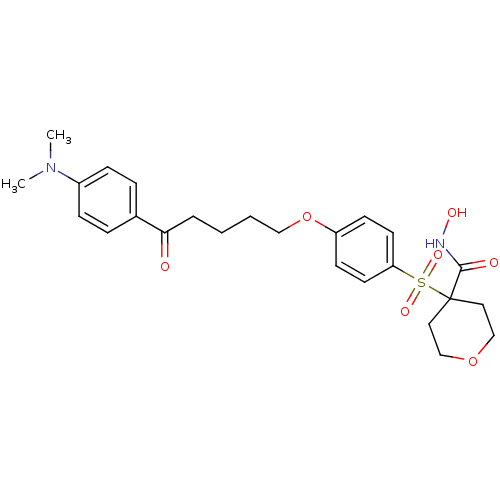
(4-(4-(5-(4-(dimethylamino)phenyl)-5-oxopentyloxy)p...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C25H32N2O7S/c1-27(2)20-8-6-19(7-9-20)23(28)5-3-4-16-34-21-10-12-22(13-11-21)35(31,32)25(24(29)26-30)14-17-33-18-15-25/h6-13,30H,3-5,14-18H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076589

(3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...)Show InChI InChI=1S/C15H16O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-3,5-10,19H,4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343054
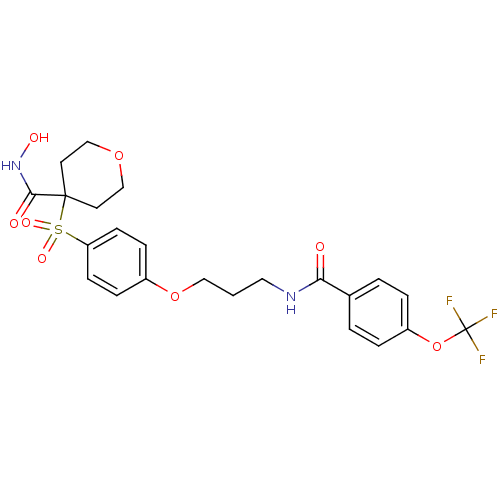
(CHEMBL1770702 | N-hydroxy-4-(4-(3-(4-(trifluoromet...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCNC(=O)c2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H25F3N2O8S/c24-23(25,26)36-18-4-2-16(3-5-18)20(29)27-12-1-13-35-17-6-8-19(9-7-17)37(32,33)22(21(30)28-31)10-14-34-15-11-22/h2-9,31H,1,10-15H2,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343066

(CHEMBL1770712 | N-hydroxy-4-(4-(4-(4-methoxyphenox...)Show SMILES COc1ccc(OCCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H29NO8S/c1-29-18-4-6-19(7-5-18)31-14-2-3-15-32-20-8-10-21(11-9-20)33(27,28)23(22(25)24-26)12-16-30-17-13-23/h4-11,26H,2-3,12-17H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343058
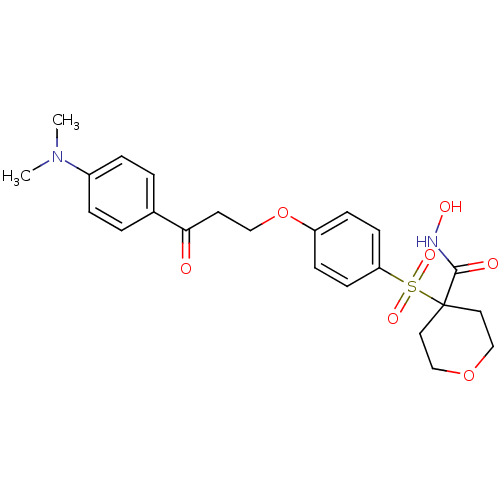
(4-(4-(3-(4-(dimethylamino)phenyl)-3-oxopropoxy)phe...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C23H28N2O7S/c1-25(2)18-5-3-17(4-6-18)21(26)11-14-32-19-7-9-20(10-8-19)33(29,30)23(22(27)24-28)12-15-31-16-13-23/h3-10,28H,11-16H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50078667

