

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176468 (CHEMBL202223 | haloxysterol B) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176465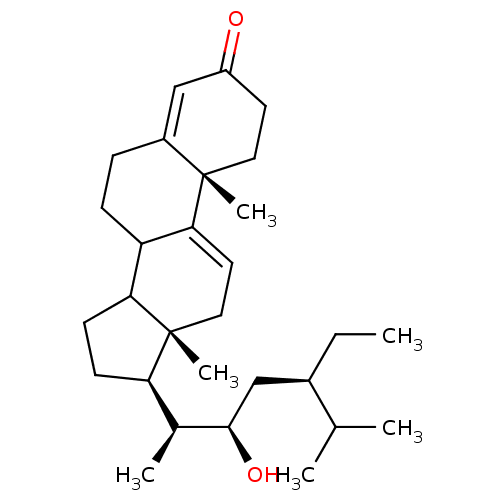 (CHEMBL382961 | haloxysterol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176467 (CHEMBL201253 | haloxysterol D) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176468 (CHEMBL202223 | haloxysterol B) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176470 (24-ethyl-cholest-7-ene-3,5,6-triol | CHEMBL201951) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176472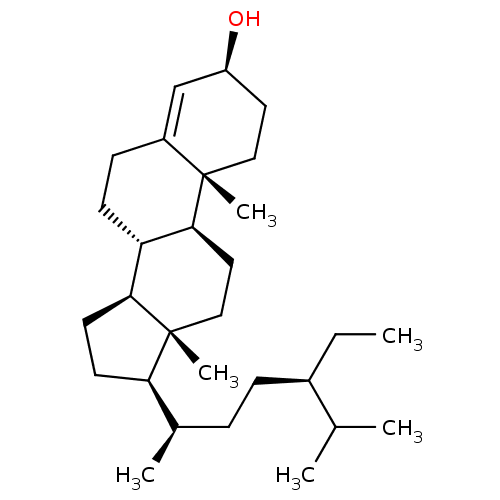 (CHEMBL202496 | lawsaritol) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176466 (24-ethylcholest-6-ene-3,5-diol | CHEMBL201866) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176466 (24-ethylcholest-6-ene-3,5-diol | CHEMBL201866) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176469 (CHEMBL202221 | haloxysterol A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176473 ((24S)-ethylcholesta-7,9(11),22(E)-triene-3b-ol | C...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176471 (5alpha,8alpha-epidioxy-(24S)-ethylcholesta-6,9(11)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176469 (CHEMBL202221 | haloxysterol A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176470 (24-ethyl-cholest-7-ene-3,5,6-triol | CHEMBL201951) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176472 (CHEMBL202496 | lawsaritol) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176467 (CHEMBL201253 | haloxysterol D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176465 (CHEMBL382961 | haloxysterol C) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176473 ((24S)-ethylcholesta-7,9(11),22(E)-triene-3b-ol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176471 (5alpha,8alpha-epidioxy-(24S)-ethylcholesta-6,9(11)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86010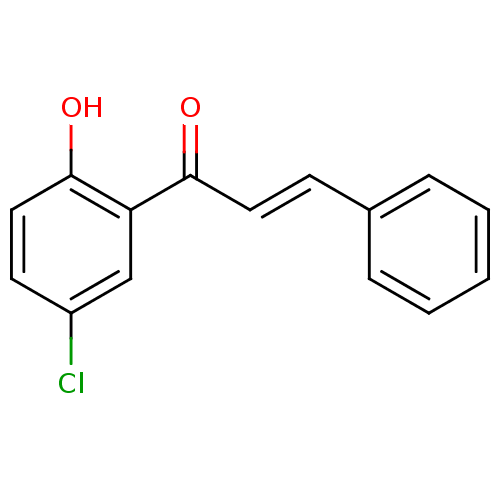 (Chalcone, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.76E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro lipoxygenase inhibition assay, lipoxygenase inhibiting activity was convenintly measured by slightly modifying the spectrometic method devel... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86012 (Chalcone, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.17E+4 | -23.