 Found 4182 hits with Last Name = 'nell' and Initial = 'p'
Found 4182 hits with Last Name = 'nell' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283704
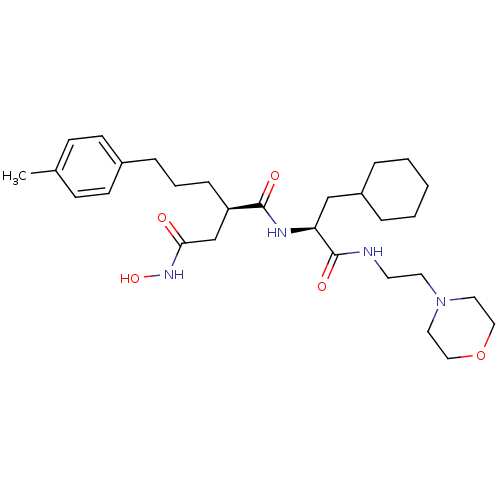
((R)-N*1*-[(S)-2-Cyclohexyl-1-(2-morpholin-4-yl-eth...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCN2CCOCC2)cc1 Show InChI InChI=1S/C29H46N4O5/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(34)32-37)28(35)31-26(20-24-6-3-2-4-7-24)29(36)30-14-15-33-16-18-38-19-17-33/h10-13,24-26,37H,2-9,14-21H2,1H3,(H,30,36)(H,31,35)(H,32,34)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283708
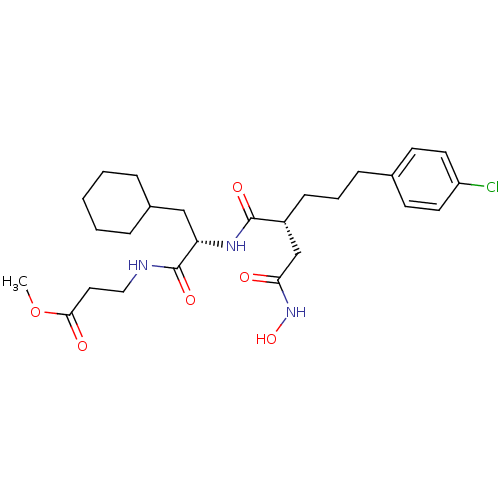
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283701
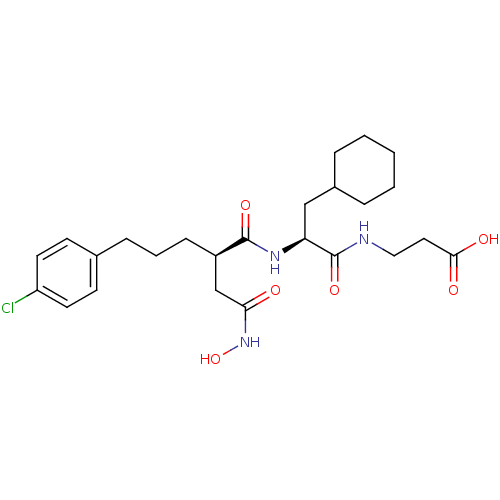
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283705
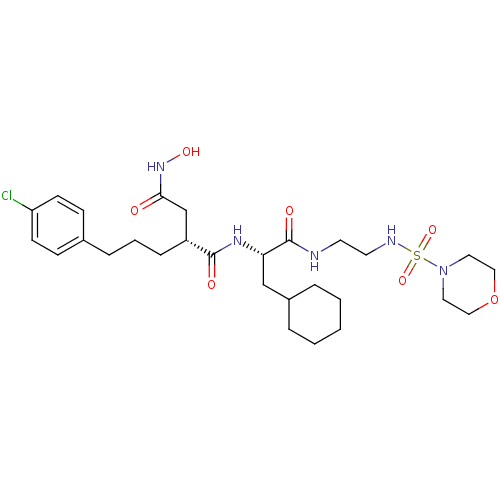
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCNS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C28H44ClN5O7S/c29-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-38)27(36)32-25(19-22-5-2-1-3-6-22)28(37)30-13-14-31-42(39,40)34-15-17-41-18-16-34/h9-12,22-23,25,31,38H,1-8,13-20H2,(H,30,37)(H,32,36)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283715
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(morpholine-4-sulf...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCNS(=O)(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C29H47N5O7S/c1-22-10-12-23(13-11-22)8-5-9-25(21-27(35)33-38)28(36)32-26(20-24-6-3-2-4-7-24)29(37)30-14-15-31-42(39,40)34-16-18-41-19-17-34/h10-13,24-26,31,38H,2-9,14-21H2,1H3,(H,30,37)(H,32,36)(H,33,35)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283703
((R)-N*1*-{(S)-2-Cyclohexyl-1-[2-(4-sulfamoyl-pheny...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C31H44N4O6S/c1-22-10-12-23(13-11-22)8-5-9-26(21-29(36)35-39)30(37)34-28(20-25-6-3-2-4-7-25)31(38)33-19-18-24-14-16-27(17-15-24)42(32,40)41/h10-17,25-26,28,39H,2-9,18-21H2,1H3,(H,33,38)(H,34,37)(H,35,36)(H2,32,40,41)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283710
