 Found 15895 hits with Last Name = 'oh' and Initial = 'c'
Found 15895 hits with Last Name = 'oh' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043393
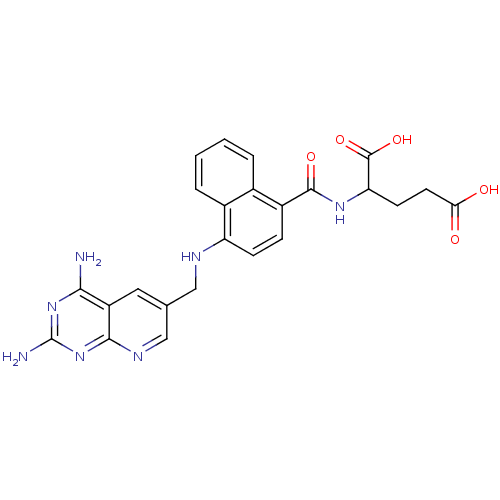
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043396
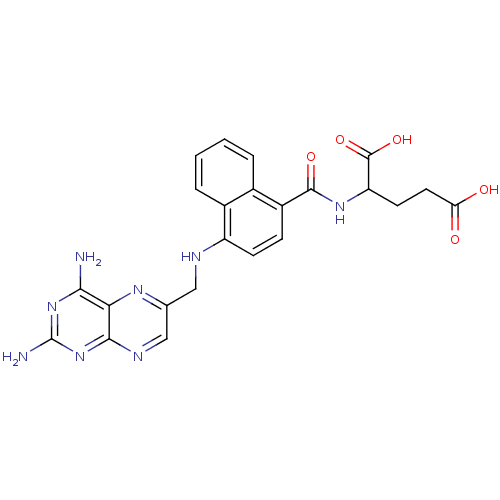
(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C23H22N8O5/c24-19-18-20(31-23(25)30-19)27-10-11(28-18)9-26-15-6-5-14(12-3-1-2-4-13(12)15)21(34)29-16(22(35)36)7-8-17(32)33/h1-6,10,16,26H,7-9H2,(H,29,34)(H,32,33)(H,35,36)(H4,24,25,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043399
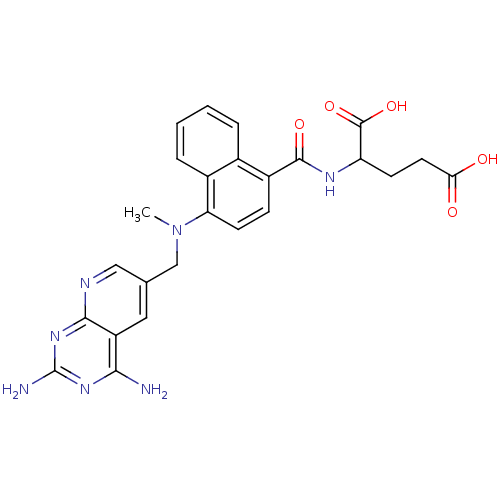
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C25H25N7O5/c1-32(12-13-10-17-21(26)30-25(27)31-22(17)28-11-13)19-8-6-16(14-4-2-3-5-15(14)19)23(35)29-18(24(36)37)7-9-20(33)34/h2-6,8,10-11,18H,7,9,12H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,26,27,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells |
J Med Chem 35: 3002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2MP527Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043395
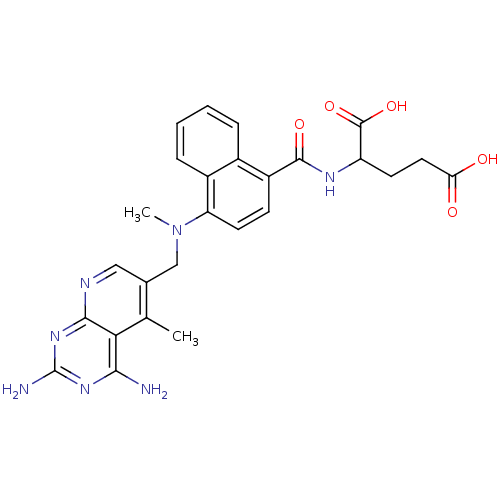
(2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1C)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C26H27N7O5/c1-13-14(11-29-23-21(13)22(27)31-26(28)32-23)12-33(2)19-9-7-17(15-5-3-4-6-16(15)19)24(36)30-18(25(37)38)8-10-20(34)35/h3-7,9,11,18H,8,10,12H2,1-2H3,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043400
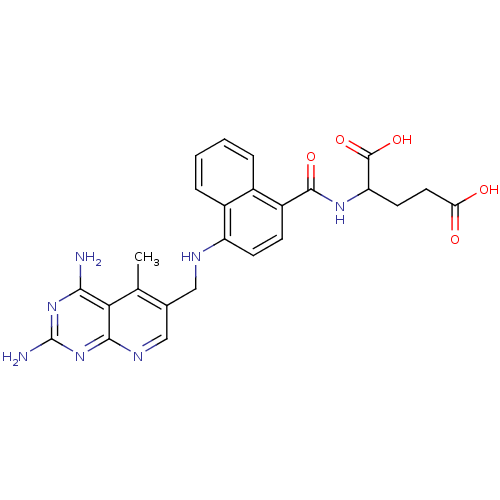
(2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...)Show SMILES Cc1c(CNc2ccc(C(=O)NC(CCC(O)=O)C(O)=O)c3ccccc23)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C25H25N7O5/c1-12-13(11-29-22-20(12)21(26)31-25(27)32-22)10-28-17-7-6-16(14-4-2-3-5-15(14)17)23(35)30-18(24(36)37)8-9-19(33)34/h2-7,11,18,28H,8-10H2,1H3,(H,30,35)(H,33,34)(H,36,37)(H4,26,27,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043398
