

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031689 (CHEMBL3360298) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031688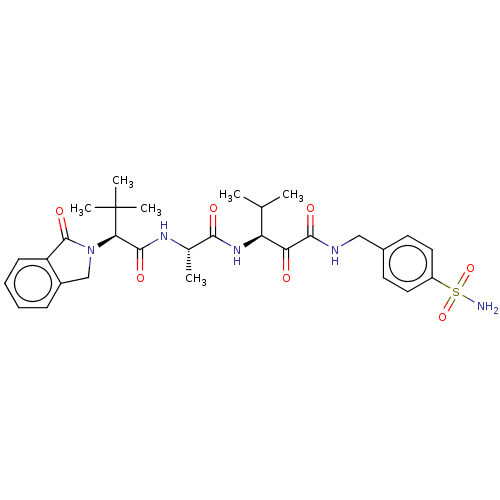 (CHEMBL3360299) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031684 (CHEMBL3360302) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031695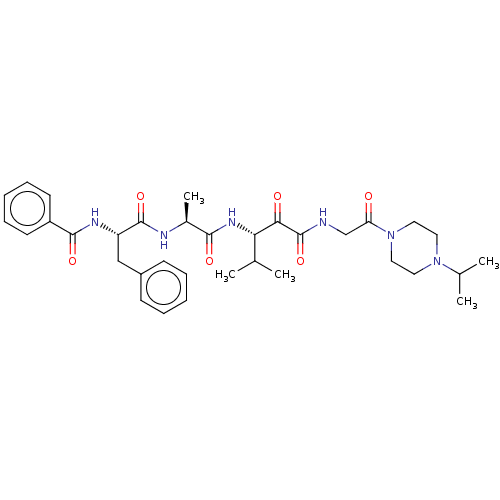 (CHEMBL3360292) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031687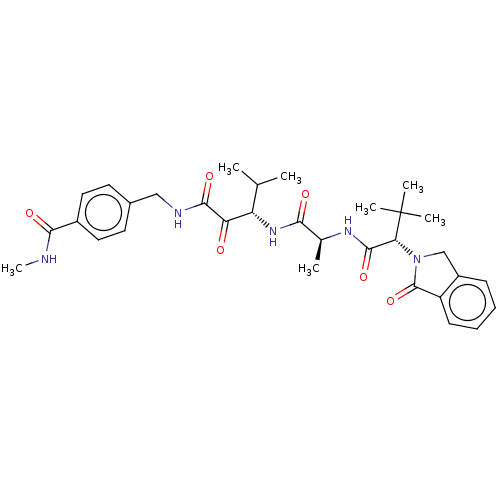 (CHEMBL3360300) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031685 (CHEMBL3360301) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031690 (CHEMBL3360297) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031693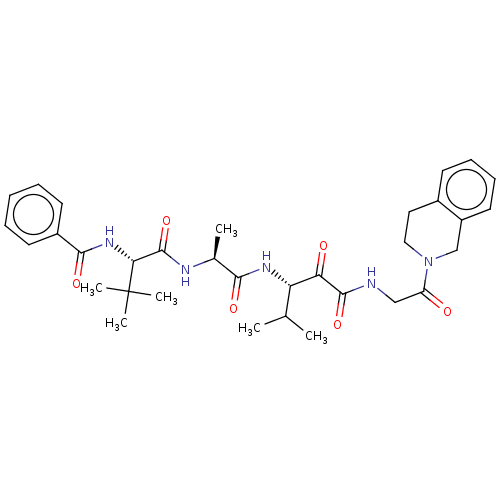 (CHEMBL3360294) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031713 (CHEMBL3360282) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031632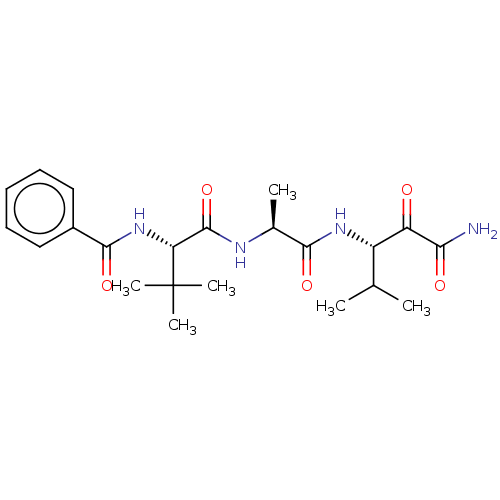 (CHEMBL3359790) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031635 (CHEMBL3359786) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103023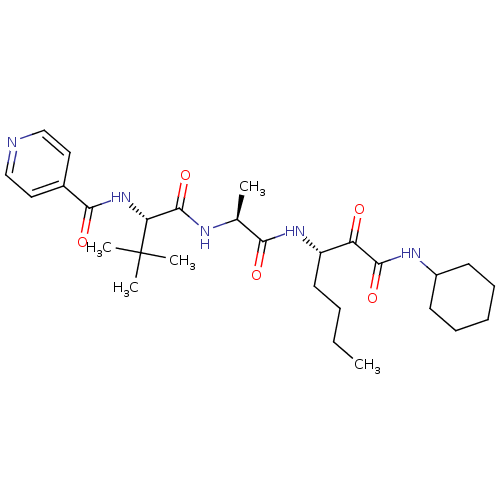 (US8541363, PVA-039) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester US Patent | Assay Description The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... | US Patent US8541363 (2013) BindingDB Entry DOI: 10.7270/Q2PC3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031668 (CHEMBL3359782) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103022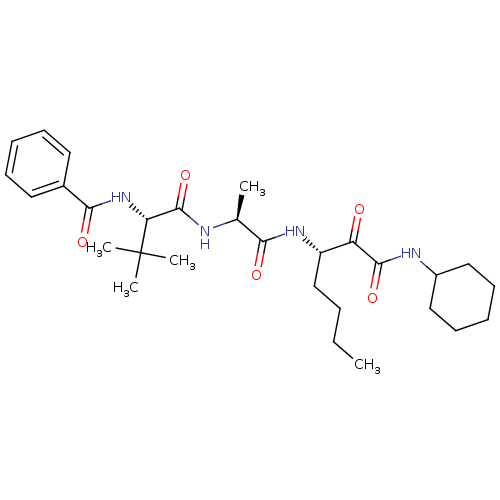 (US8541363, PVA-037) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.85 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester US Patent | Assay Description The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... | US Patent US8541363 (2013) BindingDB Entry DOI: 10.7270/Q2PC3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031719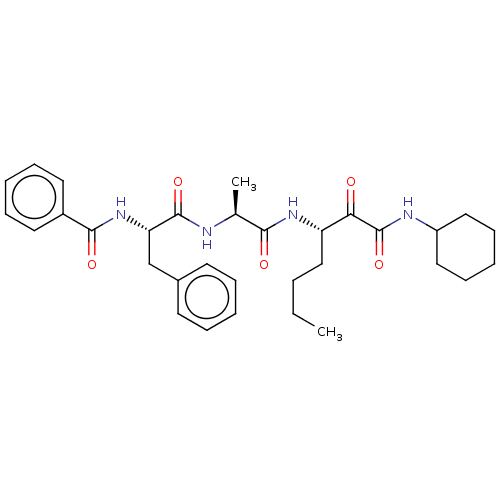 (CHEMBL3359772) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031711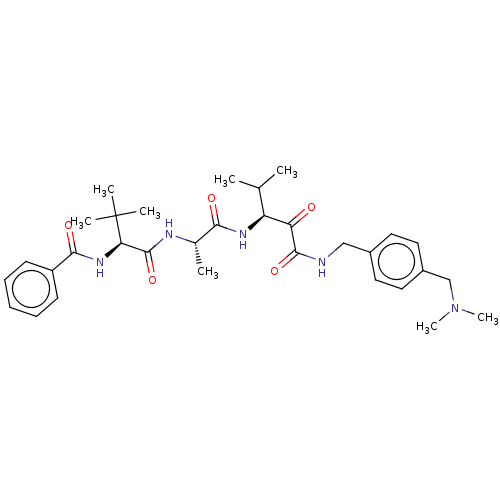 (CHEMBL3360284) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031714 (CHEMBL3360281) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031697 (CHEMBL3360290) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031694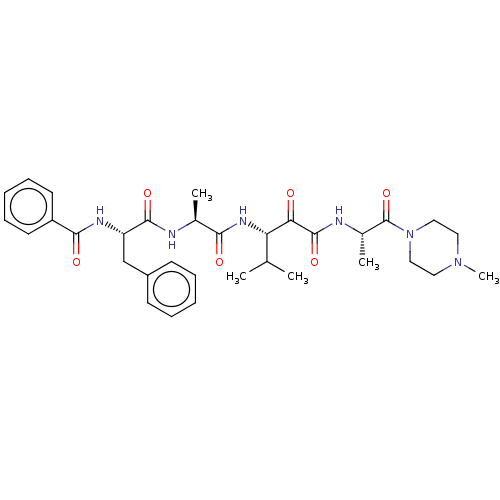 (CHEMBL3360293) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031700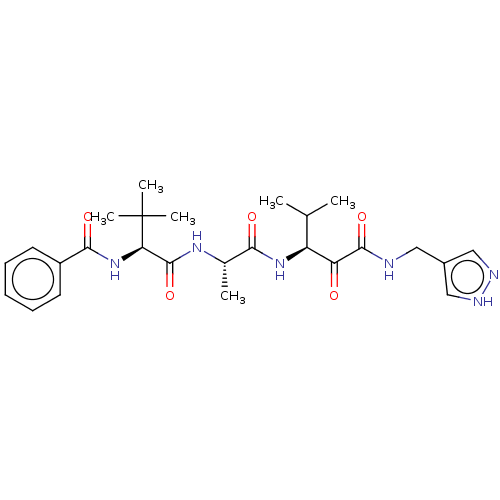 (CHEMBL3360287) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031682 (CHEMBL3359775) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103021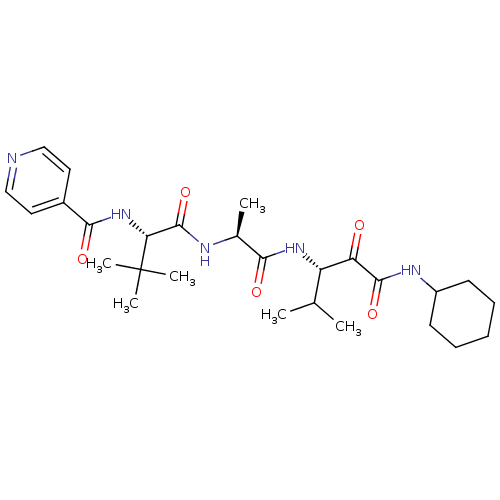 (US8541363, PVA-038) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031673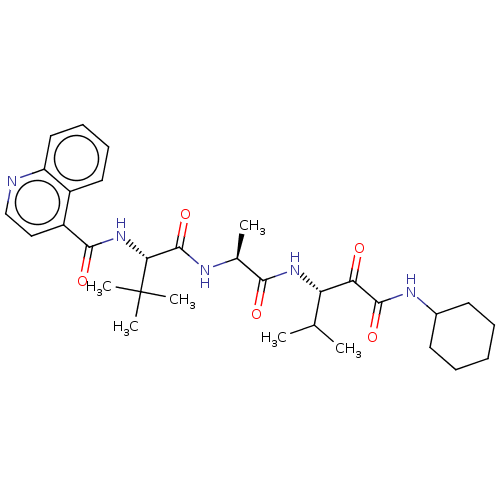 (CHEMBL3359780) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103021 (US8541363, PVA-038) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester US Patent | Assay Description The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... | US Patent US8541363 (2013) BindingDB Entry DOI: 10.7270/Q2PC3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031696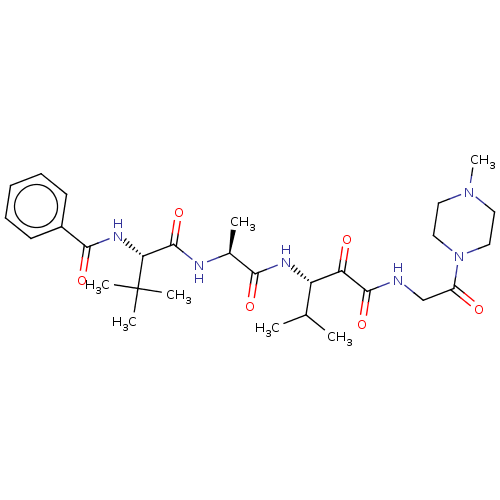 (CHEMBL3360291) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031698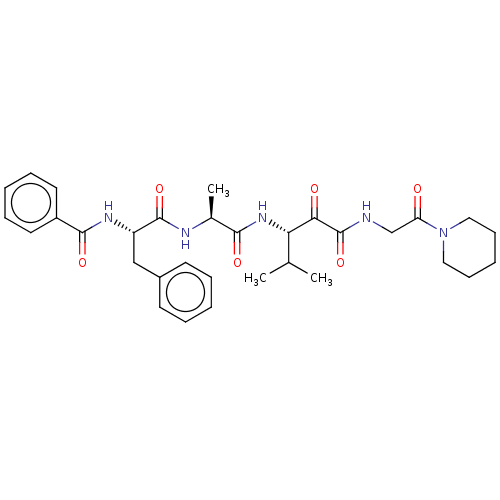 (CHEMBL3360289) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103020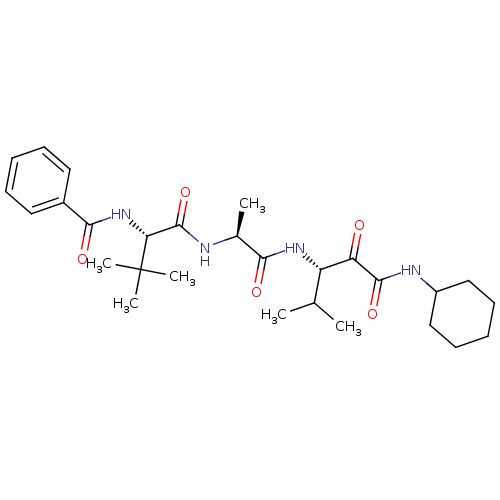 (US8541363, PVA-026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester US Patent | Assay Description The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... | US Patent US8541363 (2013) BindingDB Entry DOI: 10.7270/Q2PC3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM103020 (US8541363, PVA-026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidase 1 (Dermatophagoides pteronyssinus (European house dus...) | BDBM50031699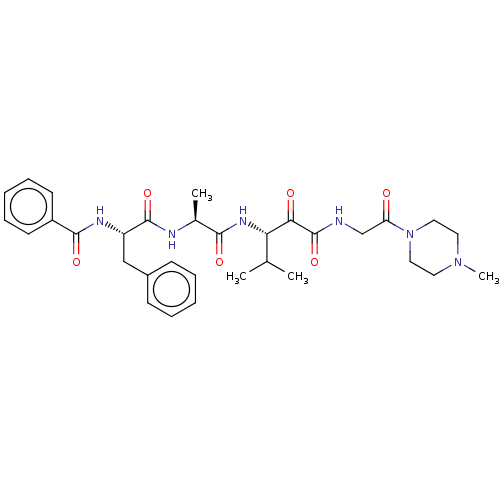 (CHEMBL3360288) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd. Curated by ChEMBL | Assay Description Inhibition of house dust mite Derp-1 after 20 mins | J Med Chem 57: 9447-62 (2014) Article DOI: 10.1021/jm501102h BindingDB Entry DOI: 10.7270/Q2R212ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM245931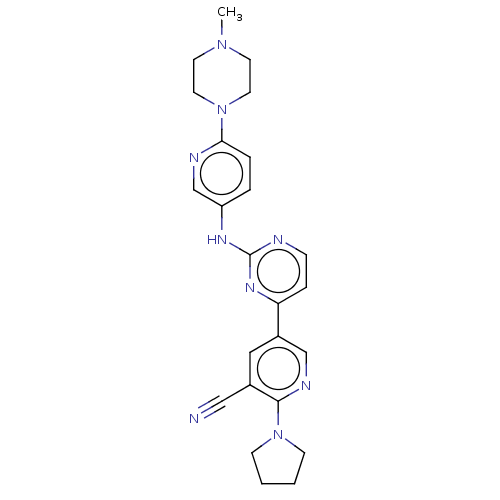 (US9433622, DMX- 3) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM245964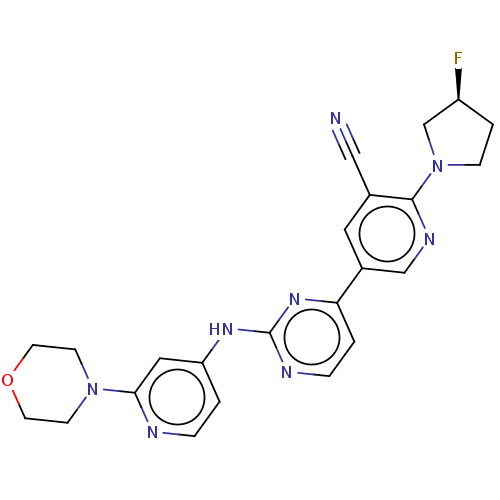 (US9433622, DMX- 61) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM245966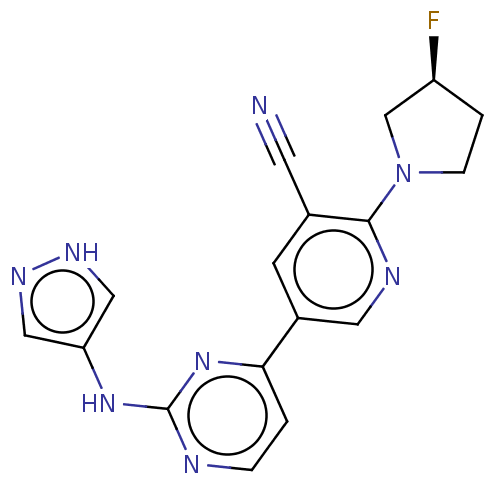 (US9433622, DMX- 63) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM245971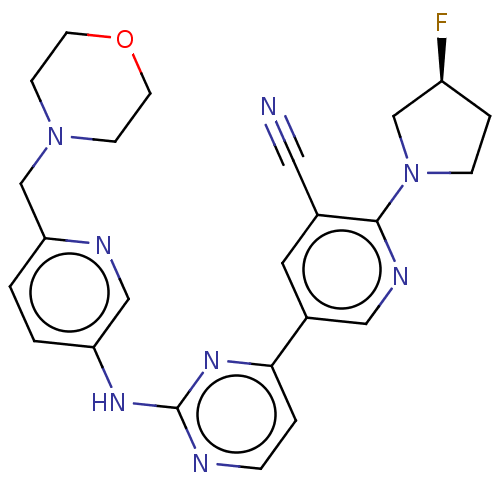 (US9433622, DMX- 68) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246030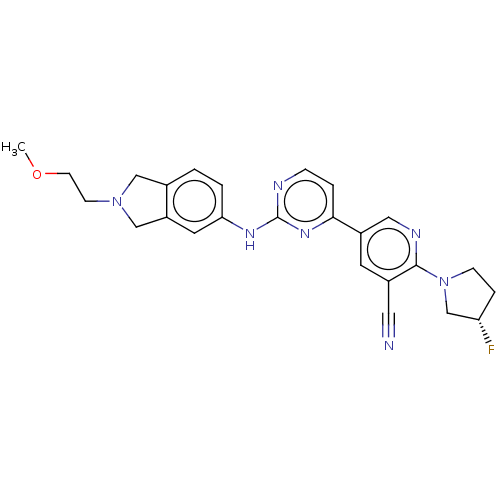 (US9433622, DMX- 127) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246032 (US9433622, DMX- 37) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246044 (US9433622, DMX- 49) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246076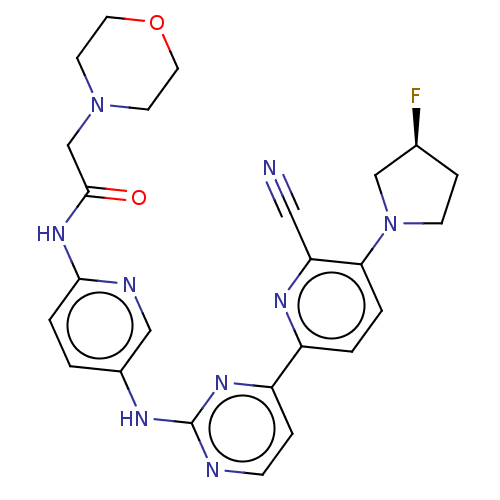 (US9433622, DMX- 142) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246083 (US9433622, DMX- 149) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246087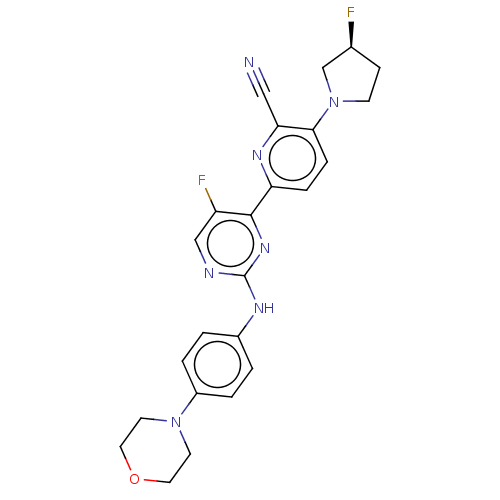 (US9433622, DMX- 153) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM246094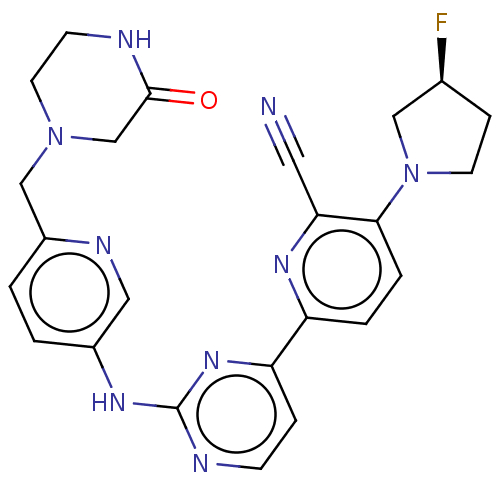 (US9433622, DMX- 156) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM245933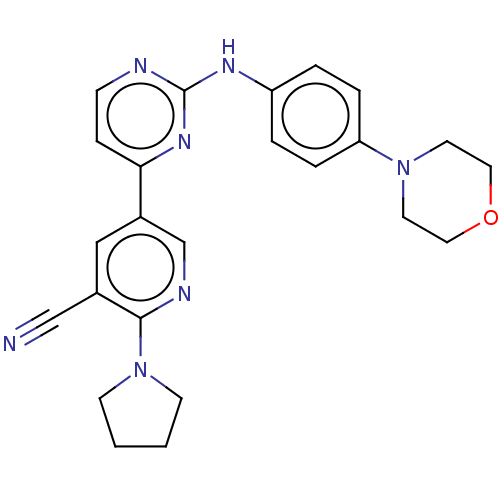 (US9433622, DMX- 5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM246005 (US9433622, DMX- 102) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM246032 (US9433622, DMX- 37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM246039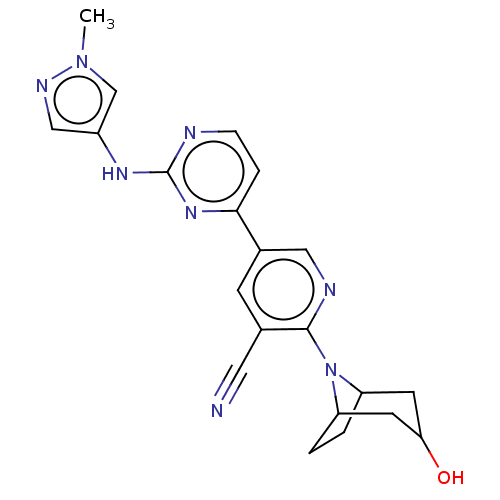 (US9433622, DMX- 44) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM246092 (US9433622, DMX- 154) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM246094 (US9433622, DMX- 156) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM245950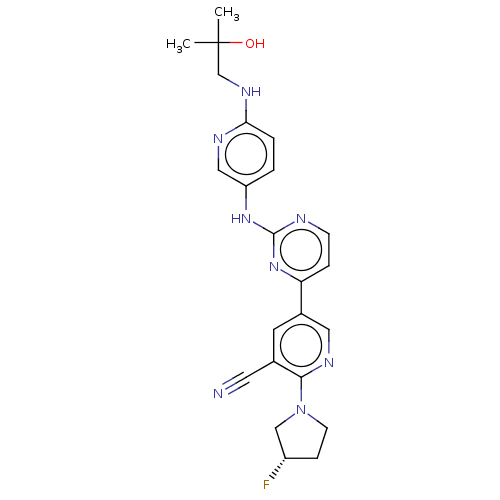 (US9433622, DMX- 22) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM245953 (US9433622, DMX- 25) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM245952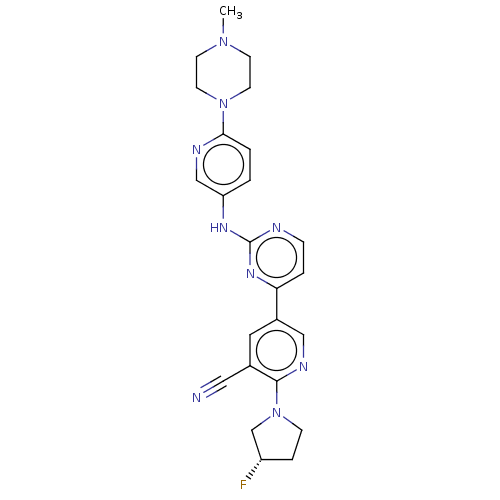 (US9433622, DMX- 24) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM245956 (US9433622, DMX- 28) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Case Western Reserve University US Patent | Assay Description Kinase inhibition assays (10 μL) were performed at 20° C. in 384-well plate format. Compound IC50 values were determined at the apparent Km for ... | US Patent US9433622 (2016) BindingDB Entry DOI: 10.7270/Q2PZ57R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 546 total ) | Next | Last >> |