(3-(4-Phenoxy-benzenesulfonyl)-cyclohexanethiol | C...)Show InChI InChI=1S/C18H20O3S2/c19-23(20,18-8-4-7-16(22)13-18)17-11-9-15(10-12-17)21-14-5-2-1-3-6-14/h1-3,5-6,9-12,16,18,22H,4,7-8,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196751
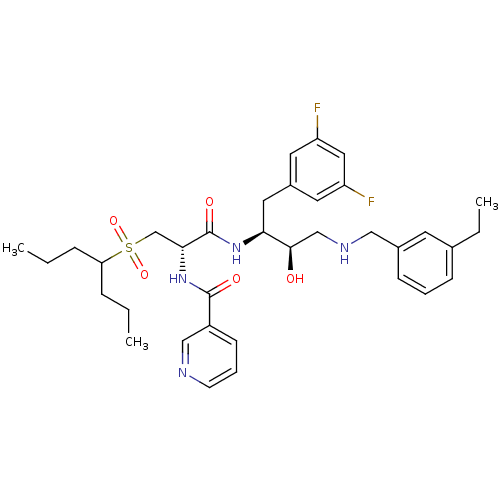
(CHEMBL231862 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C35H46F2N4O5S/c1-4-9-30(10-5-2)47(45,46)23-32(41-34(43)27-13-8-14-38-21-27)35(44)40-31(18-26-16-28(36)19-29(37)17-26)33(42)22-39-20-25-12-7-11-24(6-3)15-25/h7-8,11-17,19,21,30-33,39,42H,4-6,9-10,18,20,22-23H2,1-3H3,(H,40,44)(H,41,43)/t31-,32+,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343064

(CHEMBL1770710 | N-hydroxy-4-(4-(4-oxo-4-(piperidin...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCC(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C21H30N2O7S/c24-19(23-12-2-1-3-13-23)5-4-14-30-17-6-8-18(9-7-17)31(27,28)21(20(25)22-26)10-15-29-16-11-21/h6-9,26H,1-5,10-16H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343053
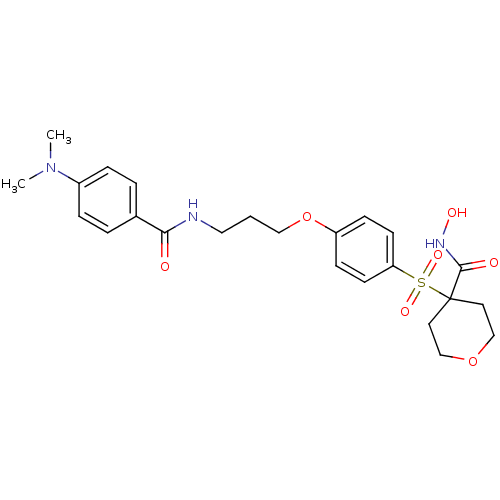
(4-(4-(3-(4-(dimethylamino)benzamido)propoxy)phenyl...)Show SMILES CN(C)c1ccc(cc1)C(=O)NCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C24H31N3O7S/c1-27(2)19-6-4-18(5-7-19)22(28)25-14-3-15-34-20-8-10-21(11-9-20)35(31,32)24(23(29)26-30)12-16-33-17-13-24/h4-11,30H,3,12-17H2,1-2H3,(H,25,28)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343052
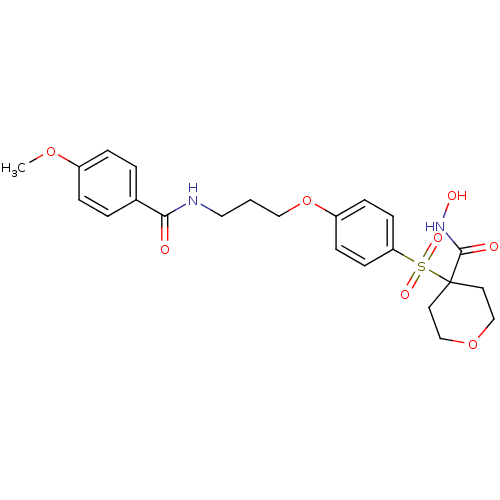
(CHEMBL1770700 | N-hydroxy-4-(4-(3-(4-methoxybenzam...)Show SMILES COc1ccc(cc1)C(=O)NCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C23H28N2O8S/c1-31-18-5-3-17(4-6-18)21(26)24-13-2-14-33-19-7-9-20(10-8-19)34(29,30)23(22(27)25-28)11-15-32-16-12-23/h3-10,28H,2,11-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409
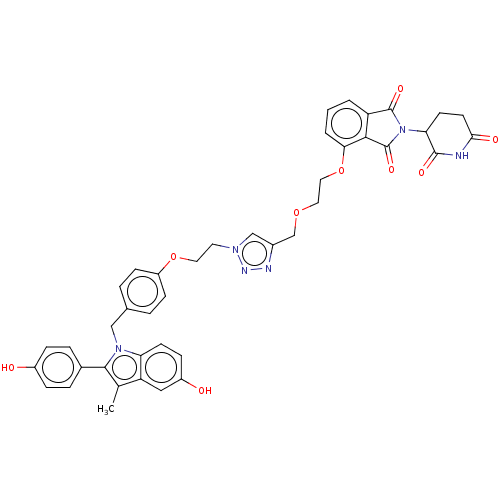
(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343060