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-morpholin-4-yl-but...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCCCN2CCOCC2)cc1 Show InChI InChI=1S/C31H50N4O5/c1-24-12-14-25(15-13-24)10-7-11-27(23-29(36)34-39)30(37)33-28(22-26-8-3-2-4-9-26)31(38)32-16-5-6-17-35-18-20-40-21-19-35/h12-15,26-28,39H,2-11,16-23H2,1H3,(H,32,38)(H,33,37)(H,34,36)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283713
((R)-N*1*-[(S)-2-Cyclohexyl-1-(4-dimethylamino-buty...)Show SMILES CN(C)CCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(C)cc1)CC(=O)NO Show InChI InChI=1S/C29H48N4O4/c1-22-14-16-23(17-15-22)12-9-13-25(21-27(34)32-37)28(35)31-26(20-24-10-5-4-6-11-24)29(36)30-18-7-8-19-33(2)3/h14-17,24-26,37H,4-13,18-21H2,1-3H3,(H,30,36)(H,31,35)(H,32,34)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283711
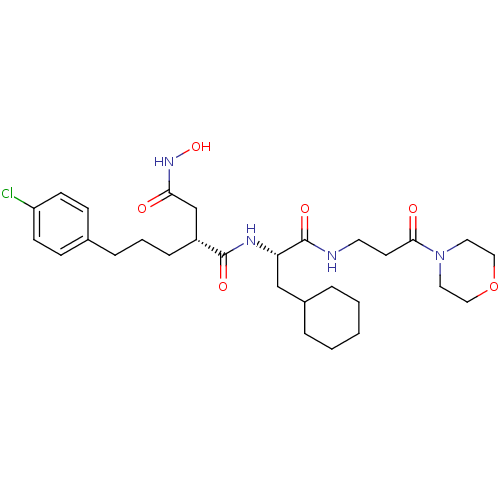
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C29H43ClN4O6/c30-24-11-9-21(10-12-24)7-4-8-23(20-26(35)33-39)28(37)32-25(19-22-5-2-1-3-6-22)29(38)31-14-13-27(36)34-15-17-40-18-16-34/h9-12,22-23,25,39H,1-8,13-20H2,(H,31,38)(H,32,37)(H,33,35)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283707
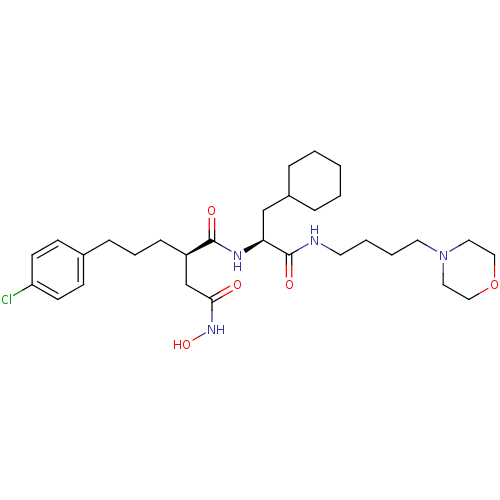
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCCCN1CCOCC1 Show InChI InChI=1S/C30H47ClN4O5/c31-26-13-11-23(12-14-26)9-6-10-25(22-28(36)34-39)29(37)33-27(21-24-7-2-1-3-8-24)30(38)32-15-4-5-16-35-17-19-40-20-18-35/h11-14,24-25,27,39H,1-10,15-22H2,(H,32,38)(H,33,37)(H,34,36)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101495
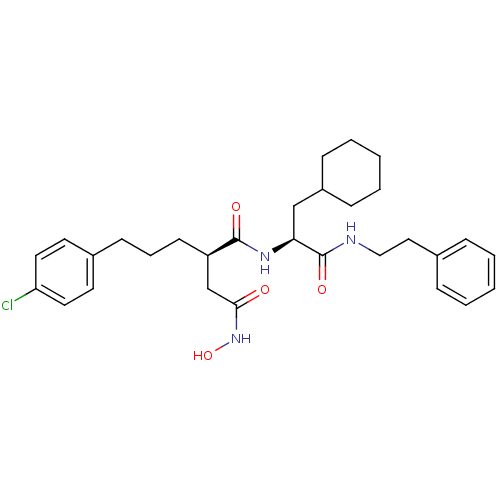
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-((S)-2-cyc...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40ClN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283702
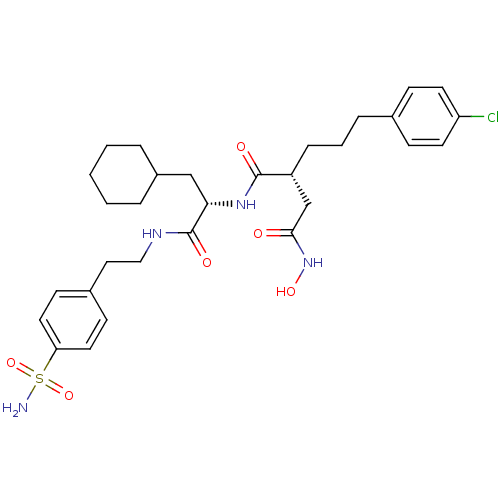
