

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642916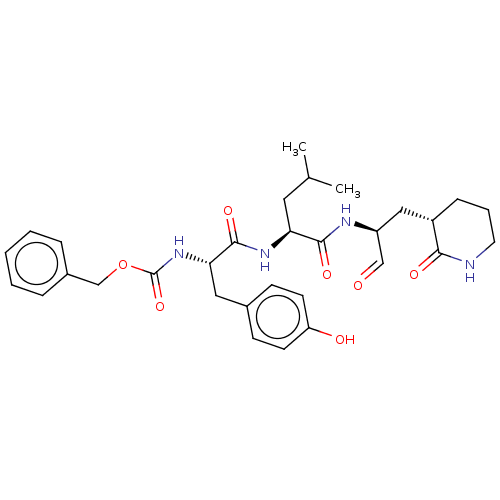 (US11859014, Compound 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM50273966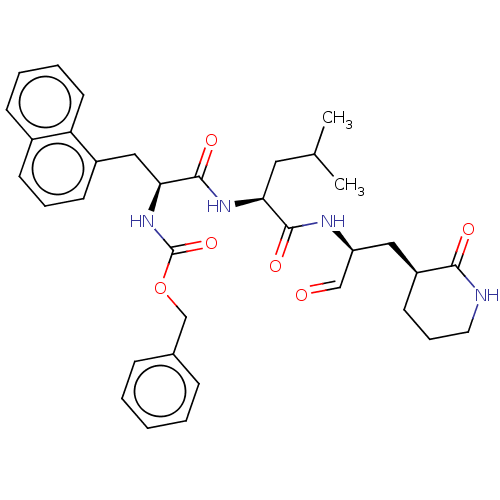 (CHEMBL4129318 | US11859014, Compound 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642927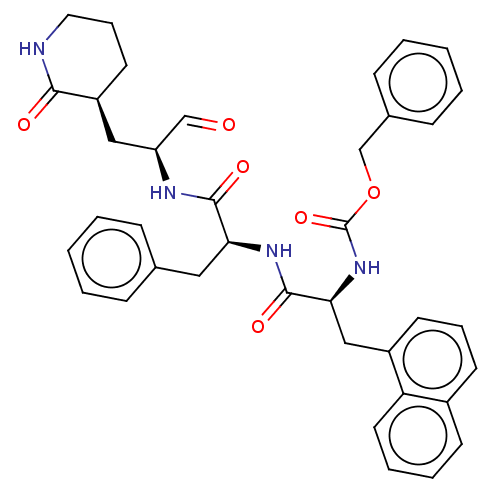 (US11859014, Compound 83) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642919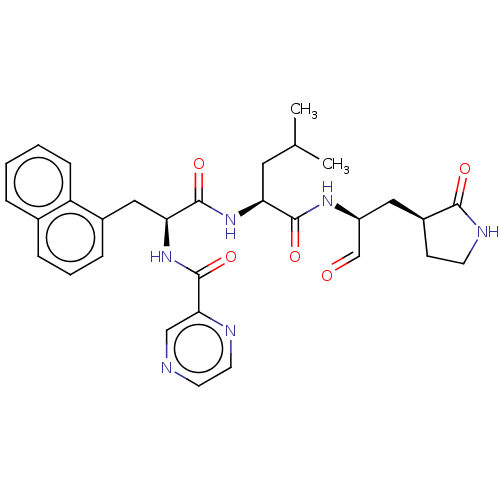 (US11859014, Compound 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642916 (US11859014, Compound 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642920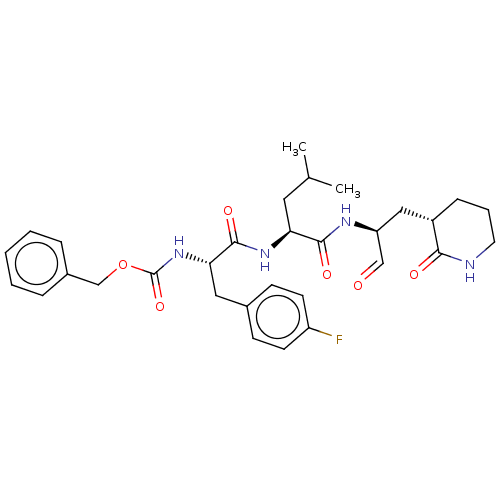 (US11859014, Compound 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642918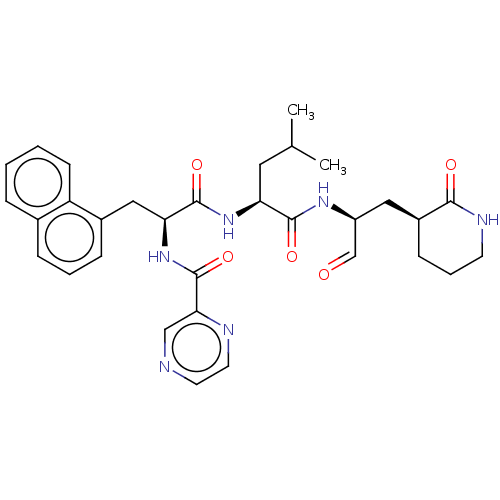 (US11859014, Compound 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 528 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642919 (US11859014, Compound 