(4-(4-(4-(4-(dimethylamino)phenylamino)-4-oxobutoxy...)Show SMILES CN(C)c1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H31N3O7S/c1-27(2)19-7-5-18(6-8-19)25-22(28)4-3-15-34-20-9-11-21(12-10-20)35(31,32)24(23(29)26-30)13-16-33-17-14-24/h5-12,30H,3-4,13-17H2,1-2H3,(H,25,28)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343065
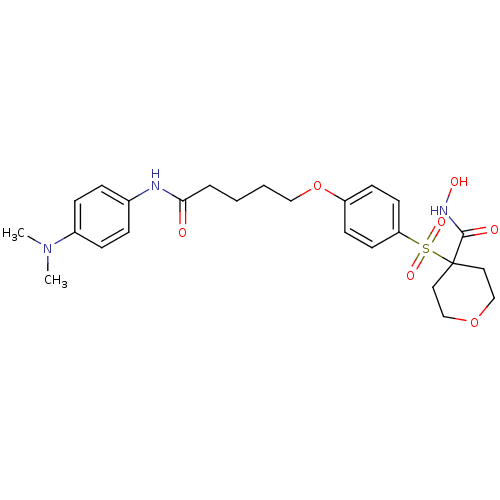
(4-(4-(5-(4-(dimethylamino)phenylamino)-5-oxopentyl...)Show SMILES CN(C)c1ccc(NC(=O)CCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C25H33N3O7S/c1-28(2)20-8-6-19(7-9-20)26-23(29)5-3-4-16-35-21-10-12-22(13-11-21)36(32,33)25(24(30)27-31)14-17-34-18-15-25/h6-13,31H,3-5,14-18H2,1-2H3,(H,26,29)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343065

(4-(4-(5-(4-(dimethylamino)phenylamino)-5-oxopentyl...)Show SMILES CN(C)c1ccc(NC(=O)CCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C25H33N3O7S/c1-28(2)20-8-6-19(7-9-20)26-23(29)5-3-4-16-35-21-10-12-22(13-11-21)36(32,33)25(24(30)27-31)14-17-34-18-15-25/h6-13,31H,3-5,14-18H2,1-2H3,(H,26,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196747
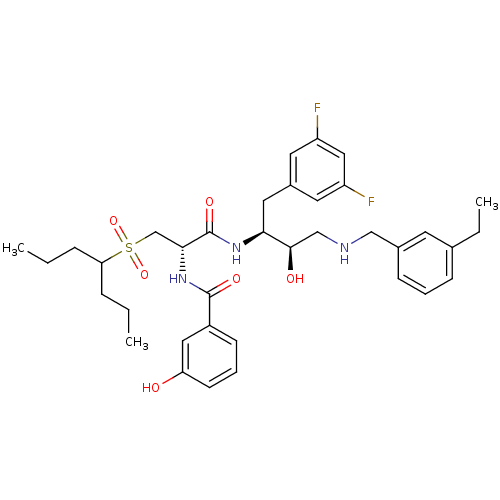
(CHEMBL266566 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1cccc(O)c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H47F2N3O6S/c1-4-9-31(10-5-2)48(46,47)23-33(41-35(44)27-13-8-14-30(42)19-27)36(45)40-32(18-26-16-28(37)20-29(38)17-26)34(43)22-39-21-25-12-7-11-24(6-3)15-25/h7-8,11-17,19-20,31-34,39,42-43H,4-6,9-10,18,21-23H2,1-3H3,(H,40,45)(H,41,44)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196746
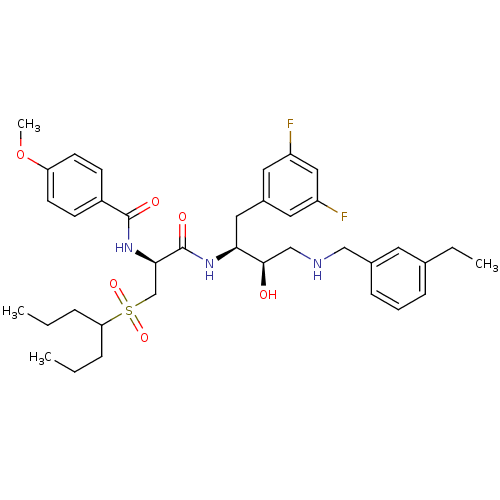
(CHEMBL231861 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1ccc(OC)cc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O6S/c1-5-9-32(10-6-2)49(46,47)24-34(42-36(44)28-13-15-31(48-4)16-14-28)37(45)41-33(20-27-18-29(38)21-30(39)19-27)35(43)23-40-22-26-12-8-11-25(7-3)17-26/h8,11-19,21,32-35,40,43H,5-7,9-10,20,22-24H2,1-4H3,(H,41,45)(H,42,44)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196755