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro lipoxygenase inhibition assay, lipoxygenase inhibiting activity was convenintly measured by slightly modifying the spectrometic method devel... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176468 (CHEMBL202223 | haloxysterol B) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176465 (CHEMBL382961 | haloxysterol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176470 (24-ethyl-cholest-7-ene-3,5,6-triol | CHEMBL201951) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176468 (CHEMBL202223 | haloxysterol B) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176467 (CHEMBL201253 | haloxysterol D) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176466 (24-ethylcholest-6-ene-3,5-diol | CHEMBL201866) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176466 (24-ethylcholest-6-ene-3,5-diol | CHEMBL201866) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176472 (CHEMBL202496 | lawsaritol) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176473 ((24S)-ethylcholesta-7,9(11),22(E)-triene-3b-ol | C...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176469 (CHEMBL202221 | haloxysterol A) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176471 (5alpha,8alpha-epidioxy-(24S)-ethylcholesta-6,9(11)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176469 (CHEMBL202221 | haloxysterol A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | J Nat Prod 69: 1429-34 (2006) Article DOI: 10.1021/np0680174 BindingDB Entry DOI: 10.7270/Q2CN73N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50241737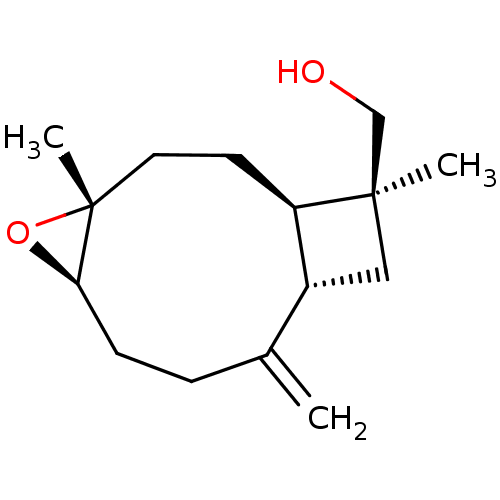 ((1R,4R,5R,9S,11S)-4,5-epoxycaryophyllan-8(13)-en-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | J Nat Prod 69: 1429-34 (2006) Article DOI: 10.1021/np0680174 BindingDB Entry DOI: 10.7270/Q2CN73N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176470 (24-ethyl-cholest-7-ene-3,5,6-triol | CHEMBL201951) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176472 (CHEMBL202496 | lawsaritol) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86002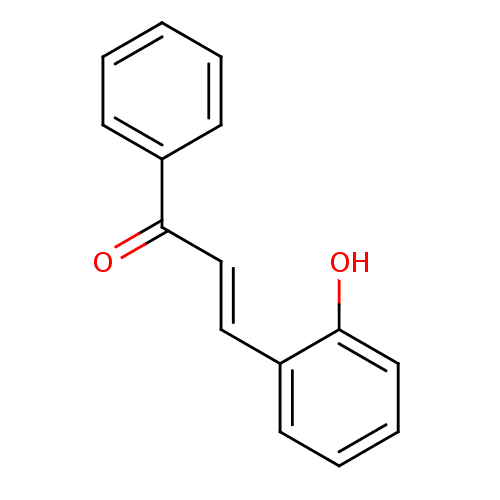 ((E)-1-phenyl-3-(2-hydroxyphenyl)-2-propen-1-one (8...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro cholinesterase inhibition assay using electric-eel acetylcholinesterase, horse-serum butyrylcholinesterse. The IC50 values were calculated ... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176467 (CHEMBL201253 | haloxysterol D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50176465 (CHEMBL382961 | haloxysterol C) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against BuChE from horse serum | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176473 ((24S)-ethylcholesta-7,9(11),22(E)-triene-3b-ol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86006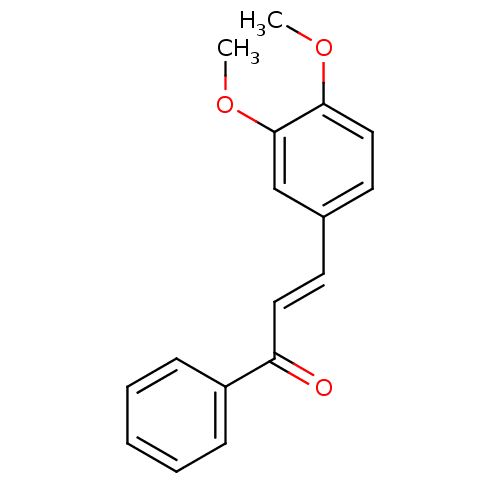 (Chalcone, 5) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro cholinesterase inhibition assay using electric-eel acetylcholinesterase, horse-serum butyrylcholinesterse. The IC50 values were calculated ... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50241740 ((1R,4R,5R,8S, 9S,13S)-caryolane-5,8,13-triol | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | J Nat Prod 69: 1429-34 (2006) Article DOI: 10.1021/np0680174 BindingDB Entry DOI: 10.7270/Q2CN73N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50241739 ((1R,4R,5R,8S,9S)-4,5-epoxycaryophyllan-13-ol | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | J Nat Prod 69: 1429-34 (2006) Article DOI: 10.1021/np0680174 BindingDB Entry DOI: 10.7270/Q2CN73N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50176471 (5alpha,8alpha-epidioxy-(24S)-ethylcholesta-6,9(11)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibitory activity against AChE from electric eel | Bioorg Med Chem Lett 16: 573-80 (2005) Article DOI: 10.1016/j.bmcl.2005.10.042 BindingDB Entry DOI: 10.7270/Q29P3170 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM86009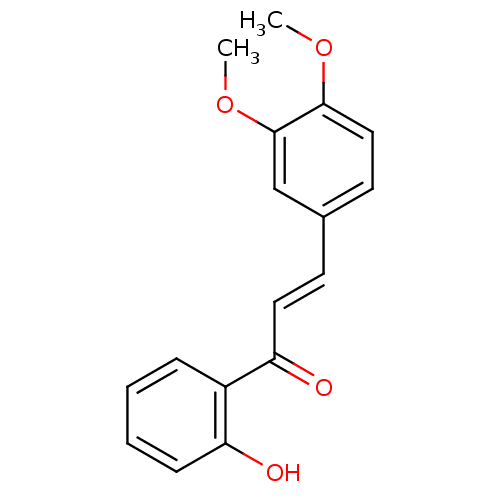 (Chalcone, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro cholinesterase inhibition assay using electric-eel acetylcholinesterase, horse-serum butyrylcholinesterse. The IC50 values were calculated ... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86005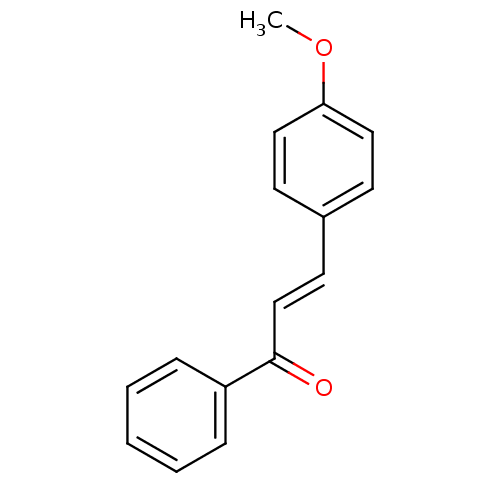 (Chalcone, 4) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Quaid-i-Azam University | Assay Description In vitro cholinesterase inhibition assay using electric-eel acetylcholinesterase, horse-serum butyrylcholinesterse. The IC50 values were calculated ... | J Enzyme Inhib Med Chem 20: 41-7 (2005) Article DOI: 10.1080/14756360400015231 BindingDB Entry DOI: 10.7270/Q2FX781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |