

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213387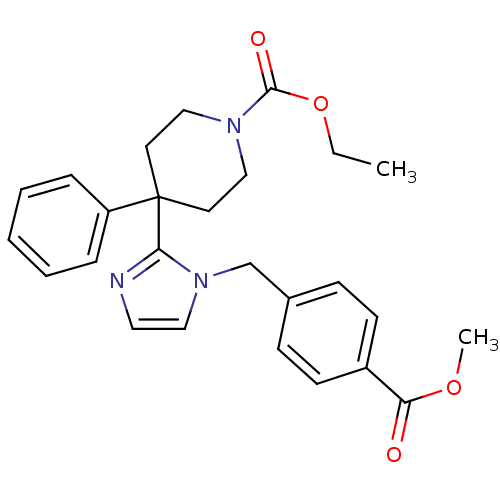 (CHEMBL393965 | ethyl 4-(1-(4-(methoxycarbonyl)benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213393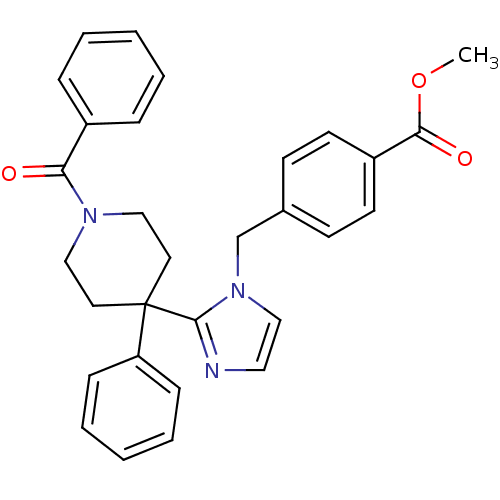 (CHEMBL232548 | methyl 4-((2-(1-benzoyl-4-phenylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213403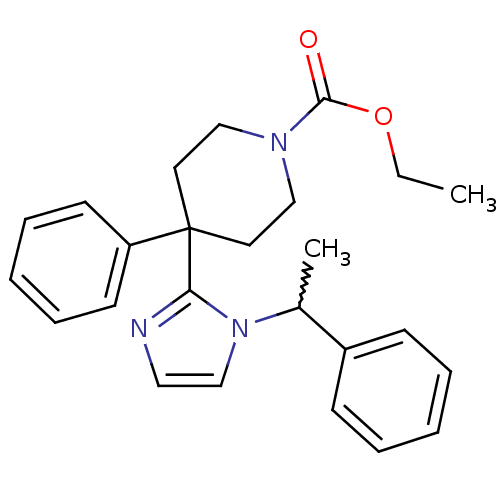 (CHEMBL391852 | ethyl 4-phenyl-4-(1-(1-phenylethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016752 (CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213380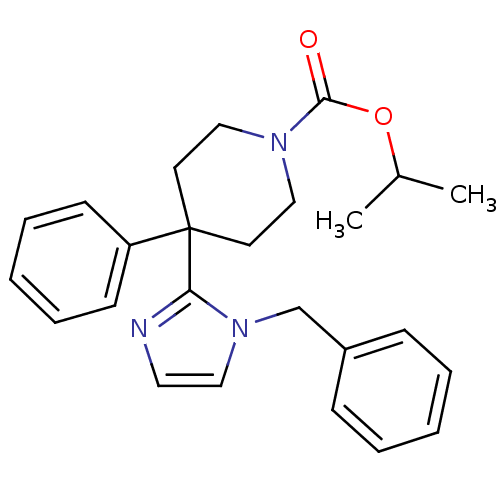 (CHEMBL400436 | isopropyl 4-(1-benzyl-1H-imidazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213374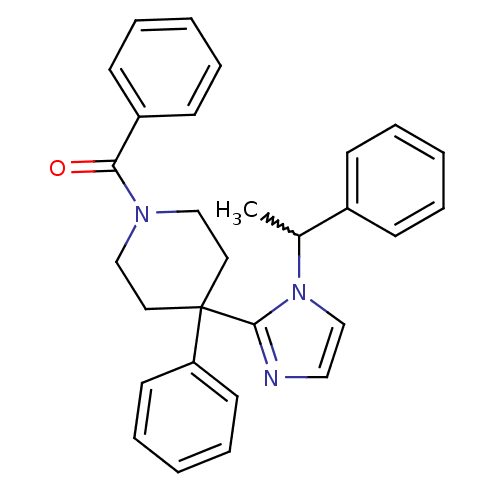 (CHEMBL437397 | phenyl(4-phenyl-4-(1-(1-phenylethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213404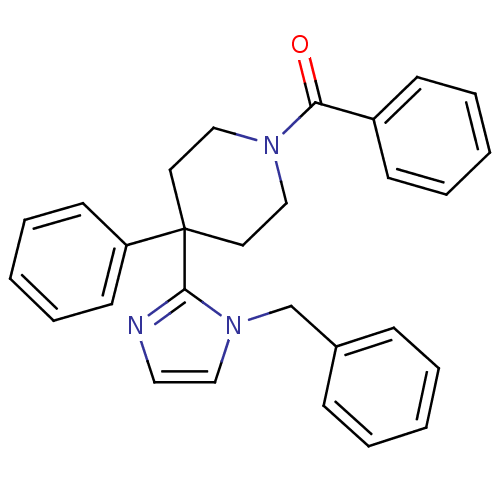 ((4-(1-benzyl-1H-imidazol-2-yl)-4-phenylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213372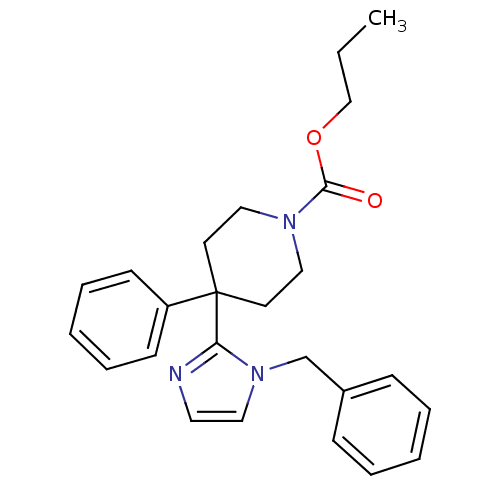 (CHEMBL399205 | propyl 4-(1-benzyl-1H-imidazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016752 (CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50175717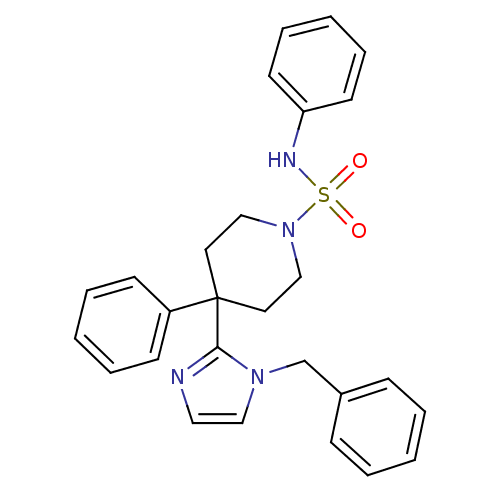 (4-(1-benzyl-1H-imidazol-2-yl)-N,4-diphenylpiperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 16: 146-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.025 BindingDB Entry DOI: 10.7270/Q2ZK5G70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016760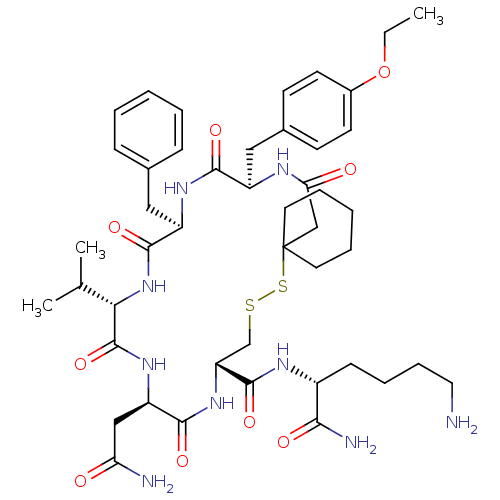 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213406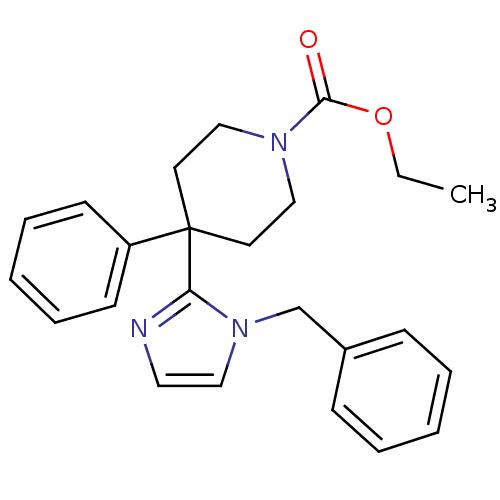 (CHEMBL232740 | ethyl 4-(1-benzyl-1H-imidazol-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213376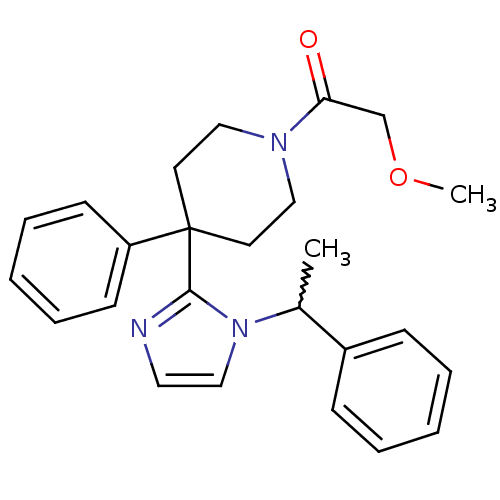 (2-methoxy-1-(4-phenyl-4-(1-(1-phenylethyl)-1H-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016756 (CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016753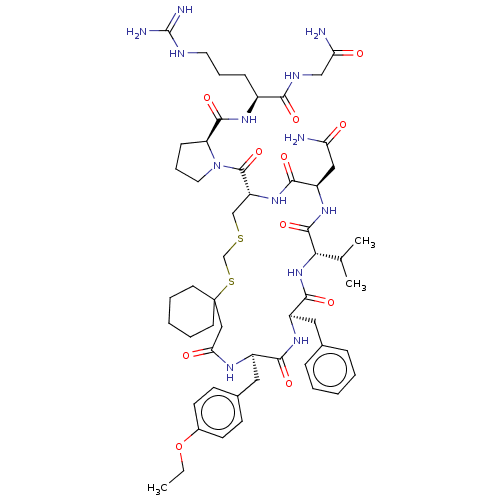 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016753 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016757 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016757 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020655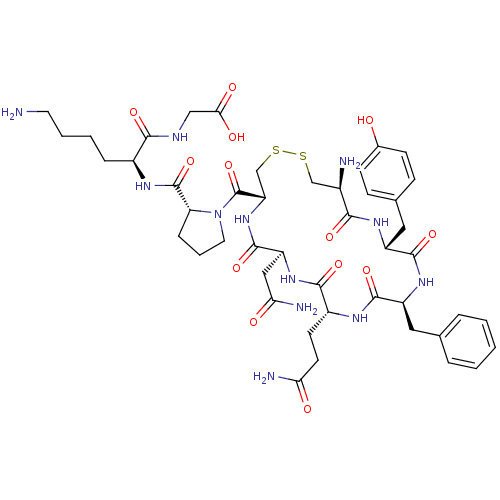 (CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020655 (CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of hog kidney renin | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213400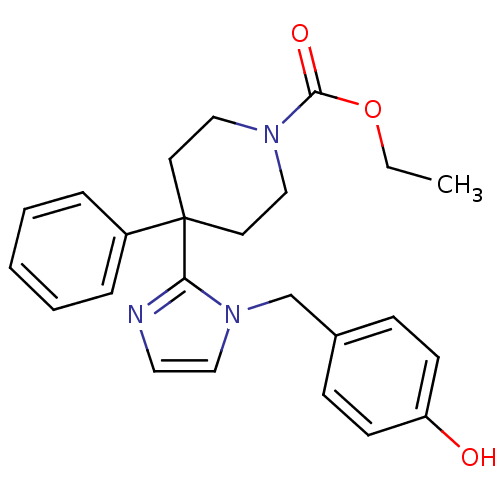 (CHEMBL391623 | ethyl 4-(1-(4-hydroxybenzyl)-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016747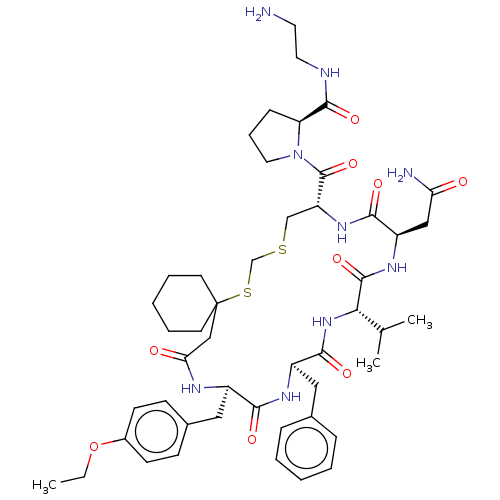 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016747 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213397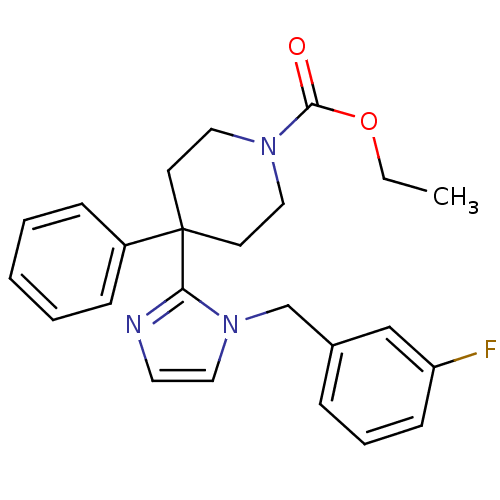 (CHEMBL231783 | ethyl 4-(1-(3-fluorobenzyl)-1H-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213381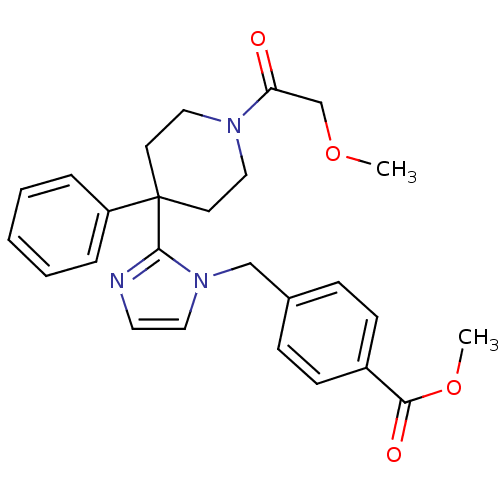 (CHEMBL232137 | methyl 4-((2-(1-(2-methoxyacetyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016756 (CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653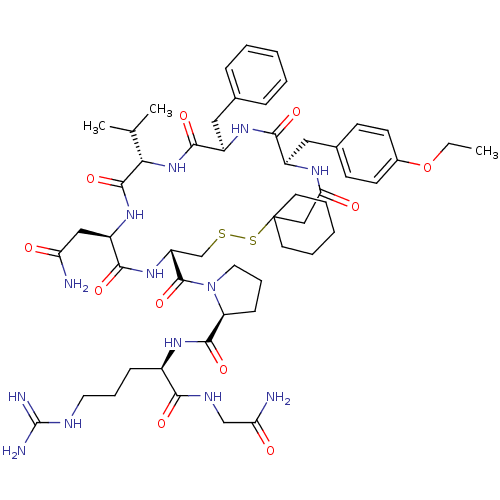 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016755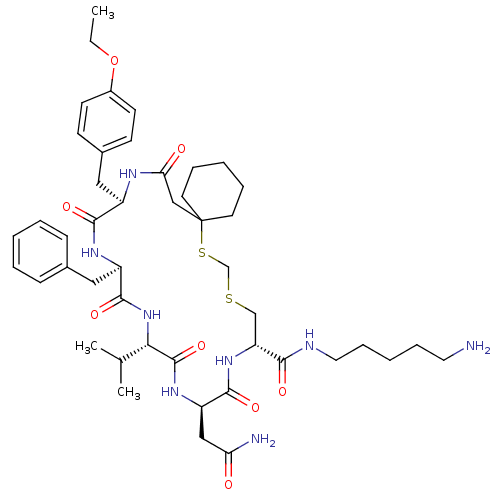 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016755 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213386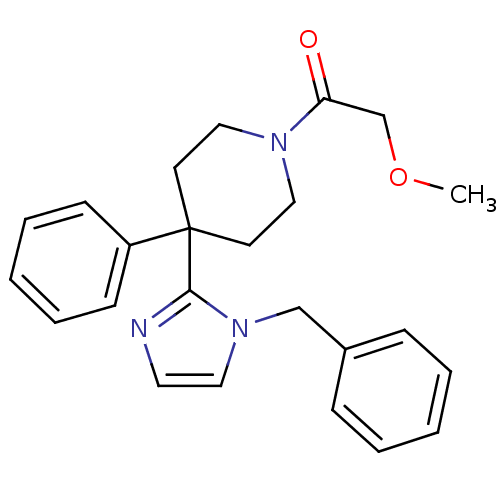 (1-(4-(1-benzyl-1H-imidazol-2-yl)-4-phenylpiperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213378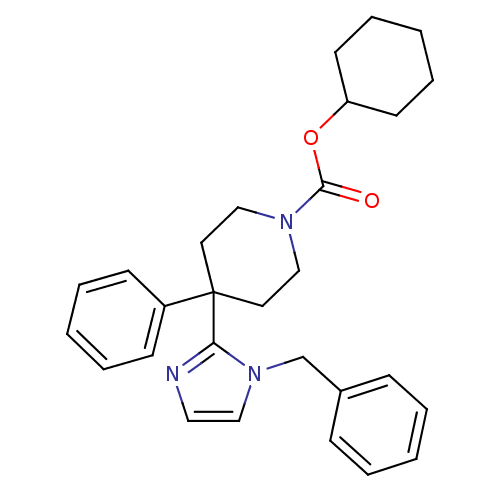 (CHEMBL233143 | cyclohexyl 4-(1-benzyl-1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016763 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016763 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016754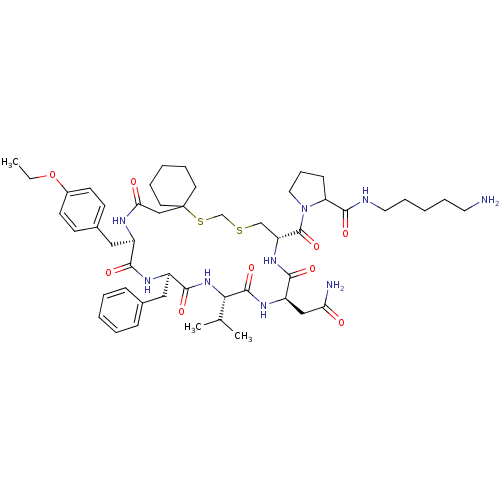 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50175723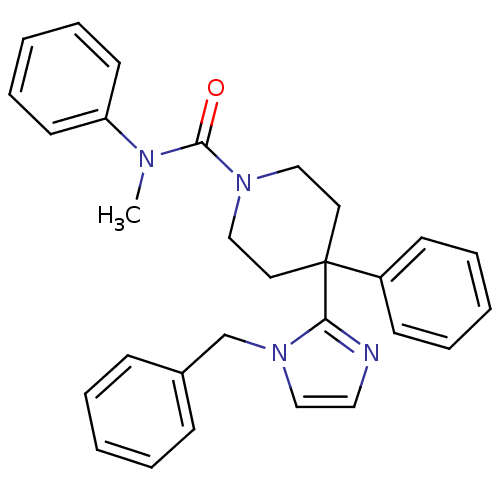 (4-(1-benzyl-1H-imidazol-2-yl)-N-methyl-N,4-dipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 16: 146-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.025 BindingDB Entry DOI: 