(CHEMBL266567 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C32H40F2N4O5S/c1-3-5-12-44(42,43)21-29(38-31(40)25-10-7-11-35-19-25)32(41)37-28(16-24-14-26(33)17-27(34)15-24)30(39)20-36-18-23-9-6-8-22(4-2)13-23/h6-11,13-15,17,19,28-30,36,39H,3-5,12,16,18,20-21H2,1-2H3,(H,37,41)(H,38,40)/t28-,29+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50076593
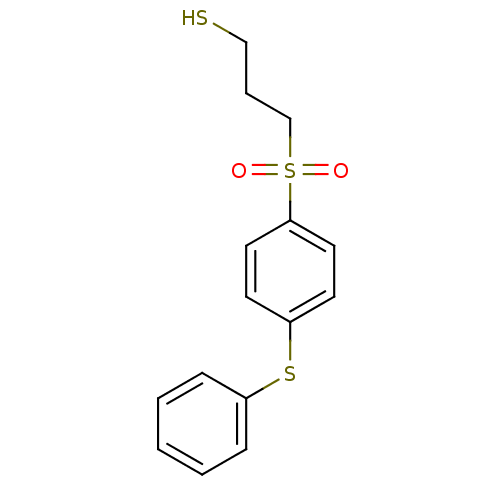
(3-(4-Phenylsulfanyl-benzenesulfonyl)-propane-1-thi...)Show InChI InChI=1S/C15H16O2S3/c16-20(17,12-4-11-18)15-9-7-14(8-10-15)19-13-5-2-1-3-6-13/h1-3,5-10,18H,4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-13 (hMMP-13) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409

(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343061

(CHEMBL1770157 | N-hydroxy-4-(4-(4-(4-methoxyphenyl...)Show SMILES COc1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O8S/c1-31-18-6-4-17(5-7-18)24-21(26)3-2-14-33-19-8-10-20(11-9-19)34(29,30)23(22(27)25-28)12-15-32-16-13-23/h4-11,28H,2-3,12-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343065

(4-(4-(5-(4-(dimethylamino)phenylamino)-5-oxopentyl...)Show SMILES CN(C)c1ccc(NC(=O)CCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C25H33N3O7S/c1-28(2)20-8-6-19(7-9-20)26-23(29)5-3-4-16-35-21-10-12-22(13-11-21)36(32,33)25(24(30)27-31)14-17-34-18-15-25/h6-13,31H,3-5,14-18H2,1-2H3,(H,26,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196749
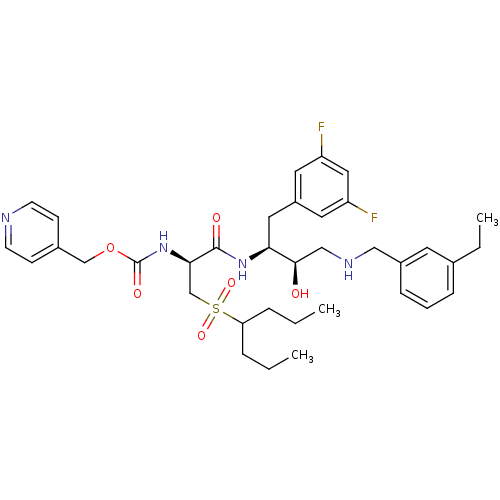
(CHEMBL394270 | pyridin-4-ylmethyl (S)-1-((2S,3R)-4...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OCc1ccncc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H48F2N4O6S/c1-4-8-31(9-5-2)49(46,47)24-33(42-36(45)48-23-26-12-14-39-15-13-26)35(44)41-32(19-28-17-29(37)20-30(38)18-28)34(43)22-40-21-27-11-7-10-25(6-3)16-27/h7,10-18,20,31-34,40,43H,4-6,8-9,19,21-24H2,1-3H3,(H,41,44)(H,42,45)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196736