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642920 (US11859014, Compound 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM50273966 (CHEMBL4129318 | US11859014, Compound 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642918 (US11859014, Compound 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM222139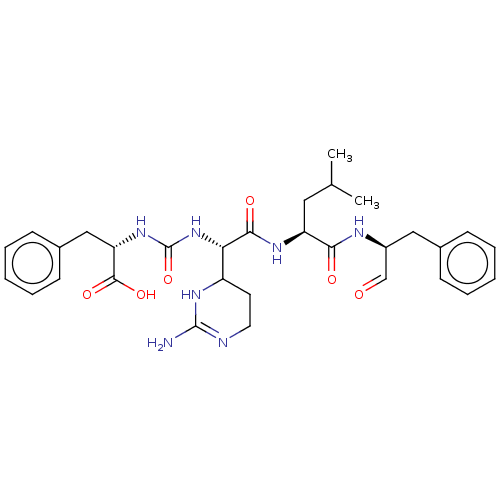 (Chymostatin | US11859014, Compound chymostatin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM50273979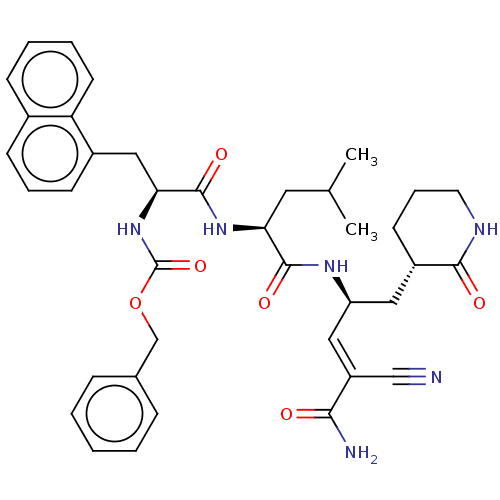 (CHEMBL4128616 | US11859014, Compound 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642922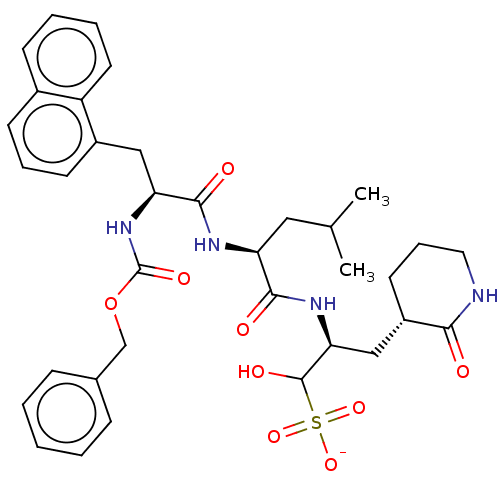 (US11859014, Compound 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM50273979 (CHEMBL4128616 | US11859014, Compound 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642927 (US11859014, Compound 83) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM11243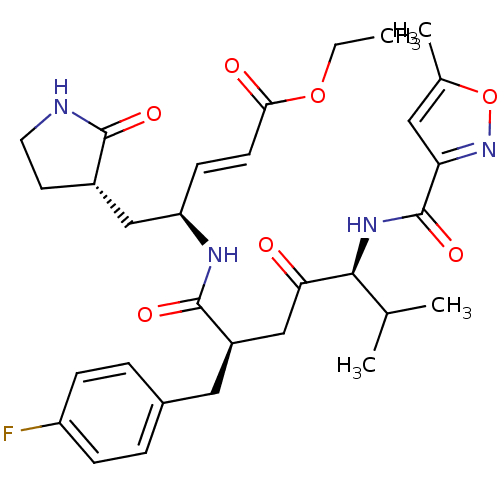 (AG7088 | CHEMBL20210 | US11859014, Compound rupint...