

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140128 (US8901315, 256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140095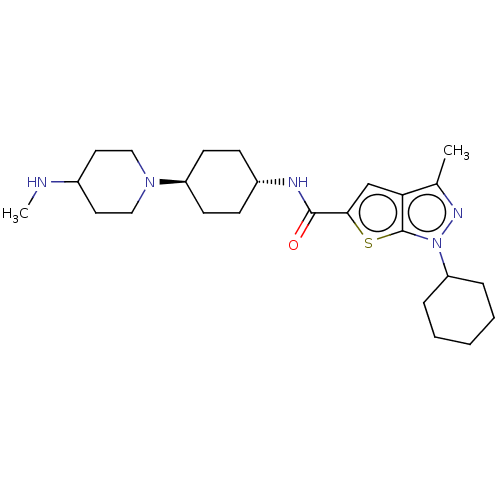 (US8901315, 179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140100 (US8901315, 190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140142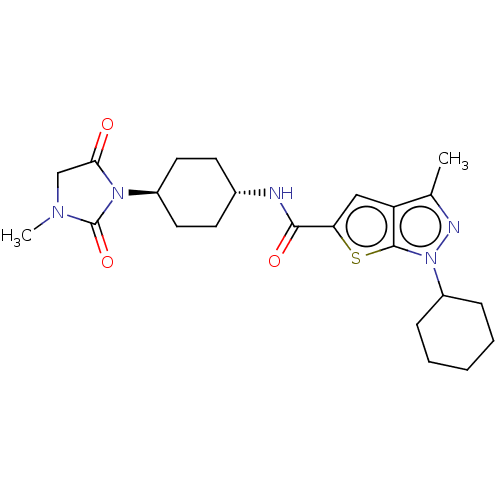 (US8901315, 275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140168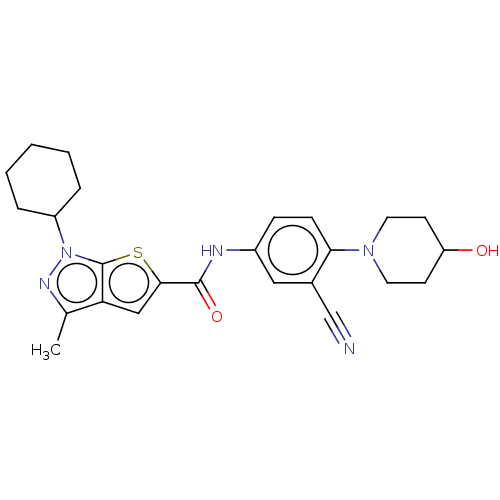 (US8901315, 373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140155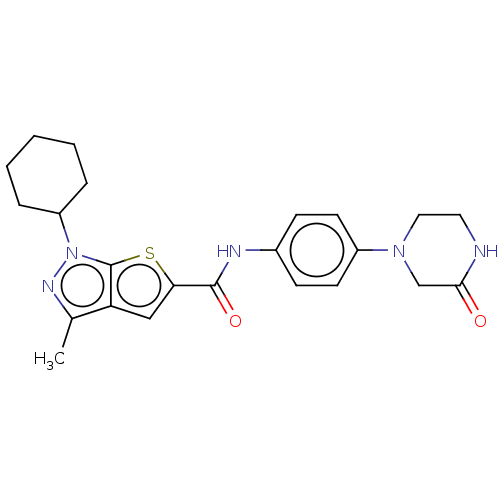 (US8901315, 341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140108 (US8901315, 