(CHEMBL231863 | benzyl (S)-1-((2S,3R)-4-(3-ethylben...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O6S/c1-4-11-32(12-5-2)49(46,47)25-34(42-37(45)48-24-27-13-8-7-9-14-27)36(44)41-33(20-29-18-30(38)21-31(39)19-29)35(43)23-40-22-28-16-10-15-26(6-3)17-28/h7-10,13-19,21,32-35,40,43H,4-6,11-12,20,22-25H2,1-3H3,(H,41,44)(H,42,45)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196745

(CHEMBL414145 | methyl (S)-1-((2S,3R)-4-(3-ethylben...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OC)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H45F2N3O6S/c1-5-9-26(10-6-2)43(40,41)20-28(36-31(39)42-4)30(38)35-27(16-23-14-24(32)17-25(33)15-23)29(37)19-34-18-22-12-8-11-21(7-3)13-22/h8,11-15,17,26-29,34,37H,5-7,9-10,16,18-20H2,1-4H3,(H,35,38)(H,36,39)/t27-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409

(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343063

(CHEMBL1770697 | N-hydroxy-4-(4-(4-(methyl(4-(trifl...)Show SMILES CN(C(=O)CCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H27F3N2O8S/c1-29(17-4-6-19(7-5-17)37-24(25,26)27)21(30)3-2-14-36-18-8-10-20(11-9-18)38(33,34)23(22(31)28-32)12-15-35-16-13-23/h4-11,32H,2-3,12-16H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196740
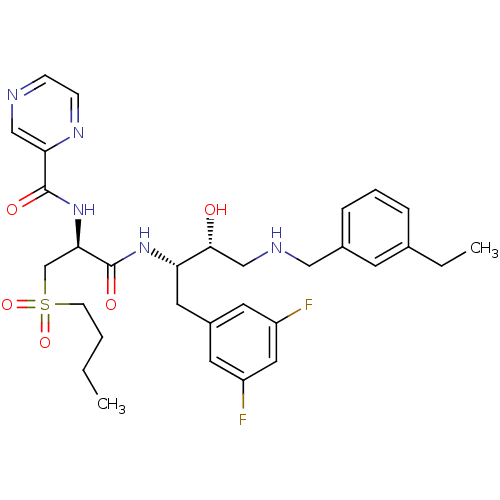
(CHEMBL409875 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cnccn1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H39F2N5O5S/c1-3-5-11-44(42,43)20-28(38-30(40)27-18-34-9-10-36-27)31(41)37-26(15-23-13-24(32)16-25(33)14-23)29(39)19-35-17-22-8-6-7-21(4-2)12-22/h6-10,12-14,16,18,26,28-29,35,39H,3-5,11,15,17,19-20H2,1-2H3,(H,37,41)(H,38,40)/t26-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50076589

(3-(4-Phenoxy-benzenesulfonyl)-propane-1-thiol | CH...)Show InChI InChI=1S/C15H16O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-3,5-10,19H,4,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloproteinase-8 (hMMP-8) |
Bioorg Med Chem Lett 9: 1757-60 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FVN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196733

(CHEMBL414776 | pyridin-3-ylmethyl (S)-1-((2S,3R)-4...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)OCc1cccnc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H48F2N4O6S/c1-4-9-31(10-5-2)49(46,47)24-33(42-36(45)48-23-27-13-8-14-39-21-27)35(44)41-32(18-28-16-29(37)19-30(38)17-28)34(43)22-40-20-26-12-7-11-25(6-3)15-26/h7-8,11-17,19,21,31-34,40,43H,4-6,9-10,18,20,22-24H2,1-3H3,(H,41,44)(H,42,45)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196734