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448175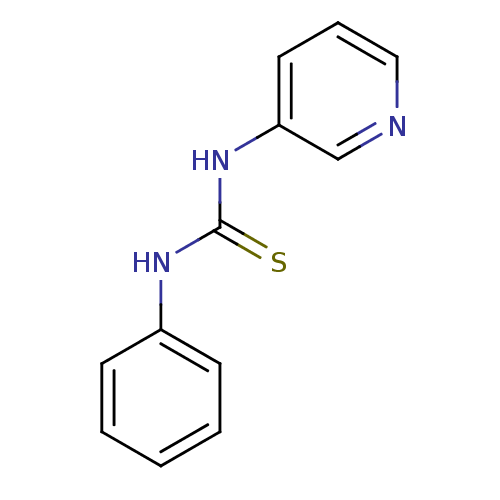 (CHEMBL3120552) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysi... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448176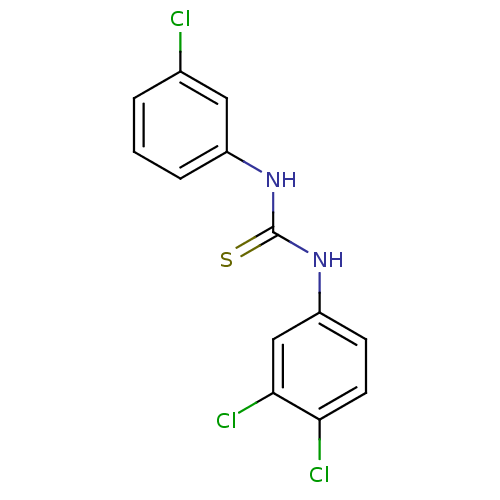 (CHEMBL3120570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysi... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448174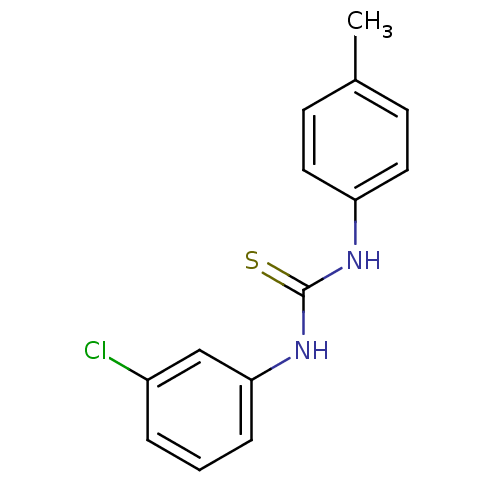 (CHEMBL3120548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Non-competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot ana... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642922 (US11859014, Compound 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448177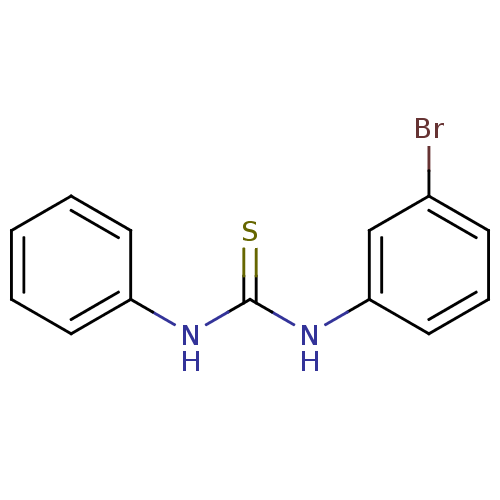 (CHEMBL1576403) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448178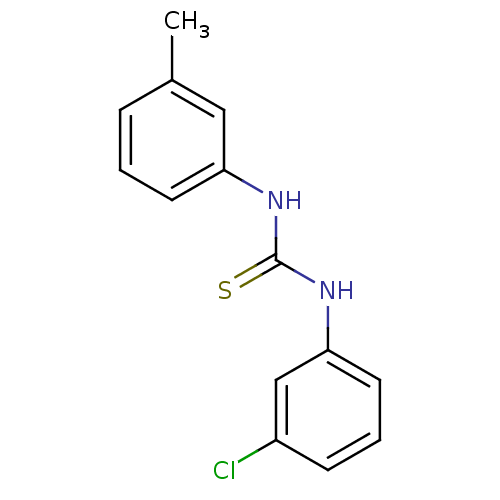 (CHEMBL3120574) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448179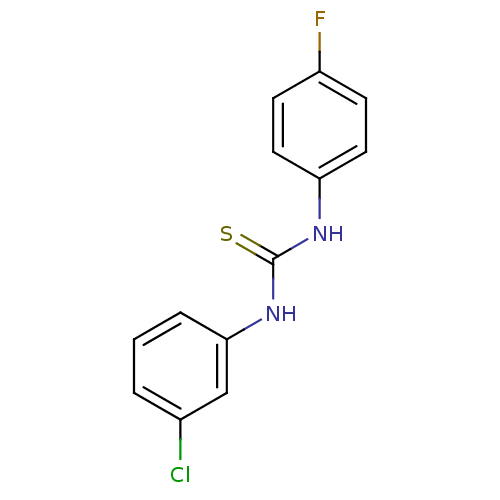 (CHEMBL1544729) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448184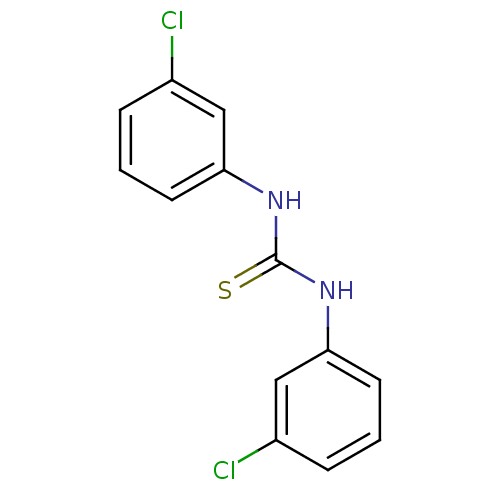 (CHEMBL3120558) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448182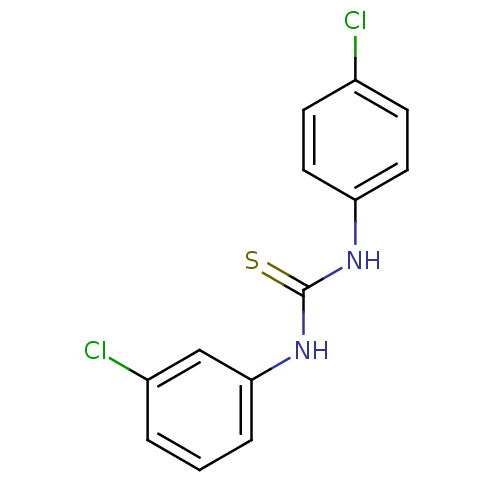 (CHEMBL3120560) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448180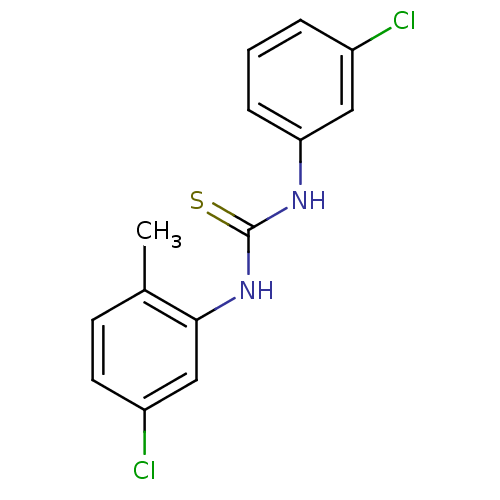 (CHEMBL3120571) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448185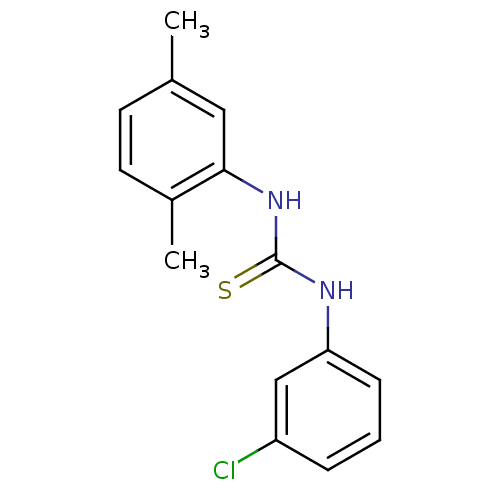 (CHEMBL3120566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50229993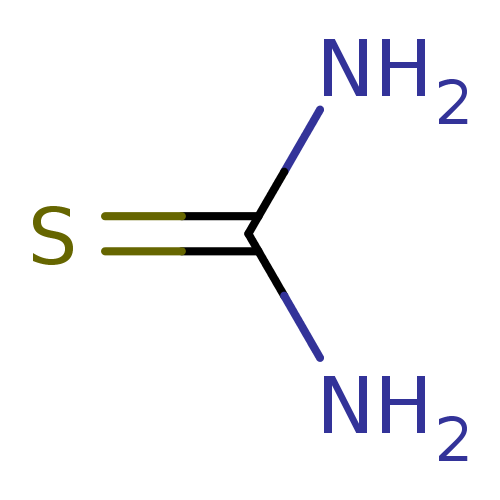 (2-thiourea | CHEMBL260876 | Thiocarbamid | Thiohar...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysi... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642926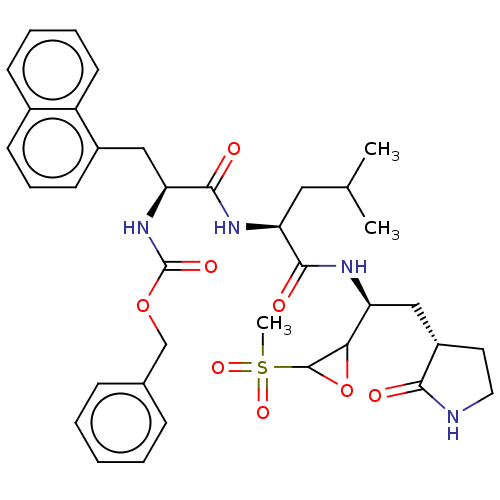 (US11859014, Compound 67) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of the compound towards HIV-reverse transcriptase | Bioorg Med Chem Lett 5: 1819-1824 (1995) Article DOI: 10.1016/0960-894X(95)00302-A BindingDB Entry DOI: 10.7270/Q23T9H64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Orl£ans et CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 integrase stand transfer activity expressed in Escherichia coli BL21(DE3) cells using 32P-labeled DNA substrate after... | Eur J Med Chem 104: 127-38 (2015) Article DOI: 10.1016/j.ejmech.2015.09.015 BindingDB Entry DOI: 10.7270/Q2CR5XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224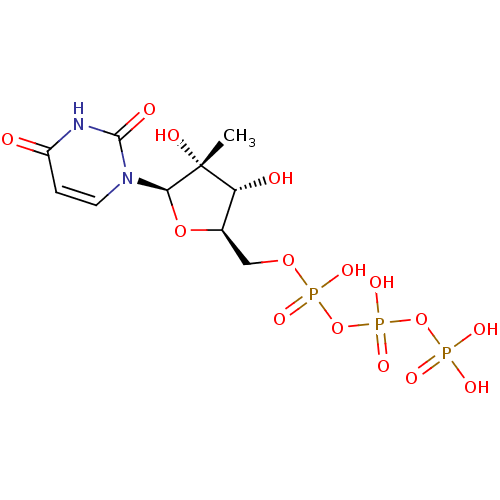 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 1a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50558593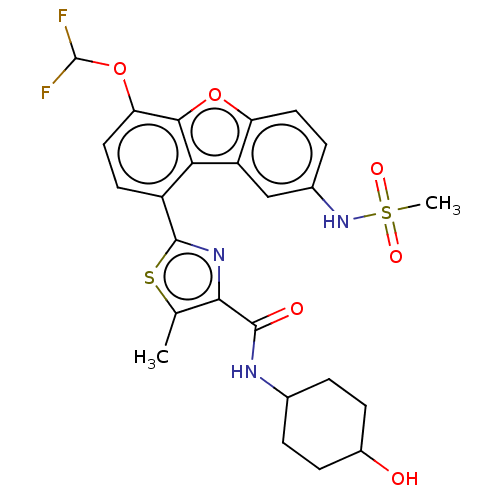 (CHEMBL4747406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50558594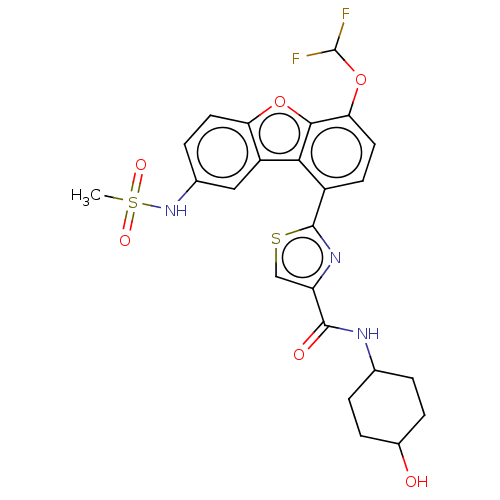 (CHEMBL4744412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GI/10360/2010/VNM) | BDBM50273967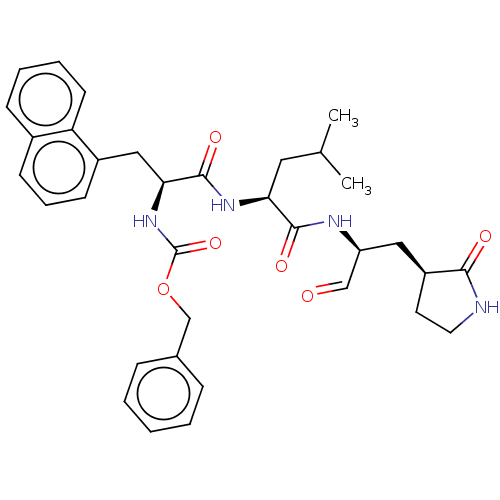 (CHEMBL2441741) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of Norovirus