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{(S)-2-cyc...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)[C@H](CC2CCCCC2)NC(=O)[C@H](CCCc2ccc(Cl)cc2)CC(=O)NO)cc1 Show InChI InChI=1S/C30H41ClN4O6S/c31-25-13-9-21(10-14-25)7-4-8-24(20-28(36)35-39)29(37)34-27(19-23-5-2-1-3-6-23)30(38)33-18-17-22-11-15-26(16-12-22)42(32,40)41/h9-16,23-24,27,39H,1-8,17-20H2,(H,33,38)(H,34,37)(H,35,36)(H2,32,40,41)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50094037
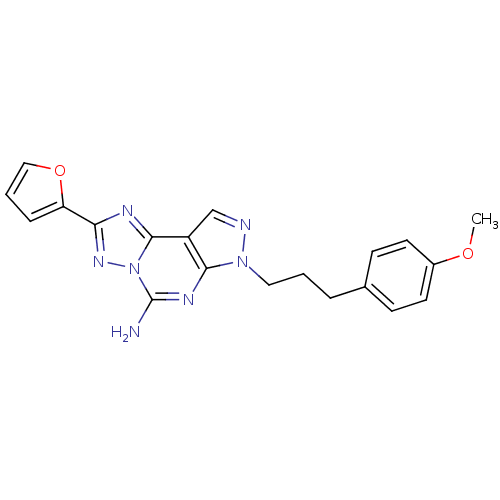
(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milano-Bicocca
Curated by ChEMBL
| Assay Description
Inhibitory activity against human adenosine A2A receptor expressed in HEK-293 cells by displacement of [3H]-SCH-58,261 |
J Med Chem 43: 4359-62 (2000)
BindingDB Entry DOI: 10.7270/Q2Z037C8 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283714
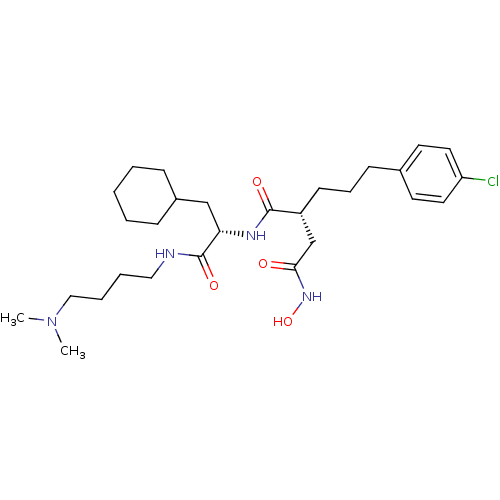
((R)-2-[3-(4-Chloro-phenyl)-propyl]-N*1*-[(S)-2-cyc...)Show SMILES CN(C)CCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C28H45ClN4O4/c1-33(2)18-7-6-17-30-28(36)25(19-22-9-4-3-5-10-22)31-27(35)23(20-26(34)32-37)12-8-11-21-13-15-24(29)16-14-21/h13-16,22-23,25,37H,3-12,17-20H2,1-2H3,(H,30,36)(H,31,35)(H,32,34)/t23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101526
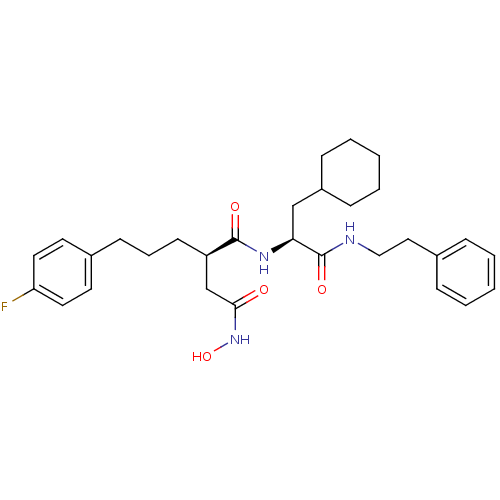
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(F)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H40FN3O4/c31-26-16-14-23(15-17-26)12-7-13-25(21-28(35)34-38)29(36)33-27(20-24-10-5-2-6-11-24)30(37)32-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,38H,2,5-7,10-13,18-21H2,(H,32,37)(H,33,36)(H,34,35)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101530
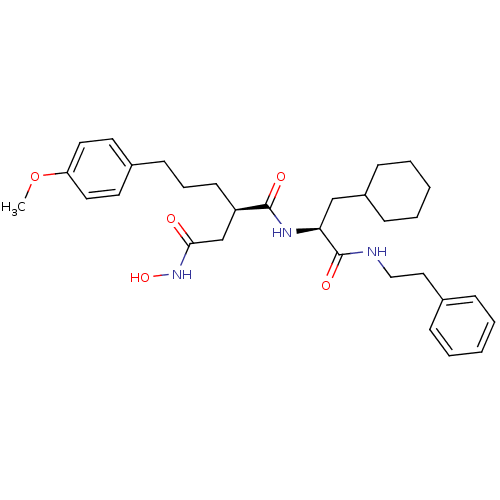
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES COc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O5/c1-39-27-17-15-24(16-18-27)13-8-14-26(22-29(35)34-38)30(36)33-28(21-25-11-6-3-7-12-25)31(37)32-20-19-23-9-4-2-5-10-23/h2,4-5,9-10,15-18,25-26,28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492
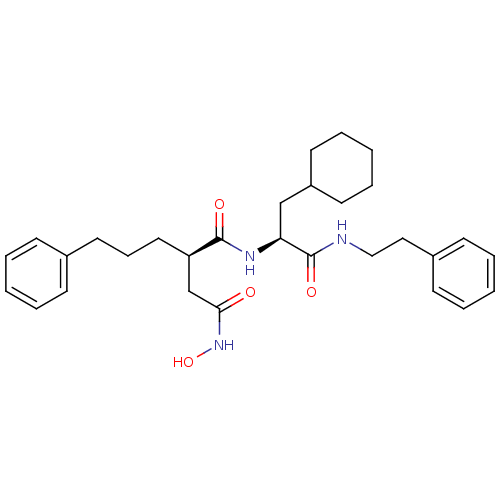
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101492