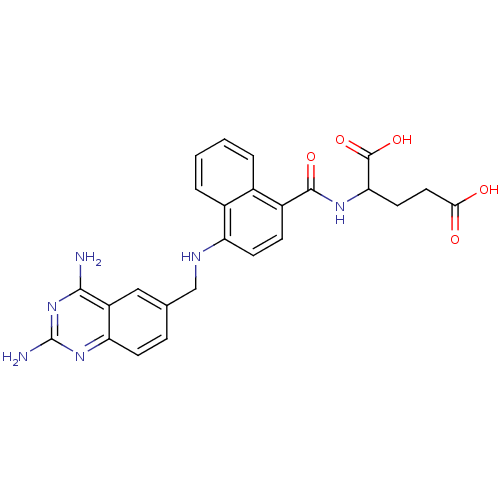
(2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)ccc2n1 Show InChI InChI=1S/C25H24N6O5/c26-22-17-11-13(5-7-19(17)30-25(27)31-22)12-28-18-8-6-16(14-3-1-2-4-15(14)18)23(34)29-20(24(35)36)9-10-21(32)33/h1-8,11,20,28H,9-10,12H2,(H,29,34)(H,32,33)(H,35,36)(H4,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043394
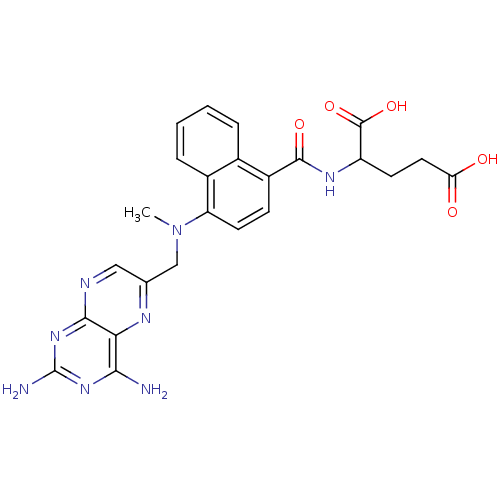
(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C24H24N8O5/c1-32(11-12-10-27-21-19(28-12)20(25)30-24(26)31-21)17-8-6-15(13-4-2-3-5-14(13)17)22(35)29-16(23(36)37)7-9-18(33)34/h2-6,8,10,16H,7,9,11H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,25,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043397
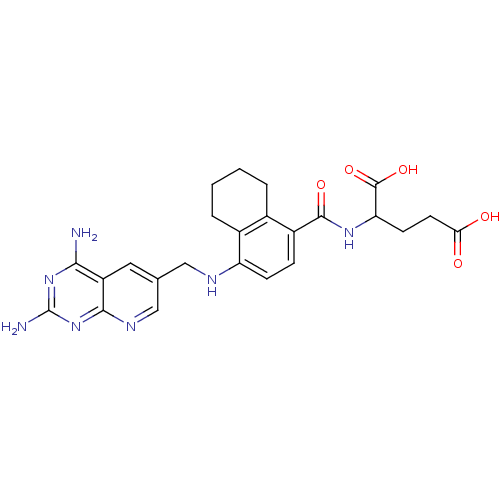
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4CCCCc34)cnc2n1 Show InChI InChI=1S/C24H27N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h5-6,9,11,18,27H,1-4,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
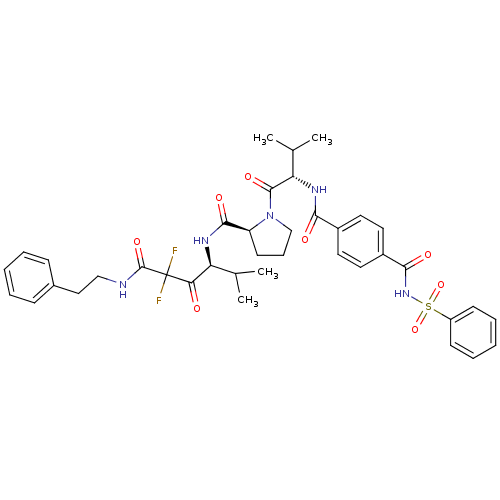
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50546794

(CHEMBL4777335)Show SMILES CC(C)(C)CC(=O)N[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccccn1)C(=O)NC1(CC1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01803
BindingDB Entry DOI: 10.7270/Q2KD22XQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50004544
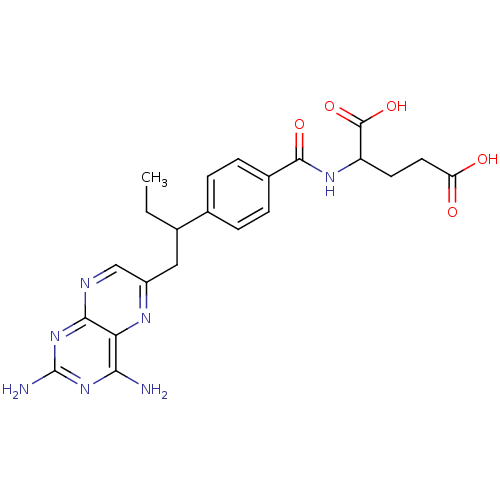
(2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...)Show SMILES CCC(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-2-11(9-14-10-25-19-17(26-14)18(23)28-22(24)29-19)12-3-5-13(6-4-12)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,10-11,15H,2,7-9H2,1H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells |
J Med Chem 35: 3002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2MP527Z |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50279775
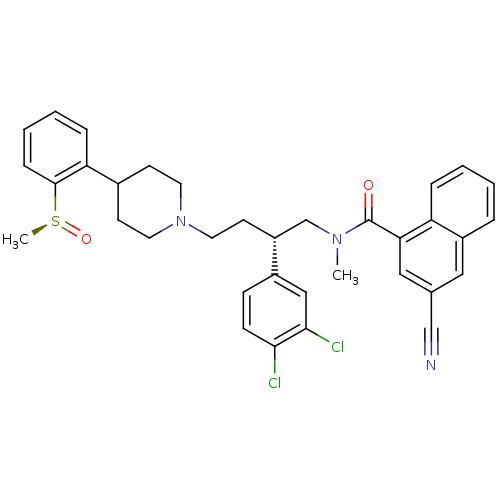
((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C(=O)c1cc(cc2ccccc12)C#N Show InChI InChI=1S/C35H35Cl2N3O2S/c1-39(35(41)31-20-24(22-38)19-27-7-3-4-8-29(27)31)23-28(26-11-12-32(36)33(37)21-26)15-18-40-16-13-25(14-17-40)30-9-5-6-10-34(30)43(2)42/h3-12,19-21,25,28H,13-18,23H2,1-2H3/t28-,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells |
Bioorg Med Chem Lett 11: 2769-73 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1M4W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Rattus norvegicus (Rat)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50279775

((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C(=O)c1cc(cc2ccccc12)C#N Show InChI InChI=1S/C35H35Cl2N3O2S/c1-39(35(41)31-20-24(22-38)19-27-7-3-4-8-29(27)31)23-28(26-11-12-32(36)33(37)21-26)15-18-40-16-13-25(14-17-40)30-9-5-6-10-34(30)43(2)42/h3-12,19-21,25,28H,13-18,23H2,1-2H3/t28-,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells |
J Med Chem 45: 3972-83 (2002)
BindingDB Entry DOI: 10.7270/Q2862H6T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50105595
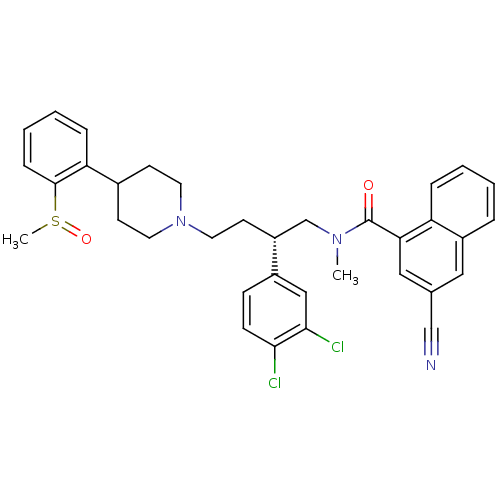
((R,S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)c1ccccc1S(C)=O)c1ccc(Cl)c(Cl)c1)C(=O)c1cc(cc2ccccc12)C#N Show InChI InChI=1S/C35H35Cl2N3O2S/c1-39(35(41)31-20-24(22-38)19-27-7-3-4-8-29(27)31)23-28(26-11-12-32(36)33(37)21-26)15-18-40-16-13-25(14-17-40)30-9-5-6-10-34(30)43(2)42/h3-12,19-21,25,28H,13-18,23H2,1-2H3/t28-,43?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 307-15 (2001)
BindingDB Entry DOI: 10.7270/Q2H130JX |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419142