203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140126 (US8901315, 247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140099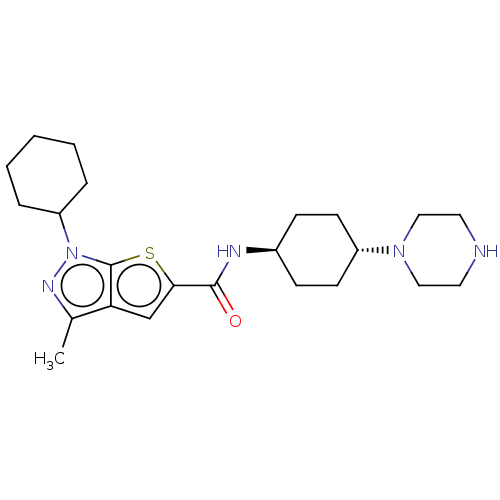 (US8901315, 188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140098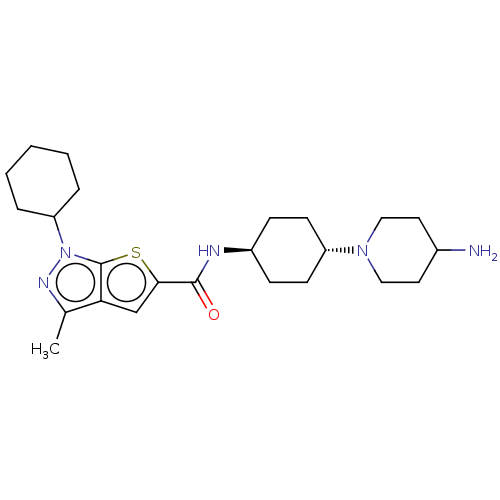 (US8901315, 184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140169 (US8901315, 375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140121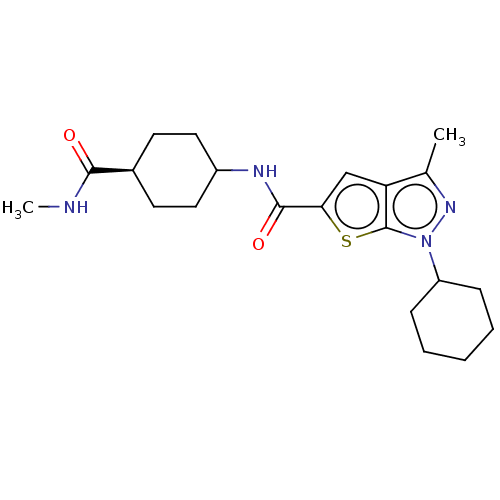 (US8901315, 237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140122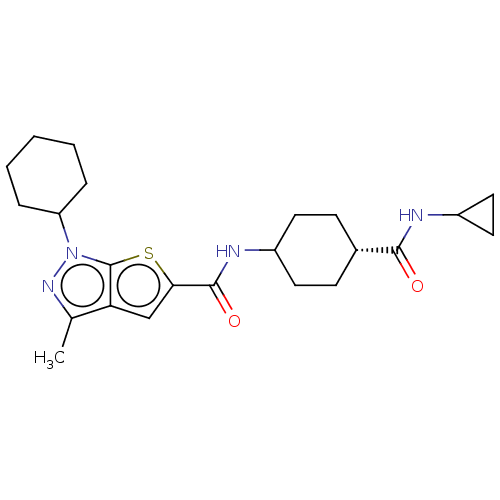 (US8901315, 238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140068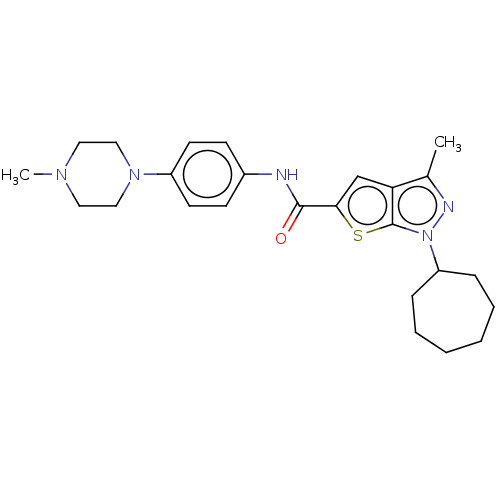 (US8901315, 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140097 (US8901315, 181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140165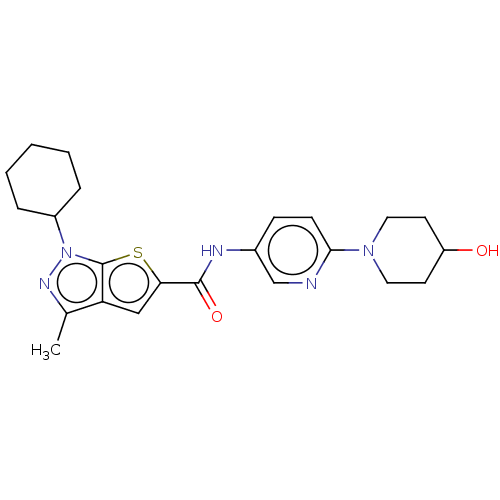 (US8901315, 370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140101 (US8901315, 191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140139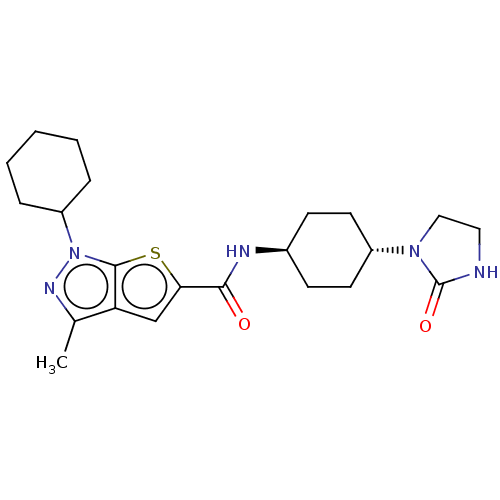 (US8901315, 271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140063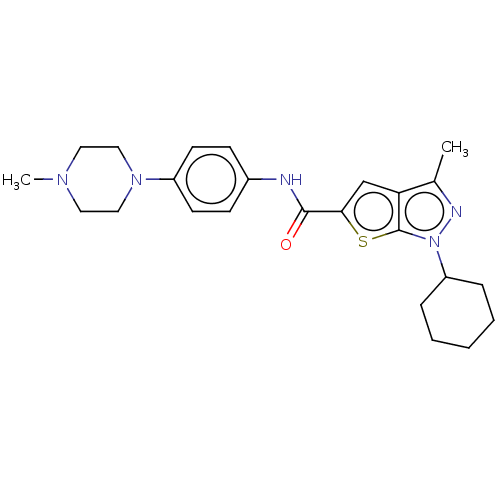 (US8901315, 106 | US8901315, 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140073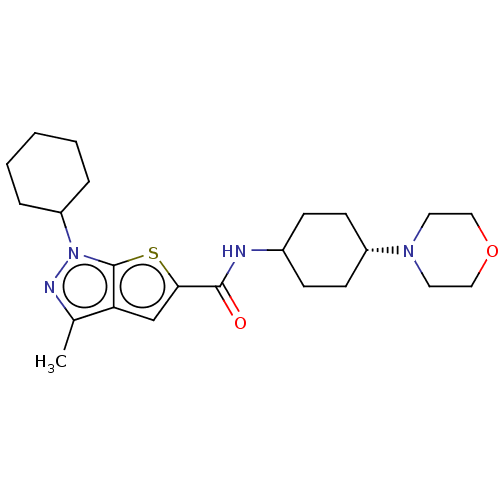 (US8901315, 133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140141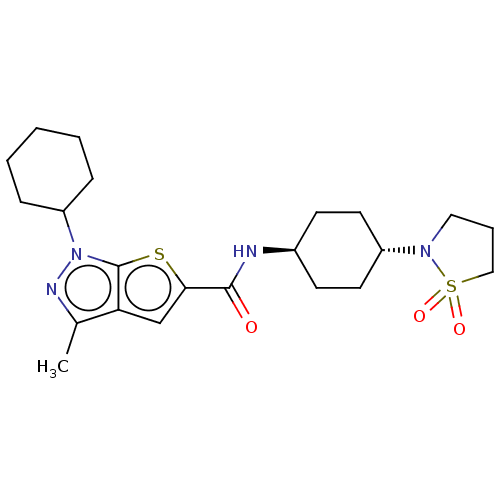 (US8901315, 274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140050 (US8901315, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140096 (US8901315, 180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140067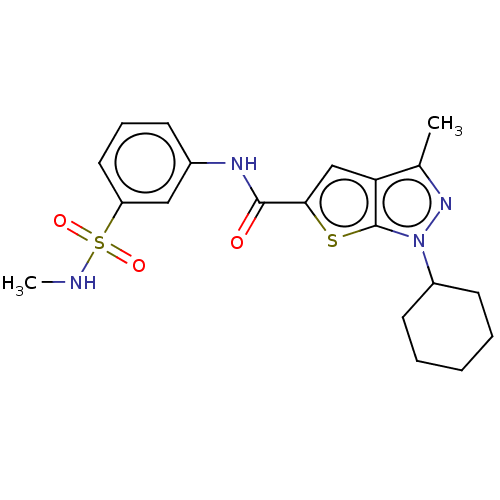 (US8901315, 111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140081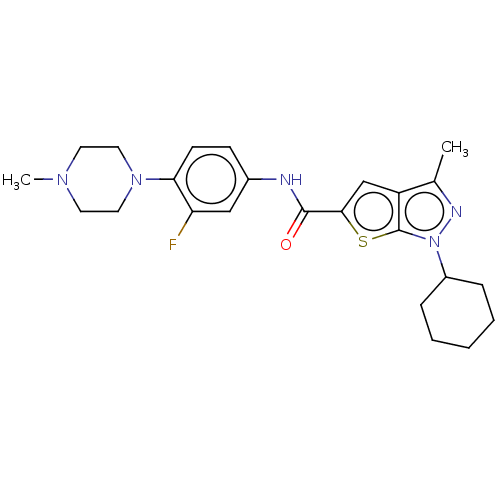 (US8901315, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140167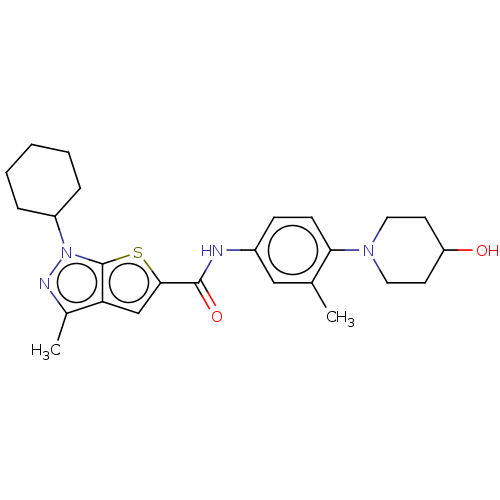 (US8901315, 372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140051 (US8901315, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140171 (US8901315, 