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c34-28(33-37)22-26(18-10-17-23-11-4-1-5-12-23)29(35)32-27(21-25-15-8-3-9-16-25)30(36)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-27,37H,3,8-10,15-22H2,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101513

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES Cc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C31H43N3O4/c1-23-15-17-25(18-16-23)13-8-14-27(22-29(35)34-38)30(36)33-28(21-26-11-6-3-7-12-26)31(37)32-20-19-24-9-4-2-5-10-24/h2,4-5,9-10,15-18,26-28,38H,3,6-8,11-14,19-22H2,1H3,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101494
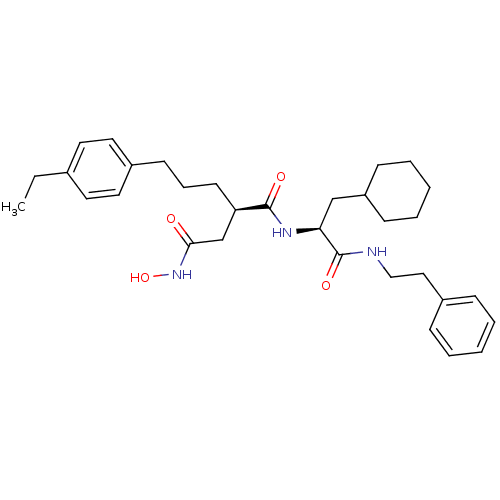
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES CCc1ccc(CCC[C@H](CC(=O)NO)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccccc2)cc1 Show InChI InChI=1S/C32H45N3O4/c1-2-24-16-18-26(19-17-24)14-9-15-28(23-30(36)35-39)31(37)34-29(22-27-12-7-4-8-13-27)32(38)33-21-20-25-10-5-3-6-11-25/h3,5-6,10-11,16-19,27-29,39H,2,4,7-9,12-15,20-23H2,1H3,(H,33,38)(H,34,37)(H,35,36)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50176070
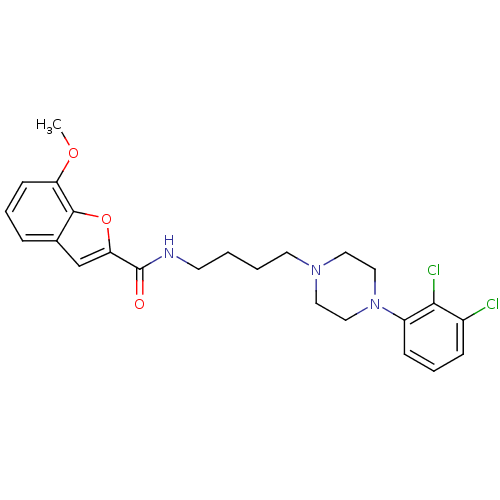
(7-Methoxy-benzofuran-2-carboxylic acid {4-[4-(2,3-...)Show SMILES COc1cccc2cc(oc12)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C24H27Cl2N3O3/c1-31-20-9-4-6-17-16-21(32-23(17)20)24(30)27-10-2-3-11-28-12-14-29(15-13-28)19-8-5-7-18(25)22(19)26/h4-9,16H,2-3,10-15H2,1H3,(H,27,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milano/Bicocca
Curated by ChEMBL
| Assay Description
Binding affinity for rat dopamine D3 receptor using [11C] radiotracer |
J Med Chem 48: 7018-23 (2005)
Article DOI: 10.1021/jm050171k
BindingDB Entry DOI: 10.7270/Q2H994SR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160851
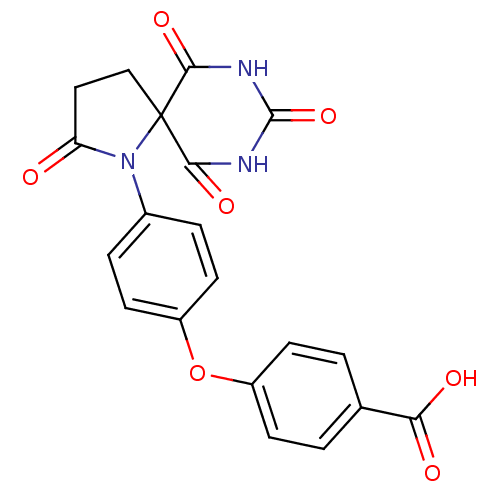
(4-[4-(2,6,8,10-Tetraoxo-1,7,9-triaza-spiro[4.5]dec...)Show SMILES OC(=O)c1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C20H15N3O7/c24-15-9-10-20(17(27)21-19(29)22-18(20)28)23(15)12-3-7-14(8-4-12)30-13-5-1-11(2-6-13)16(25)26/h1-8H,9-10H2,(H,25,26)(H2,21,22,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101528
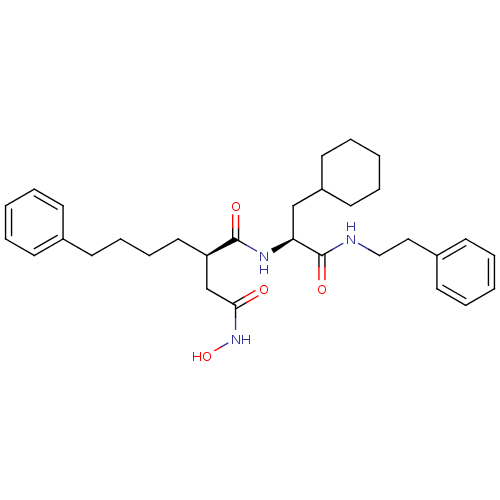
((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H43N3O4/c35-29(34-38)23-27(19-11-10-16-24-12-4-1-5-13-24)30(36)33-28(22-26-17-8-3-9-18-26)31(37)32-21-20-25-14-6-2-7-15-25/h1-2,4-7,12-15,26-28,38H,3,8-11,16-23H2,(H,32,37)(H,33,36)(H,34,35)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101510