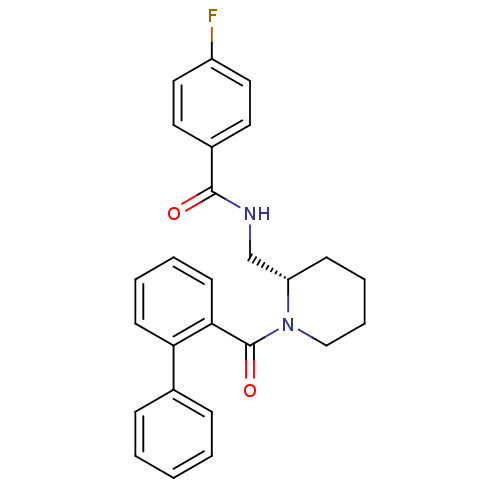
(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642632

(US20230416245, Compound 70)Show SMILES CCNc1cc(nc2n(cnc12)[C@@H]1[C@H]2C[C@@]2([C@@H](O)[C@H]1O)C(=O)NC)C#N |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50065136
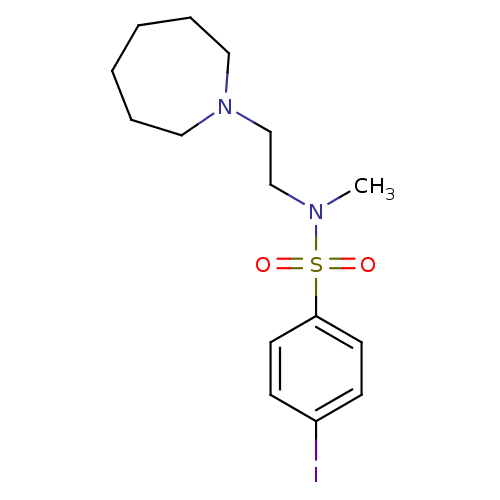
(CHEMBL81455 | N-(2-Azepan-1-yl-ethyl)-4-iodo-N-met...)Show InChI InChI=1S/C15H23IN2O2S/c1-17(12-13-18-10-4-2-3-5-11-18)21(19,20)15-8-6-14(16)7-9-15/h6-9H,2-5,10-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The George Washington University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-(+)-pentazocine binding to sigma-1 receptor in guinea pig brain membranes |
J Med Chem 41: 2445-50 (1998)
Article DOI: 10.1021/jm9800447
BindingDB Entry DOI: 10.7270/Q27P8XHM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50118098
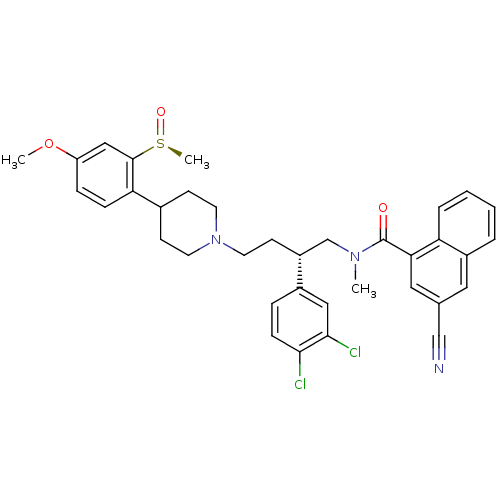
((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...)Show SMILES COc1ccc(C2CCN(CC[C@H](CN(C)C(=O)c3cc(cc4ccccc34)C#N)c3ccc(Cl)c(Cl)c3)CC2)c(c1)[S@](C)=O Show InChI InChI=1S/C36H37Cl2N3O3S/c1-40(36(42)32-19-24(22-39)18-27-6-4-5-7-30(27)32)23-28(26-8-11-33(37)34(38)20-26)14-17-41-15-12-25(13-16-41)31-10-9-29(44-2)21-35(31)45(3)43/h4-11,18-21,25,28H,12-17,23H2,1-3H3/t28-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells |
J Med Chem 45: 3972-83 (2002)
BindingDB Entry DOI: 10.7270/Q2862H6T |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419136
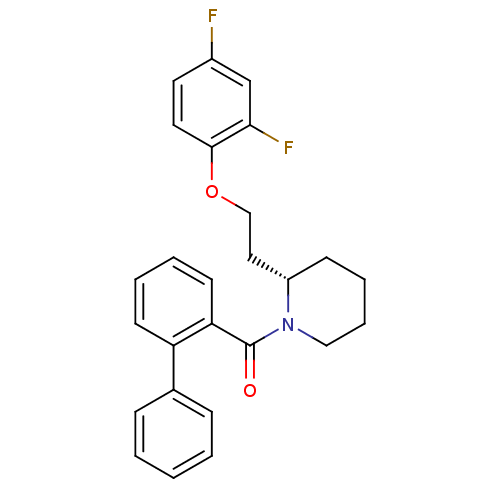
(CHEMBL1830961)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2ccccc2-c2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H25F2NO2/c27-20-13-14-25(24(28)18-20)31-17-15-21-10-6-7-16-29(21)26(30)23-12-5-4-11-22(23)19-8-2-1-3-9-19/h1-5,8-9,11-14,18,21H,6-7,10,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50118100