379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140094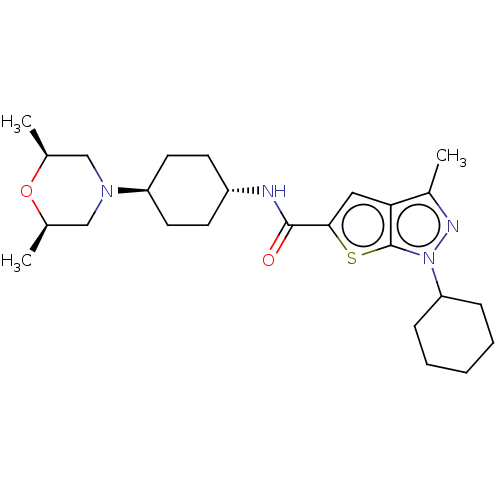 (US8901315, 177) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140118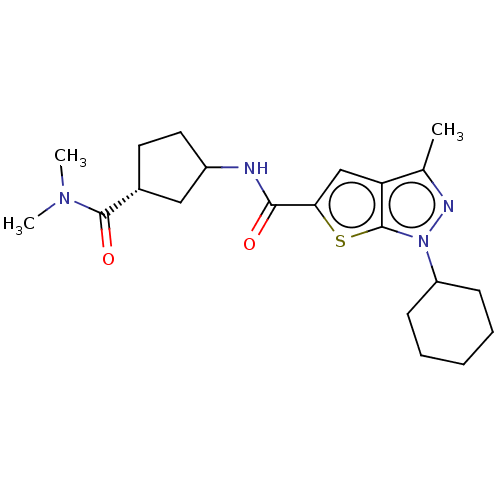 (US8901315, 234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140076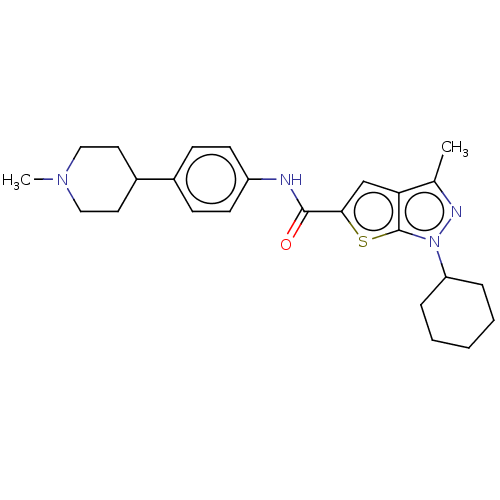 (US8901315, 142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140085 (US8901315, 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140037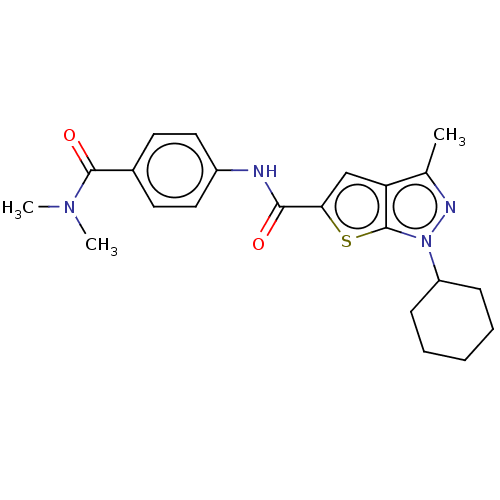 (US8901315, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140140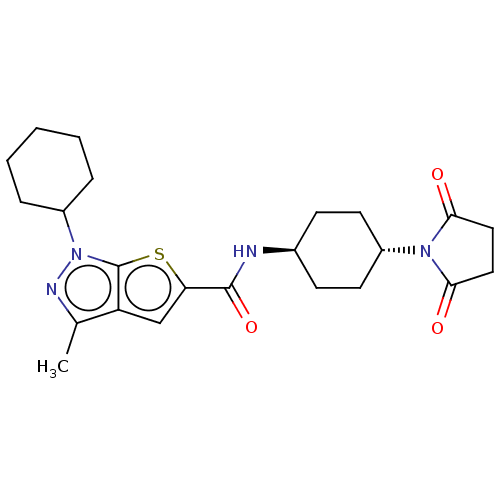 (US8901315, 273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140065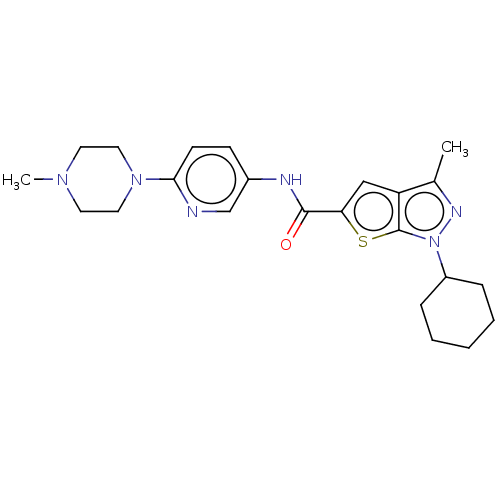 (US8901315, 109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140123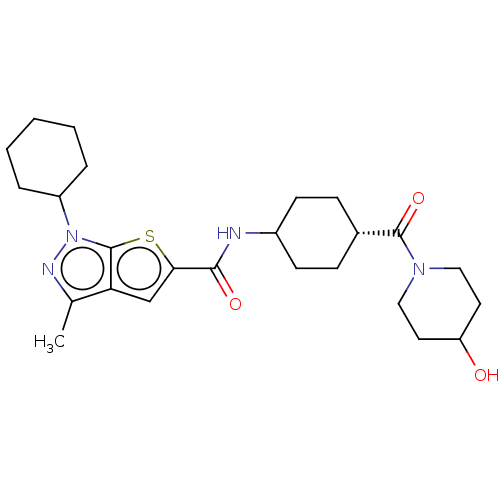 (US8901315, 239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140088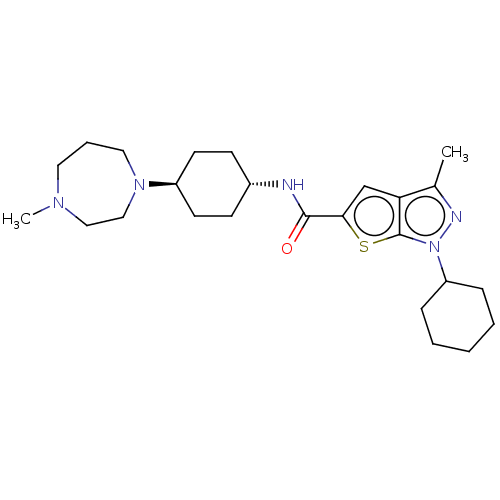 (US8901315, 167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140138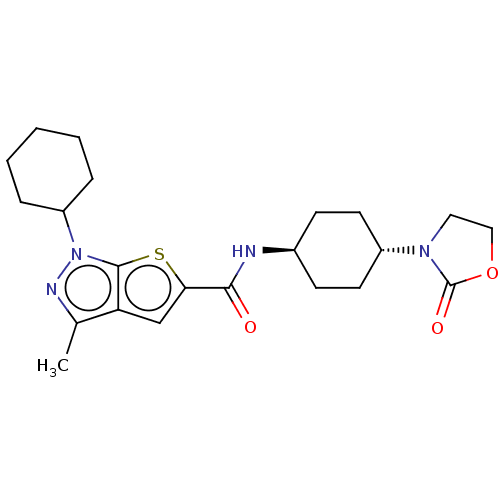 (US8901315, 270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140130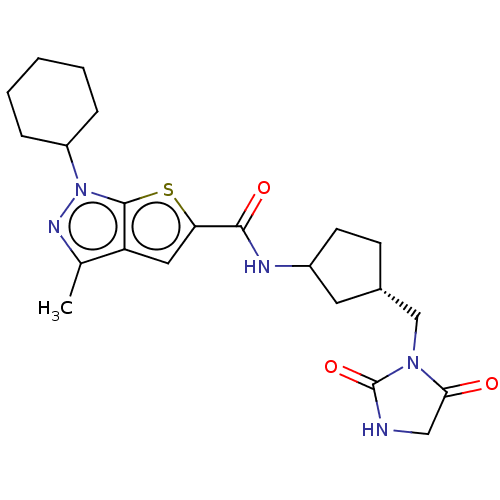 (US8901315, 258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140087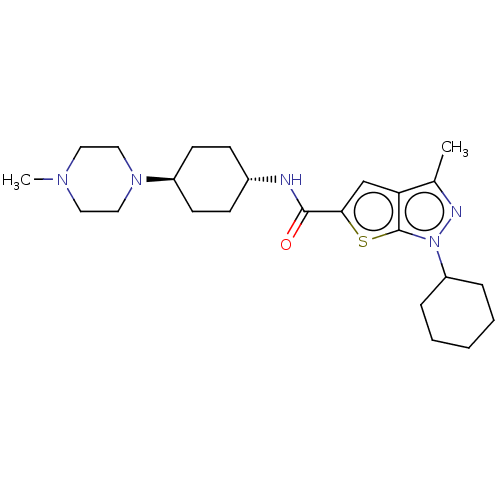 (US8901315, 166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140105 (US8901315, 