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(cc1)C(F)(F)F)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H40F3N3O4/c32-31(33,34)26-16-14-23(15-17-26)12-7-13-25(21-28(38)37-41)29(39)36-27(20-24-10-5-2-6-11-24)30(40)35-19-18-22-8-3-1-4-9-22/h1,3-4,8-9,14-17,24-25,27,41H,2,5-7,10-13,18-21H2,(H,35,40)(H,36,39)(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101529

((R)-N*1*-((S)-2-Cyclohexyl-1-phenethylcarbamoyl-et...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H39N3O4/c1-18(2)15-21(17-23(29)28-32)24(30)27-22(16-20-11-7-4-8-12-20)25(31)26-14-13-19-9-5-3-6-10-19/h3,5-6,9-10,18,20-22,32H,4,7-8,11-17H2,1-2H3,(H,26,31)(H,27,30)(H,28,29)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160843
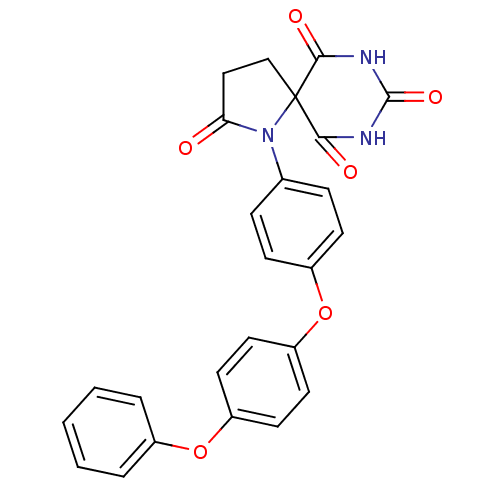
(1-[4-(4-Phenoxy-phenoxy)-phenyl]-1,7,9-triaza-spir...)Show SMILES O=C1CCC2(N1c1ccc(Oc3ccc(Oc4ccccc4)cc3)cc1)C(=O)NC(=O)NC2=O Show InChI InChI=1S/C25H19N3O6/c29-21-14-15-25(22(30)26-24(32)27-23(25)31)28(21)16-6-8-18(9-7-16)34-20-12-10-19(11-13-20)33-17-4-2-1-3-5-17/h1-13H,14-15H2,(H2,26,27,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160852
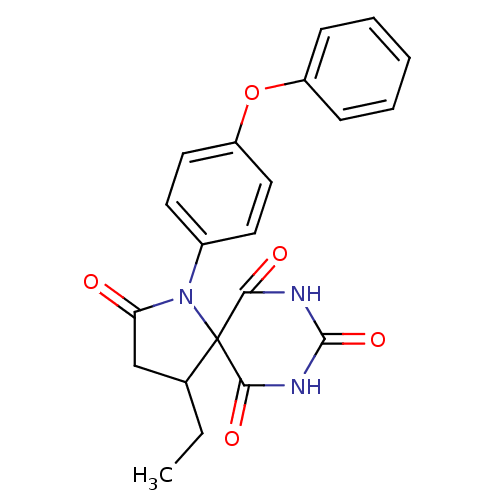
(4-ethyl-1-(4-phenoxy-phenyl)-1,7,9-triaza-spiro[4....)Show SMILES CCC1CC(=O)N(c2ccc(Oc3ccccc3)cc2)C11C(=O)NC(=O)NC1=O Show InChI InChI=1S/C21H19N3O5/c1-2-13-12-17(25)24(21(13)18(26)22-20(28)23-19(21)27)14-8-10-16(11-9-14)29-15-6-4-3-5-7-15/h3-11,13H,2,12H2,1H3,(H2,22,23,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160852