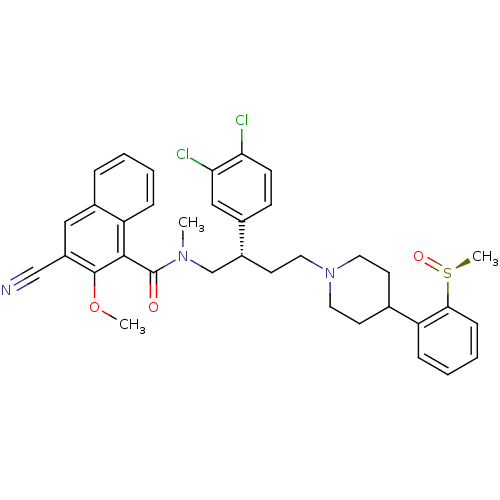
(3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...)Show SMILES COc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C36H37Cl2N3O3S/c1-40(36(42)34-30-10-5-4-8-26(30)20-28(22-39)35(34)44-2)23-27(25-12-13-31(37)32(38)21-25)16-19-41-17-14-24(15-18-41)29-9-6-7-11-33(29)45(3)43/h4-13,20-21,24,27H,14-19,23H2,1-3H3/t27-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells |
J Med Chem 45: 3972-83 (2002)
BindingDB Entry DOI: 10.7270/Q2862H6T |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50065134
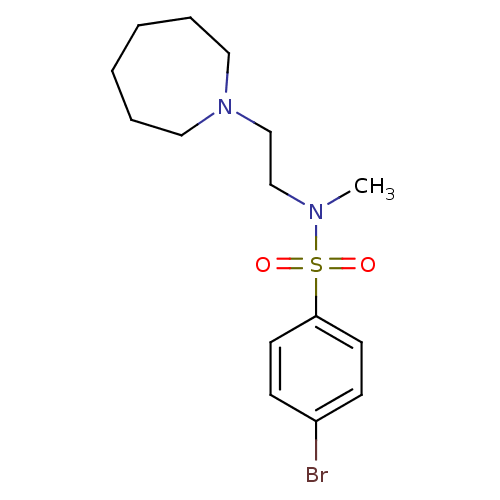
(CHEMBL309983 | N-(2-Azepan-1-yl-ethyl)-4-bromo-N-m...)Show InChI InChI=1S/C15H23BrN2O2S/c1-17(12-13-18-10-4-2-3-5-11-18)21(19,20)15-8-6-14(16)7-9-15/h6-9H,2-5,10-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The George Washington University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-(+)-pentazocine binding to sigma-1 receptor in guinea pig brain membranes |
J Med Chem 41: 2445-50 (1998)
Article DOI: 10.1021/jm9800447
BindingDB Entry DOI: 10.7270/Q27P8XHM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Glutamine synthetase
(Homo sapiens (Human)) | BDBM85288
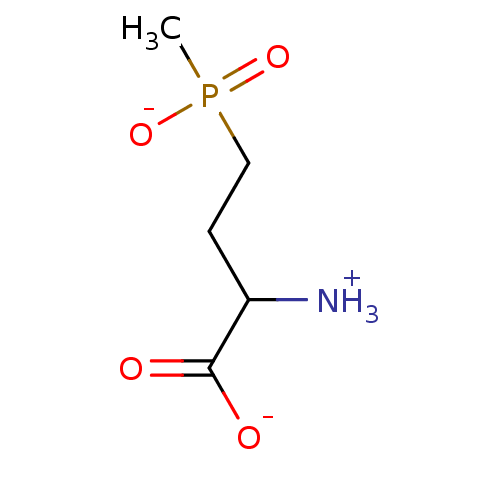
(Phosphinothricin analog, 1 (L Isomer))Show InChI InChI=1S/C5H12NO4P/c1-11(9,10)3-2-4(6)5(7)8/h4H,2-3,6H2,1H3,(H,7,8)(H,9,10)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Chevron Chemical Company
| Assay Description
The enzyme was assayed at pH 7.0 using the pyruvate kinase/lactate dehydroenase coupling system. |
Bioorg Chem 18: 154-9 (1990)
BindingDB Entry DOI: 10.7270/Q24J0CNF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642621
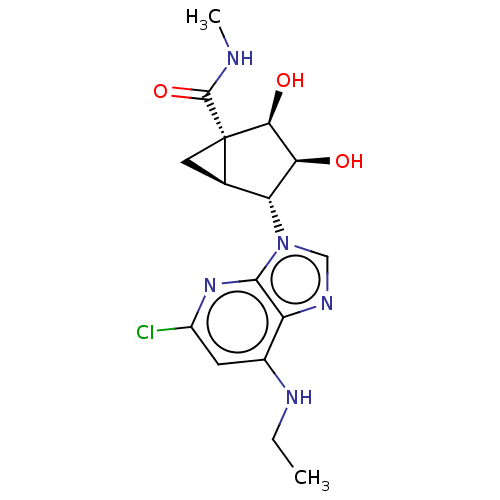