prototypic GI.1 3CL protease using HiLyte Fluor 488-labelled DFELQGPK as substrate incubated for 90 mins measured every mins ... | Bioorg Med Chem Lett 28: 2165-2170 (2018) Article DOI: 10.1016/j.bmcl.2018.05.012 BindingDB Entry DOI: 10.7270/Q2TH8Q6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50558595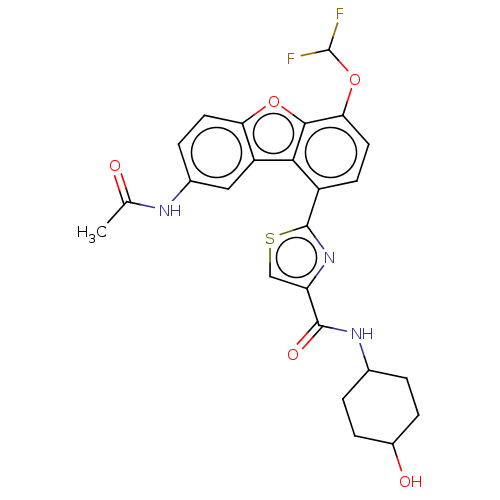 (CHEMBL4764245) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.P4_GII.4/Minerva) | BDBM642916 (US11859014, Compound 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50558598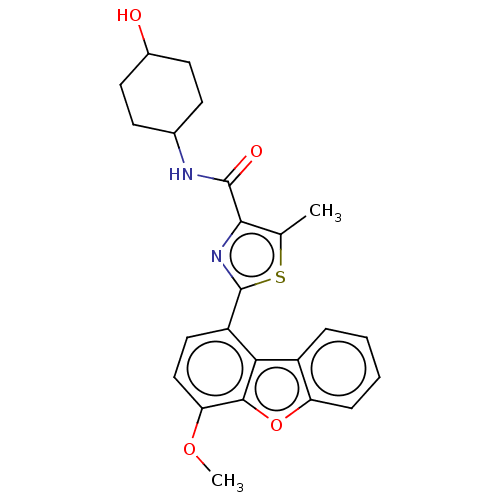 (CHEMBL4756900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 3a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 3a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50271224 (2'-C-Methyl-uridine-5'-triphosphate | CHEMBL521487) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine Curated by ChEMBL | Assay Description Inhibition of NS5B polymerase in HCV genotype 6a infected in human HuH7 replicon cells after 96 hrs by RT-PCR analysis | J Med Chem 62: 1859-1874 (2019) Article DOI: 10.1021/acs.jmedchem.8b01300 BindingDB Entry DOI: 10.7270/Q2M048WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50558600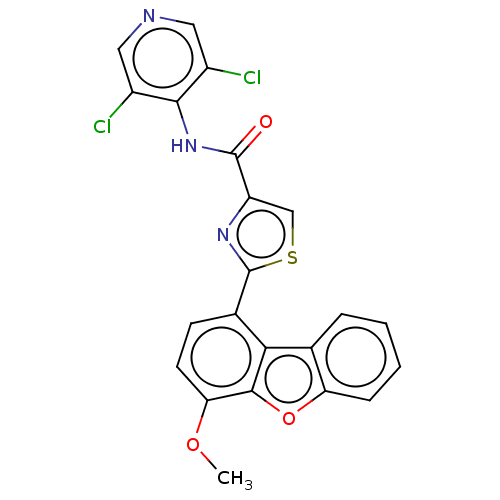 (CHEMBL4744894) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50558599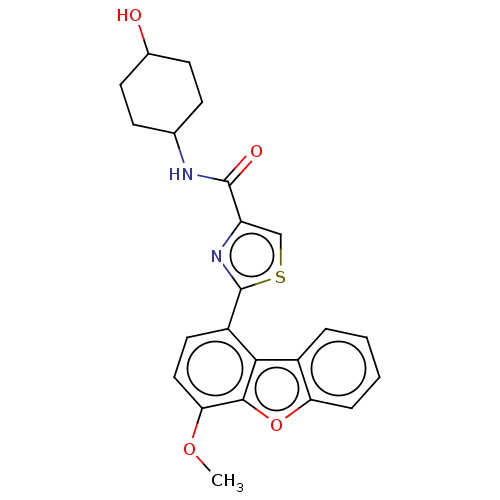 (CHEMBL4799096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.011 BindingDB Entry DOI: 10.7270/Q2N58R28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Norovirus Hu/GII.4-2002/WeertE022/2002/NL) | BDBM50273967 (CHEMBL2441741) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of Norovirus prototypic GII.4 3CL protease using HiLyte Fluor 488-labelled DFELQGPK as substrate incubated for 90 mins measured every mins... | Bioorg Med Chem Lett 28: 2165-2170 (2018) Article DOI: 10.1016/j.bmcl.2018.05.012 BindingDB Entry DOI: 10.7270/Q2TH8Q6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 815 total ) | Next | Last >> |