(4-ethyl-1-(4-phenoxy-phenyl)-1,7,9-triaza-spiro[4....)Show SMILES CCC1CC(=O)N(c2ccc(Oc3ccccc3)cc2)C11C(=O)NC(=O)NC1=O Show InChI InChI=1S/C21H19N3O5/c1-2-13-12-17(25)24(21(13)18(26)22-20(28)23-19(21)27)14-8-10-16(11-9-14)29-15-6-4-3-5-7-15/h3-11,13H,2,12H2,1H3,(H2,22,23,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160855
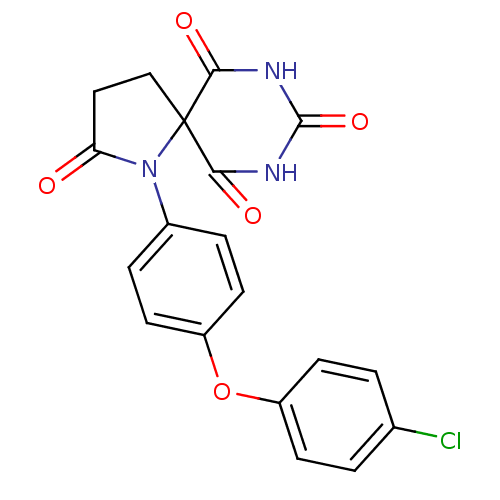
(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM86210
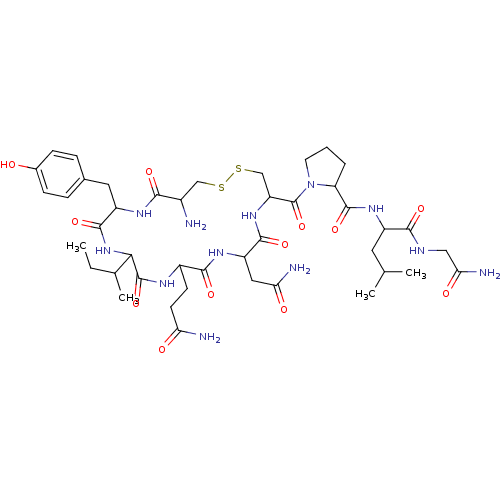
(CAS_50-56-6 | NSC_439302 | Oxytocin)Show SMILES CCC(C)C1NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(N)CSSCC(NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 253-61 (2003)
Article DOI: 10.1124/jpet.103.049395
BindingDB Entry DOI: 10.7270/Q2MC8XKT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50094037