(US20230416245, Compound 59)Show SMILES CCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@]2([C@@H](O)[C@H]1O)C(=O)NC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from morphine-insensitive Opioid receptor delta 1 (presence of 50 nM morphine) of rat brain membranes |
J Med Chem 41: 2872-81 (1998)
Article DOI: 10.1021/jm980083i
BindingDB Entry DOI: 10.7270/Q24F1RFN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
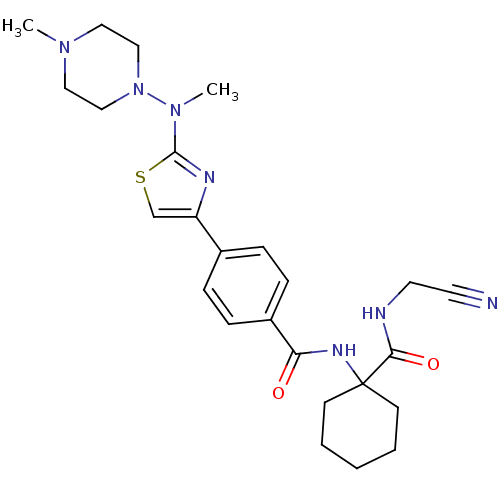
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419142

(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
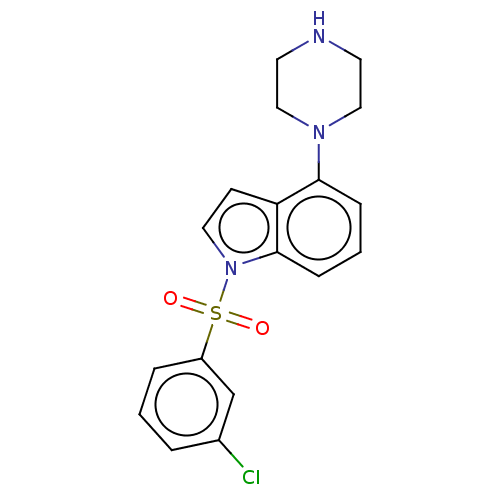
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50118099
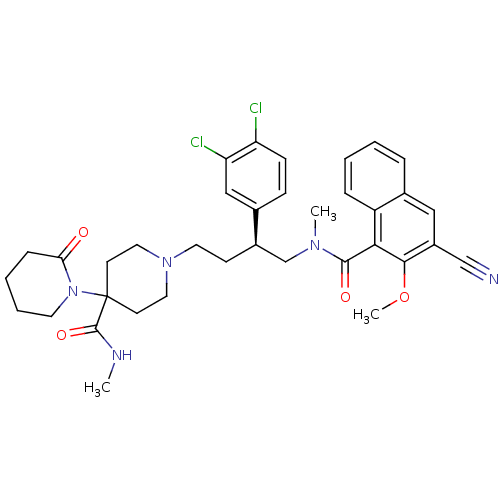
(1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...)Show SMILES CNC(=O)C1(CCN(CC[C@H](CN(C)C(=O)c2c(OC)c(cc3ccccc23)C#N)c2ccc(Cl)c(Cl)c2)CC1)N1CCCCC1=O Show InChI InChI=1S/C36H41Cl2N5O4/c1-40-35(46)36(43-16-7-6-10-31(43)44)14-18-42(19-15-36)17-13-26(24-11-12-29(37)30(38)21-24)23-41(2)34(45)32-28-9-5-4-8-25(28)20-27(22-39)33(32)47-3/h4-5,8-9,11-12,20-21,26H,6-7,10,13-19,23H2,1-3H3,(H,40,46)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells |
J Med Chem 45: 3972-83 (2002)
BindingDB Entry DOI: 10.7270/Q2862H6T |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
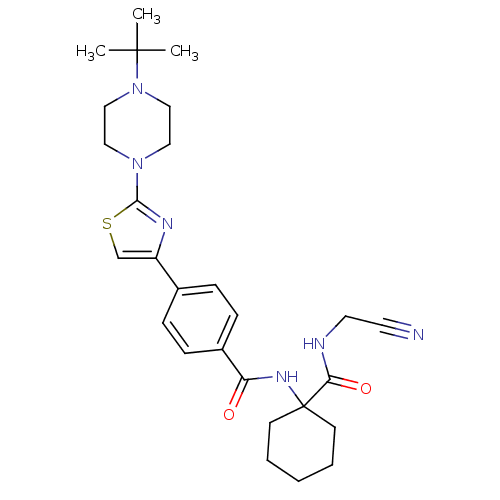
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 of rat brain membranes (DAMGO quenching mu receptor) |
J Med Chem 41: 2872-81 (1998)
Article DOI: 10.1021/jm980083i
BindingDB Entry DOI: 10.7270/Q24F1RFN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50175494
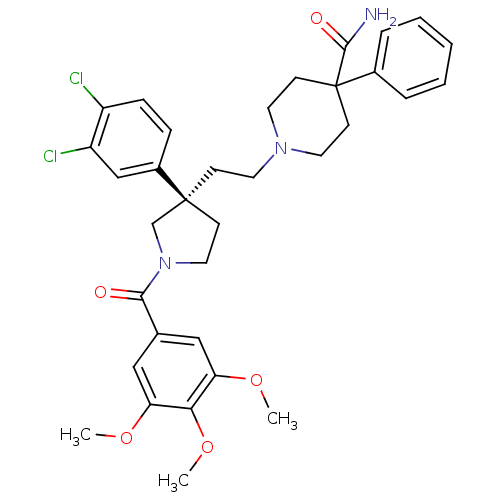
(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells |
Bioorg Med Chem Lett 11: 2769-73 (2001)
BindingDB Entry DOI: 10.7270/Q2SJ1M4W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50401082
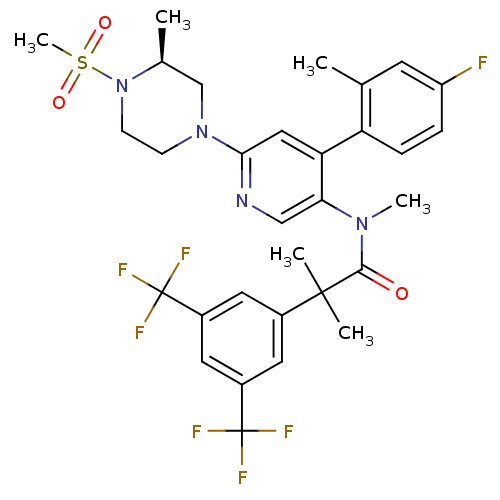