(CHEMBL409947 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cc(C)n[nH]1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C31H41F2N5O5S/c1-4-6-10-44(42,43)19-28(36-30(40)27-11-20(3)37-38-27)31(41)35-26(15-23-13-24(32)16-25(33)14-23)29(39)18-34-17-22-9-7-8-21(5-2)12-22/h7-9,11-14,16,26,28-29,34,39H,4-6,10,15,17-19H2,1-3H3,(H,35,41)(H,36,40)(H,37,38)/t26-,28+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196754
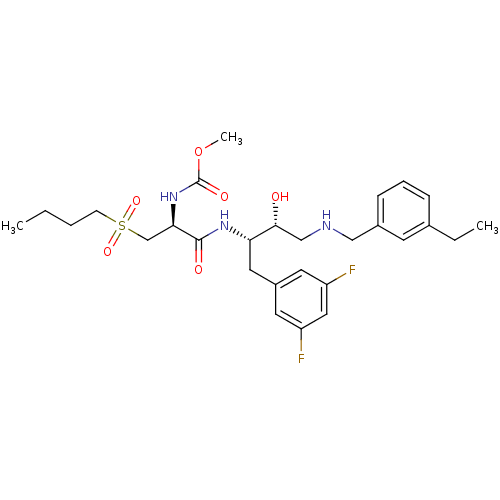
(CHEMBL393733 | methyl (S)-1-((2S,3R)-4-(3-ethylben...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)OC)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C28H39F2N3O6S/c1-4-6-10-40(37,38)18-25(33-28(36)39-3)27(35)32-24(14-21-12-22(29)15-23(30)13-21)26(34)17-31-16-20-9-7-8-19(5-2)11-20/h7-9,11-13,15,24-26,31,34H,4-6,10,14,16-18H2,1-3H3,(H,32,35)(H,33,36)/t24-,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196752

(CHEMBL412852 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1ccc(C)cc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C37H49F2N3O5S/c1-5-9-32(10-6-2)48(46,47)24-34(42-36(44)29-15-13-25(4)14-16-29)37(45)41-33(20-28-18-30(38)21-31(39)19-28)35(43)23-40-22-27-12-8-11-26(7-3)17-27/h8,11-19,21,32-35,40,43H,5-7,9-10,20,22-24H2,1-4H3,(H,41,45)(H,42,44)/t33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196744
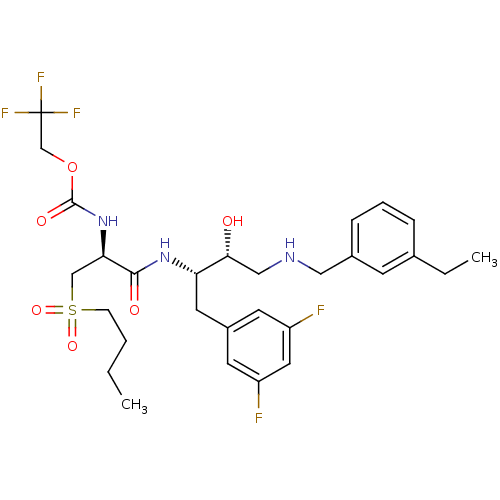
(2,2,2-trifluoroethyl (S)-1-((2S,3R)-4-(3-ethylbenz...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)OCC(F)(F)F)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C29H38F5N3O6S/c1-3-5-9-44(41,42)17-25(37-28(40)43-18-29(32,33)34)27(39)36-24(13-21-11-22(30)14-23(31)12-21)26(38)16-35-15-20-8-6-7-19(4-2)10-20/h6-8,10-12,14,24-26,35,38H,3-5,9,13,15-18H2,1-2H3,(H,36,39)(H,37,40)/t24-,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196757
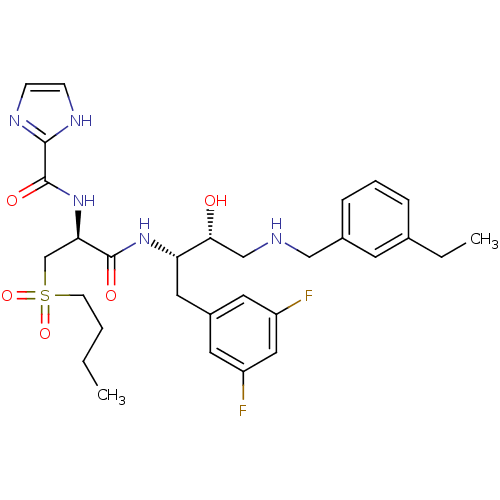
(CHEMBL391144 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1ncc[nH]1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C30H39F2N5O5S/c1-3-5-11-43(41,42)19-26(37-30(40)28-34-9-10-35-28)29(39)36-25(15-22-13-23(31)16-24(32)14-22)27(38)18-33-17-21-8-6-7-20(4-2)12-21/h6-10,12-14,16,25-27,33,38H,3-5,11,15,17-19H2,1-2H3,(H,34,35)(H,36,39)(H,37,40)/t25-,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574409

(CHEMBL4873534)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCn3cc(COCCOc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)nn3)cc2)c2ccc(O)cc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha D538G mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196753
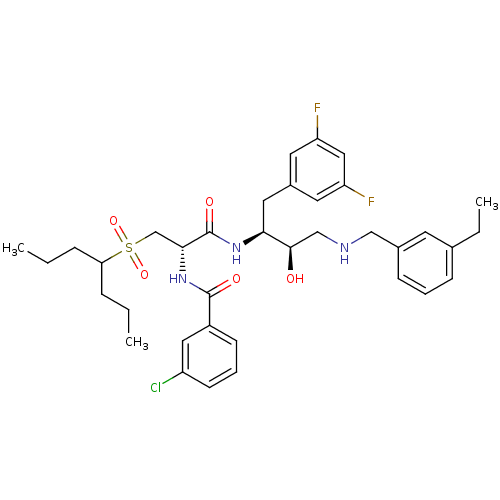
(CHEMBL266565 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCC(CCC)S(=O)(=O)C[C@@H](NC(=O)c1cccc(Cl)c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C36H46ClF2N3O5S/c1-4-9-31(10-5-2)48(46,47)23-33(42-35(44)27-13-8-14-28(37)19-27)36(45)41-32(18-26-16-29(38)20-30(39)17-26)34(43)22-40-21-25-12-7-11-24(6-3)15-25/h7-8,11-17,19-20,31-34,40,43H,4-6,9-10,18,21-23H2,1-3H3,(H,41,45)(H,42,44)/t32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM15797
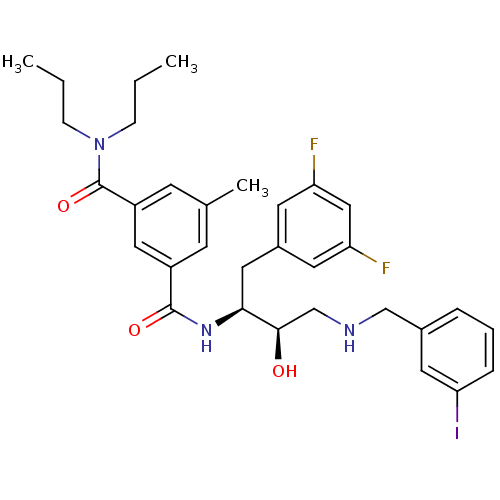
((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-i...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(I)c1 |r| Show InChI InChI=1S/C32H38F2IN3O3/c1-4-9-38(10-5-2)32(41)25-12-21(3)11-24(17-25)31(40)37-29(16-23-13-26(33)18-27(34)14-23)30(39)20-36-19-22-7-6-8-28(35)15-22/h6-8,11-15,17-18,29-30,36,39H,4-5,9-10,16,19-20H2,1-3H3,(H,37,40)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE |
Bioorg Med Chem Lett 17: 73-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.092
BindingDB Entry DOI: 10.7270/Q2FX793P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196739
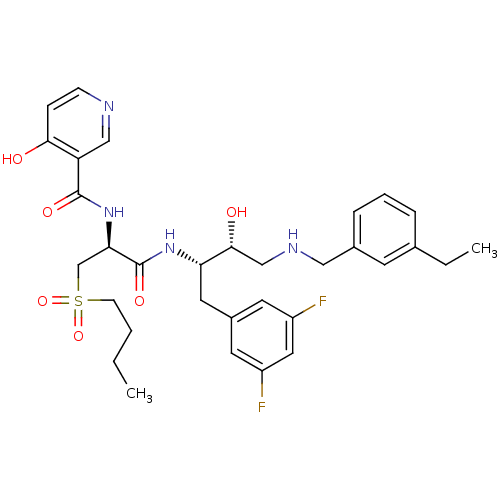
(CHEMBL266531 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1cnccc1O)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C32H40F2N4O6S/c1-3-5-11-45(43,44)20-28(38-31(41)26-18-35-10-9-29(26)39)32(42)37-27(15-23-13-24(33)16-25(34)14-23)30(40)19-36-17-22-8-6-7-21(4-2)12-22/h6-10,12-14,16,18,27-28,30,36,40H,3-5,11,15,17,19-20H2,1-2H3,(H,35,39)(H,37,42)(H,38,41)/t27-,28+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50196750
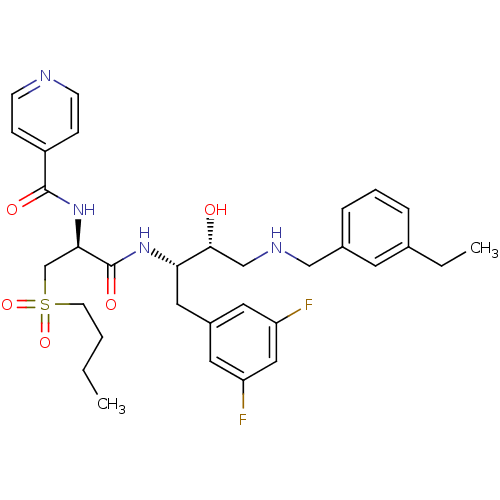