(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milano-Bicocca
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine A2A receptor in rat striatal membranes by displacement of [3H]-SCH-58,261 |
J Med Chem 43: 4359-62 (2000)
BindingDB Entry DOI: 10.7270/Q2Z037C8 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase 13 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160852

(4-ethyl-1-(4-phenoxy-phenyl)-1,7,9-triaza-spiro[4....)Show SMILES CCC1CC(=O)N(c2ccc(Oc3ccccc3)cc2)C11C(=O)NC(=O)NC1=O Show InChI InChI=1S/C21H19N3O5/c1-2-13-12-17(25)24(21(13)18(26)22-20(28)23-19(21)27)14-8-10-16(11-9-14)29-15-6-4-3-5-7-15/h3-11,13H,2,12H2,1H3,(H2,22,23,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase 13 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160852

(4-ethyl-1-(4-phenoxy-phenyl)-1,7,9-triaza-spiro[4....)Show SMILES CCC1CC(=O)N(c2ccc(Oc3ccccc3)cc2)C11C(=O)NC(=O)NC1=O Show InChI InChI=1S/C21H19N3O5/c1-2-13-12-17(25)24(21(13)18(26)22-20(28)23-19(21)27)14-8-10-16(11-9-14)29-15-6-4-3-5-7-15/h3-11,13H,2,12H2,1H3,(H2,22,23,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase 13 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50283712

(4-{2-[(S)-3-Cyclohexyl-2-((R)-2-hydroxycarbamoylme...)Show SMILES ONC(=O)C[C@@H](CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccc(cc1)S(O)(=O)=O Show InChI InChI=1S/C30H41N3O7S/c34-28(33-37)21-25(13-7-12-22-8-3-1-4-9-22)29(35)32-27(20-24-10-5-2-6-11-24)30(36)31-19-18-23-14-16-26(17-15-23)41(38,39)40/h1,3-4,8-9,14-17,24-25,27,37H,2,5-7,10-13,18-21H2,(H,31,36)(H,32,35)(H,33,34)(H,38,39,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the gelatinase-A enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50122041

(1-Methoxy-naphthalene-2-carboxylic acid {4-[4-(2,3...)Show SMILES COc1c(ccc2ccccc12)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H29Cl2N3O2/c1-33-25-20-8-3-2-7-19(20)11-12-21(25)26(32)29-13-4-5-14-30-15-17-31(18-16-30)23-10-6-9-22(27)24(23)28/h2-3,6-12H,4-5,13-18H2,1H3,(H,29,32) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milano/Bicocca
Curated by ChEMBL
| Assay Description
Binding affinity for rat dopamine D3 receptor using [11C] radiotracer |
J Med Chem 48: 7018-23 (2005)
Article DOI: 10.1021/jm050171k
BindingDB Entry DOI: 10.7270/Q2H994SR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326722
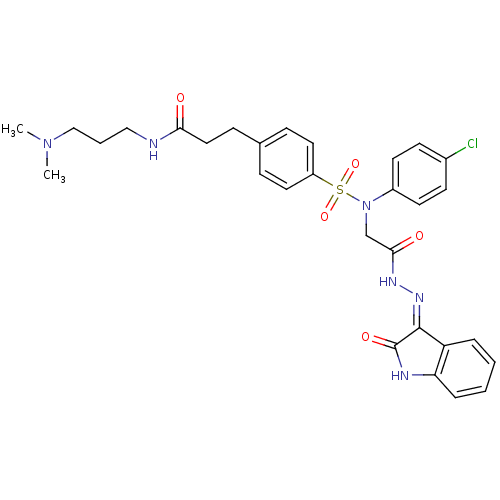
((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50326722

((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM86210

(CAS_50-56-6 | NSC_439302 | Oxytocin)Show SMILES CCC(C)C1NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(N)CSSCC(NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 253-61 (2003)
Article DOI: 10.1124/jpet.103.049395
BindingDB Entry DOI: 10.7270/Q2MC8XKT |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50160851

(4-[4-(2,6,8,10-Tetraoxo-1,7,9-triaza-spiro[4.5]dec...)Show SMILES OC(=O)c1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C20H15N3O7/c24-15-9-10-20(17(27)21-19(29)22-18(20)28)23(15)12-3-7-14(8-4-12)30-13-5-1-11(2-6-13)16(25)26/h1-8H,9-10H2,(H,25,26)(H2,21,22,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283701