(CHEMBL2203704)Show SMILES C[C@H]1CN(CCN1S(C)(=O)=O)c1cc(c(cn1)N(C)C(=O)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)-c1ccc(F)cc1C |r| Show InChI InChI=1S/C31H33F7N4O3S/c1-18-11-23(32)7-8-24(18)25-15-27(41-9-10-42(19(2)17-41)46(6,44)45)39-16-26(25)40(5)28(43)29(3,4)20-12-21(30(33,34)35)14-22(13-20)31(36,37)38/h7-8,11-16,19H,9-10,17H2,1-6H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Displacement of radioligand [3H]SP from wild type human NK1 receptor expressed in HEK293 cells |
J Med Chem 55: 5061-76 (2012)
Article DOI: 10.1021/jm2017072
BindingDB Entry DOI: 10.7270/Q2959JP4 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699
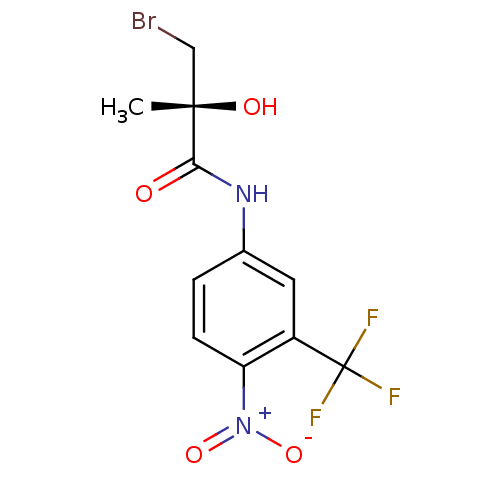
((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418481
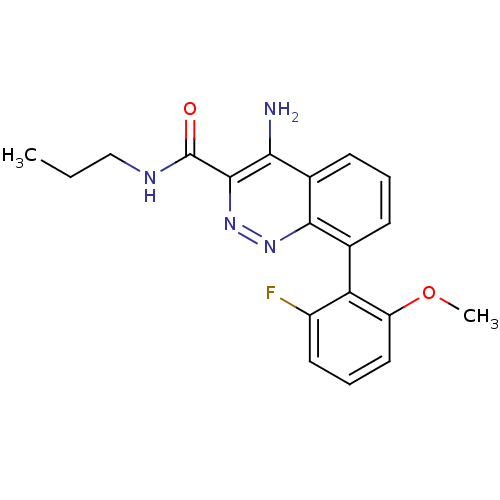
(CHEMBL1783282)Show SMILES CCCNC(=O)c1nnc2c(cccc2c1N)-c1c(F)cccc1OC |(3.52,-21.84,;2.19,-22.61,;.85,-21.84,;-.48,-22.61,;-1.81,-21.84,;-1.81,-20.3,;-3.15,-22.61,;-3.16,-24.17,;-4.5,-24.95,;-5.84,-24.18,;-7.18,-24.95,;-8.51,-24.18,;-8.51,-22.63,;-7.18,-21.86,;-5.85,-22.63,;-4.51,-21.84,;-4.52,-20.3,;-7.18,-26.49,;-5.85,-27.25,;-4.52,-26.47,;-5.85,-28.78,;-7.19,-29.56,;-8.52,-28.78,;-8.52,-27.25,;-9.85,-26.47,;-9.84,-24.93,)| Show InChI InChI=1S/C19H19FN4O2/c1-3-10-22-19(25)18-16(21)12-7-4-6-11(17(12)23-24-18)15-13(20)8-5-9-14(15)26-2/h4-9H,3,10H2,1-2H3,(H2,21,23)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
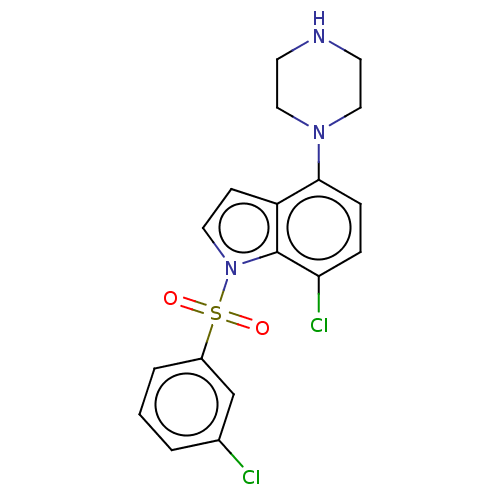
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
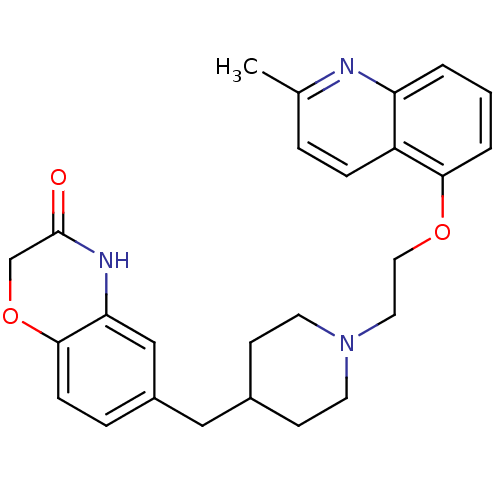
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
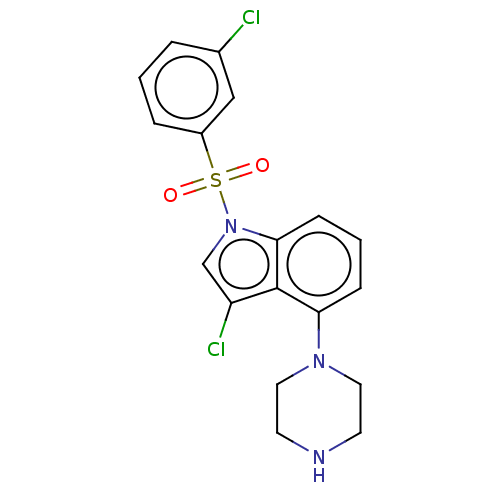
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50521576
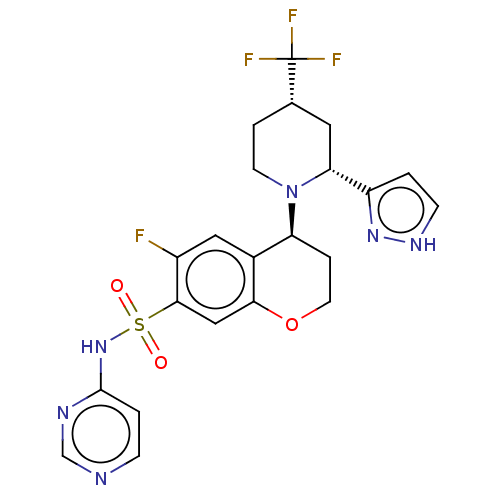