10.7270/Q2ZK5G70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213390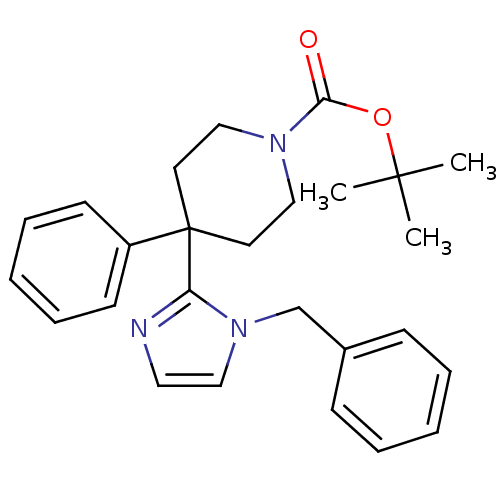 (CHEMBL392266 | tert-butyl 4-(1-benzyl-1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213398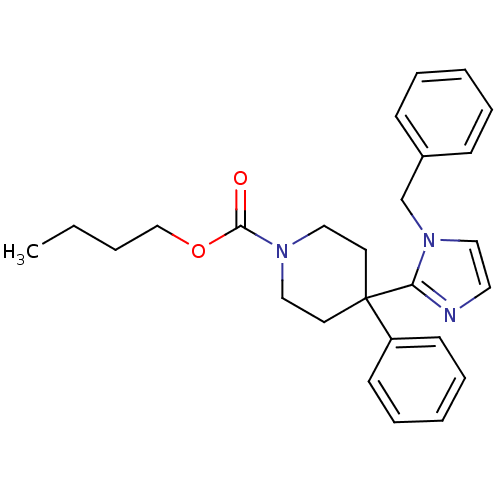 (CHEMBL232937 | butyl 4-(1-benzyl-1H-imidazol-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020656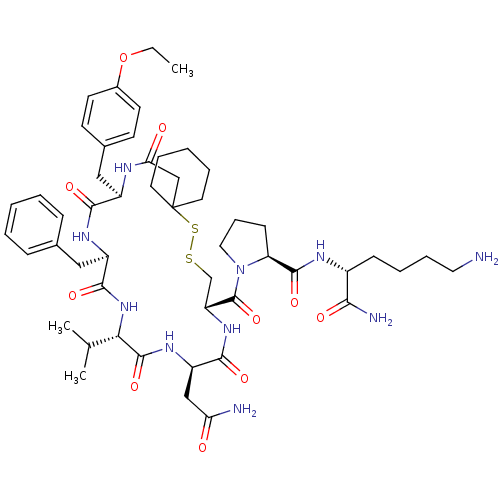 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020656 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016761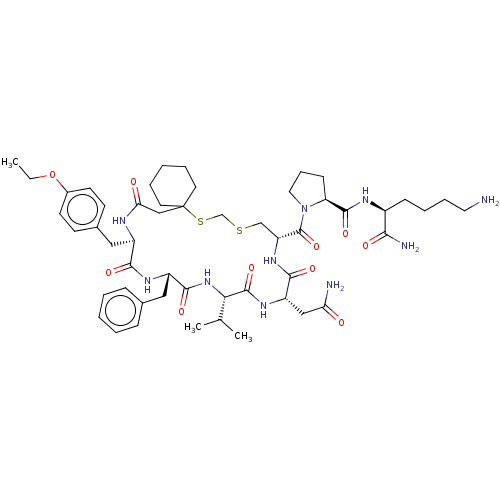 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016762 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016750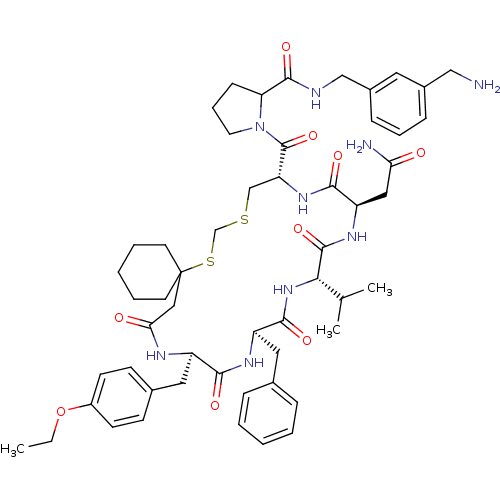 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654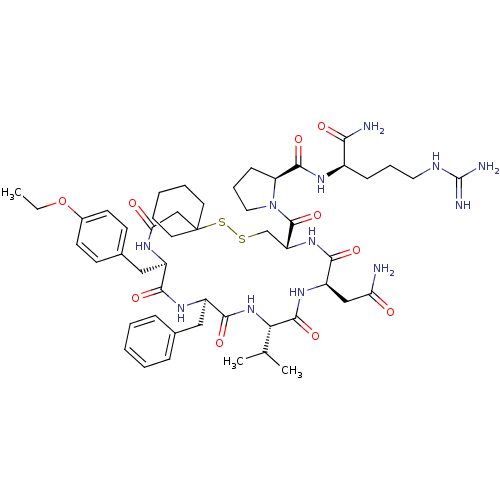 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213395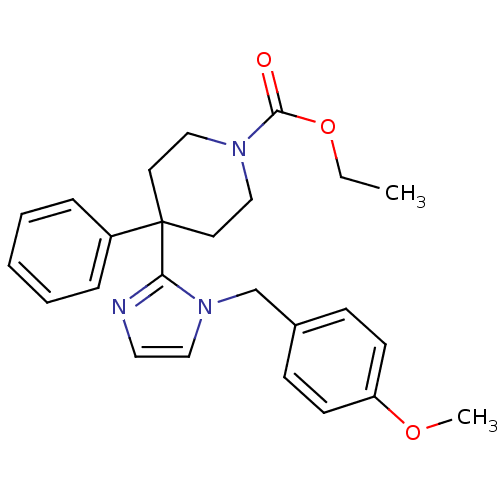 (CHEMBL231781 | ethyl 4-(1-(4-methoxybenzyl)-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50213394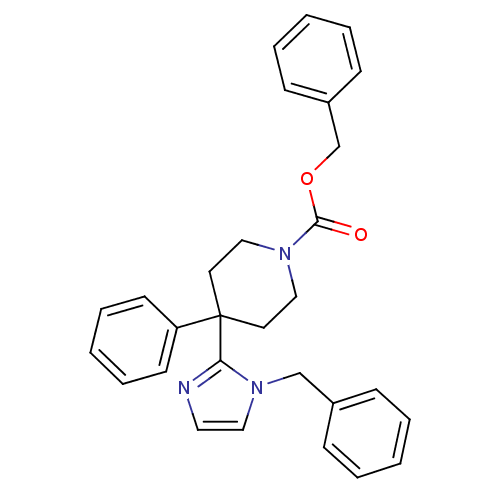 (CHEMBL233368 | benzyl 4-(1-benzyl-1H-imidazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 17: 3860-3 (2007) Article DOI: 10.1016/j.bmcl.2007.05.012 BindingDB Entry DOI: 10.7270/Q2TT4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50175719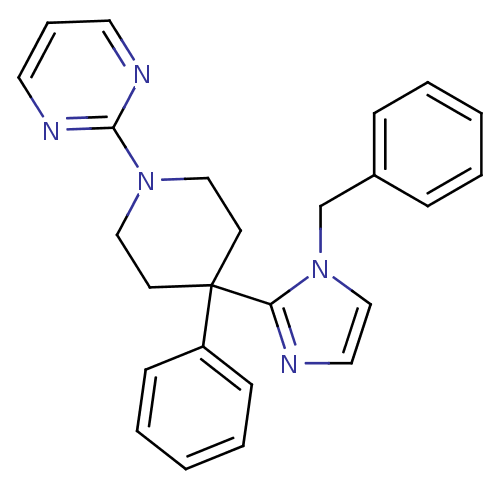 (2-(4-(1-benzyl-1H-imidazol-2-yl)-4-phenylpiperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from cloned human delta opioid receptor | Bioorg Med Chem Lett 16: 146-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.025 BindingDB Entry DOI: 10.7270/Q2ZK5G70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 317 total ) | Next | Last >> |