(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50160852

(4-ethyl-1-(4-phenoxy-phenyl)-1,7,9-triaza-spiro[4....)Show SMILES CCC1CC(=O)N(c2ccc(Oc3ccccc3)cc2)C11C(=O)NC(=O)NC1=O Show InChI InChI=1S/C21H19N3O5/c1-2-13-12-17(25)24(21(13)18(26)22-20(28)23-19(21)27)14-8-10-16(11-9-14)29-15-6-4-3-5-7-15/h3-11,13H,2,12H2,1H3,(H2,22,23,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101523
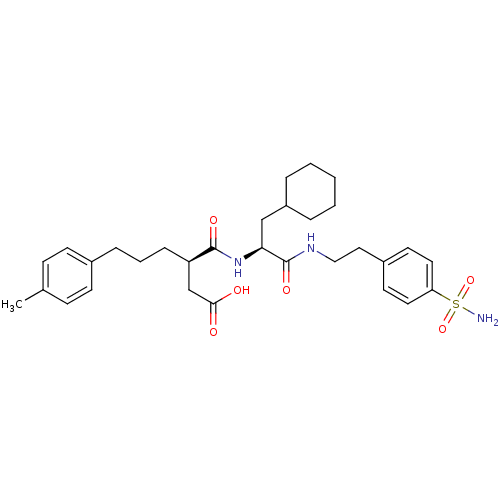
((R)-3-{(S)-2-Cyclohexyl-1-[2-(4-sulfamoyl-phenyl)-...)Show SMILES Cc1ccc(CCC[C@H](CC(O)=O)C(=O)N[C@@H](CC2CCCCC2)C(=O)NCCc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C31H43N3O6S/c1-22-10-12-23(13-11-22)8-5-9-26(21-29(35)36)30(37)34-28(20-25-6-3-2-4-7-25)31(38)33-19-18-24-14-16-27(17-15-24)41(32,39)40/h10-17,25-26,28H,2-9,18-21H2,1H3,(H,33,38)(H,34,37)(H,35,36)(H2,32,39,40)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the Gelatinase-A enzyme was determined from purified NSO cells. |
Bioorg Med Chem Lett 4: 2747-2752 (1994)
Article DOI: 10.1016/S0960-894X(01)80588-6
BindingDB Entry DOI: 10.7270/Q2JH3M3P |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50410616
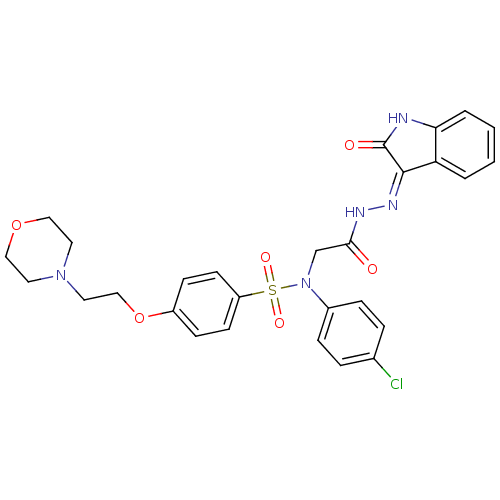
(CHEMBL2113185)Show SMILES Clc1ccc(cc1)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)S(=O)(=O)c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C28H28ClN5O6S/c29-20-5-7-21(8-6-20)34(19-26(35)31-32-27-24-3-1-2-4-25(24)30-28(27)36)41(37,38)23-11-9-22(10-12-23)40-18-15-33-13-16-39-17-14-33/h1-12H,13-19H2,(H,31,35)(H,30,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50410618
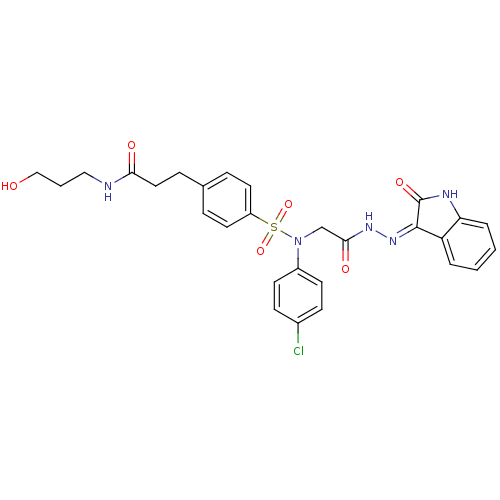
(CHEMBL2113181)Show SMILES OCCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28ClN5O6S/c29-20-9-11-21(12-10-20)34(18-26(37)32-33-27-23-4-1-2-5-24(23)31-28(27)38)41(39,40)22-13-6-19(7-14-22)8-15-25(36)30-16-3-17-35/h1-2,4-7,9-14,35H,3,8,15-18H2,(H,30,36)(H,32,37)(H,31,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
Bioorg Med Chem Lett 15: 1101-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.016
BindingDB Entry DOI: 10.7270/Q2F47NN2 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283708

(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data