200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140166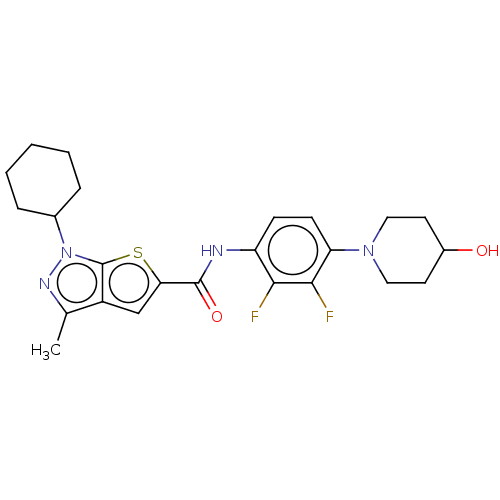 (US8901315, 371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140119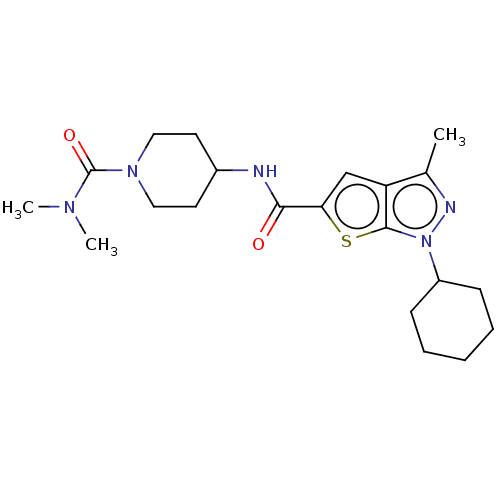 (US8901315, 235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140063 (US8901315, 106 | US8901315, 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140184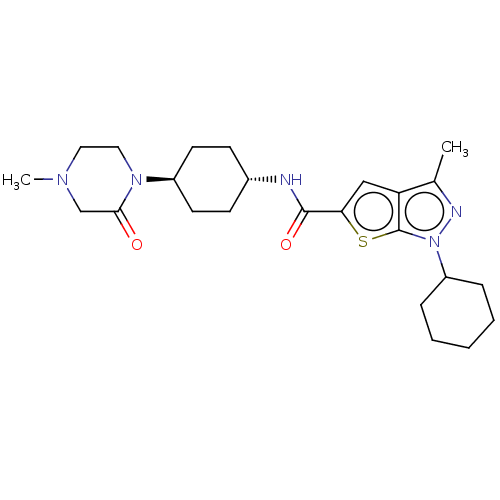 (US8901315, 416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140144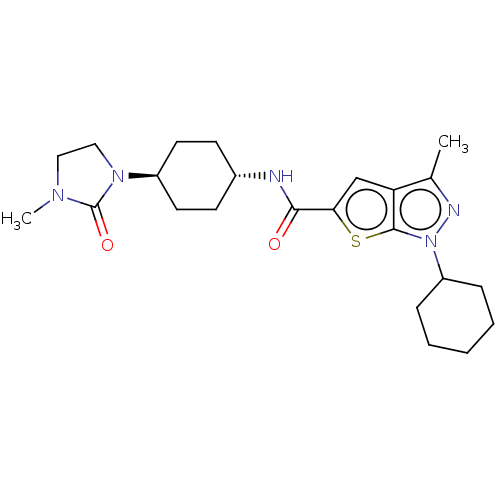 (US8901315, 280) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140114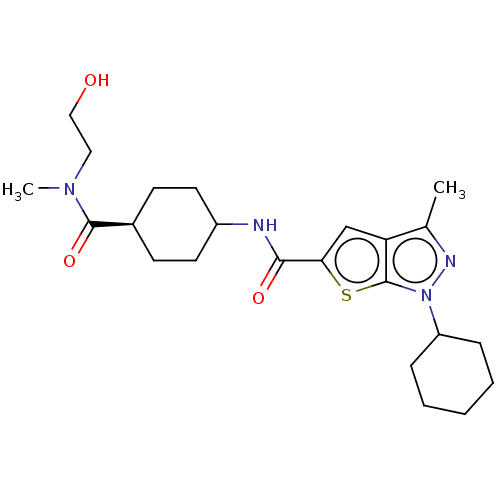 (US8901315, 