(CHEMBL4462278)Show SMILES Fc1cc2[C@H](CCOc2cc1S(=O)(=O)Nc1ccncn1)N1CC[C@@H](C[C@@H]1c1cc[nH]n1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F4N6O3S/c23-15-10-14-17(32-7-3-13(22(24,25)26)9-18(32)16-1-6-29-30-16)4-8-35-19(14)11-20(15)36(33,34)31-21-2-5-27-12-28-21/h1-2,5-6,10-13,17-18H,3-4,7-9H2,(H,29,30)(H,27,28,31)/t13-,17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Displacement of [3H]GX-545 from full length human Nav1.7 VSD4 domain expressed in HEK cell membranes measured after 20 hrs by liquid scintillation co... |
J Med Chem 62: 4091-4109 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00141
BindingDB Entry DOI: 10.7270/Q2B85CJ3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642624
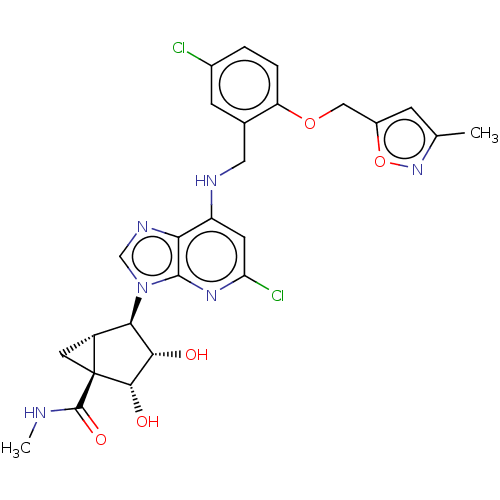
(US20230416245, Compound 62)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50118098

((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...)Show SMILES COc1ccc(C2CCN(CC[C@H](CN(C)C(=O)c3cc(cc4ccccc34)C#N)c3ccc(Cl)c(Cl)c3)CC2)c(c1)[S@](C)=O Show InChI InChI=1S/C36H37Cl2N3O3S/c1-40(36(42)32-19-24(22-39)18-27-6-4-5-7-30(27)32)23-28(26-8-11-33(37)34(38)20-26)14-17-41-15-12-25(13-16-41)31-10-9-29(44-2)21-35(31)45(3)43/h4-11,18-21,25,28H,12-17,23H2,1-3H3/t28-,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) in mouse erythroleukemia cells |
J Med Chem 45: 3972-83 (2002)
BindingDB Entry DOI: 10.7270/Q2862H6T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061028
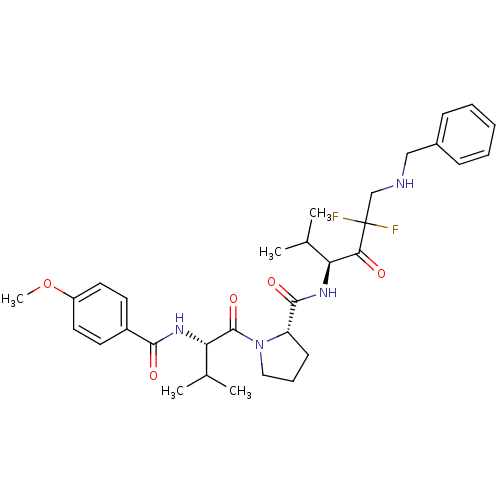
((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...)Show SMILES COc1ccc(cc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)CNCc1ccccc1 Show InChI InChI=1S/C32H42F2N4O5/c1-20(2)26(28(39)32(33,34)19-35-18-22-10-7-6-8-11-22)36-30(41)25-12-9-17-38(25)31(42)27(21(3)4)37-29(40)23-13-15-24(43-5)16-14-23/h6-8,10-11,13-16,20-21,25-27,35H,9,12,17-19H2,1-5H3,(H,36,41)(H,37,40)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data