(CHEMBL394271 | N-((S)-1-((2S,3R)-4-(3-ethylbenzyla...)Show SMILES CCCCS(=O)(=O)C[C@@H](NC(=O)c1ccncc1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(CC)c1 Show InChI InChI=1S/C32H40F2N4O5S/c1-3-5-13-44(42,43)21-29(38-31(40)25-9-11-35-12-10-25)32(41)37-28(17-24-15-26(33)18-27(34)16-24)30(39)20-36-19-23-8-6-7-22(4-2)14-23/h6-12,14-16,18,28-30,36,39H,3-5,13,17,19-21H2,1-2H3,(H,37,41)(H,38,40)/t28-,29+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE |
Bioorg Med Chem Lett 17: 78-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.091
BindingDB Entry DOI: 10.7270/Q22N51X6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574413
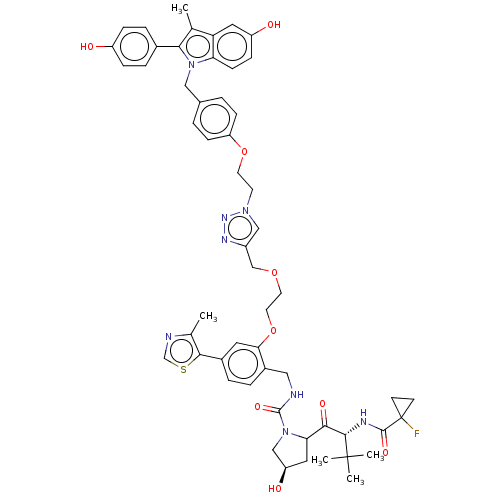
(CHEMBL4873959)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)N2C[C@H](O)CC2C(=O)[C@H](NC(=O)C2(F)CC2)C(C)(C)C)c(OCCOCc2cn(CCOc3ccc(Cn4c(c(C)c5cc(O)ccc45)-c4ccc(O)cc4)cc3)nn2)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50574412
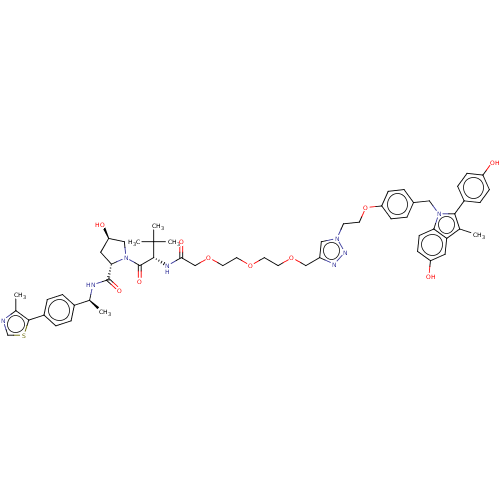
(CHEMBL4856006)Show SMILES C[C@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)COCCOCCOCc1cn(CCOc2ccc(Cn3c(c(C)c4cc(O)ccc34)-c3ccc(O)cc3)cc2)nn1)C(C)(C)C)c1ccc(cc1)-c1scnc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00127
BindingDB Entry DOI: 10.7270/Q26T0RG4 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50096851

(CHEMBL3580774)Show SMILES C[C@@H](COc1ccc(F)cc1[C@@H](C)C(F)(F)F)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O |r| Show InChI InChI=1S/C24H24F4N4O3/c1-14-11-30(13-29-14)19-5-6-20-23(34)31(8-9-32(20)22(19)33)15(2)12-35-21-7-4-17(25)10-18(21)16(3)24(26,27)28/h4-7,10-11,13,15-16H,8-9,12H2,1-3H3/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Modulation of human gamma secretase overexpressed in CHO cells co-expressing wild type human APP assessed as inhibition of amyloid beta-42 production... |
ACS Med Chem Lett 6: 596-601 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00070
BindingDB Entry DOI: 10.7270/Q26975BS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data