225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140106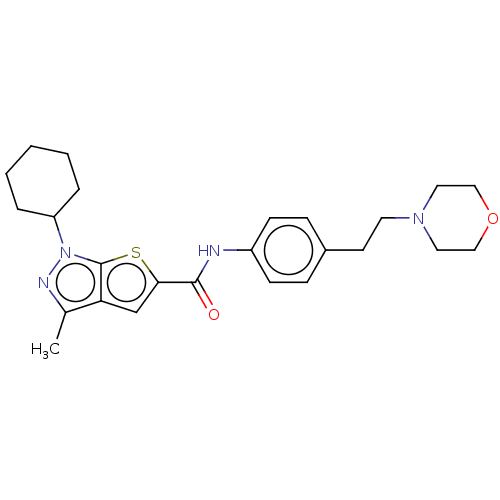 (US8901315, 201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140176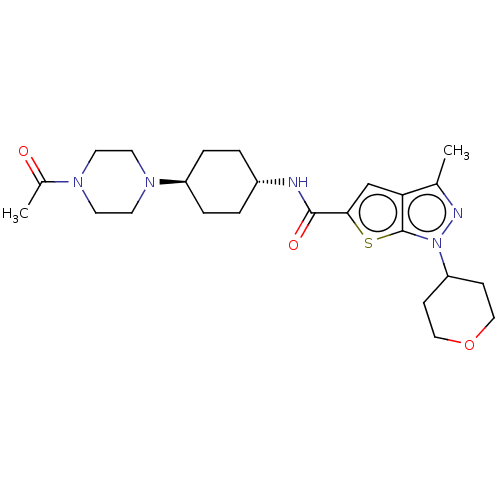 (US8901315, 395) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM140023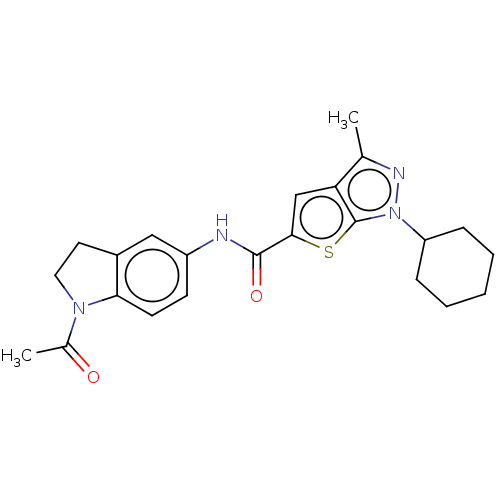 (US8901315, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Daiichi Sankyo Company, Limited US Patent | Assay Description The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention was performed by the following method, which was modifi... | US Patent US8901315 (2014) BindingDB Entry DOI: 10.7270/Q2GM860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 458 total ) | Next | Last >> |