

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50054502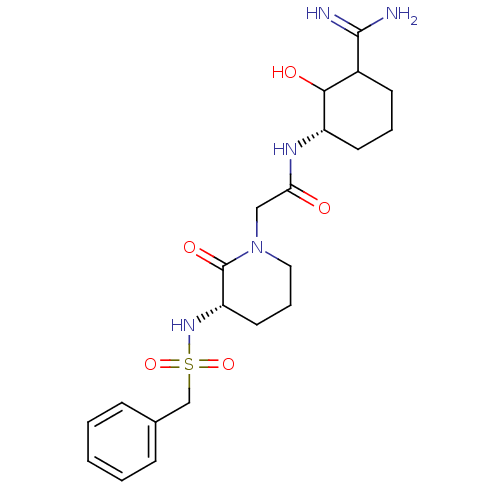 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR2 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated with enzyme followed by peptide substrate addition by ca... | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR3 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR4 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50589205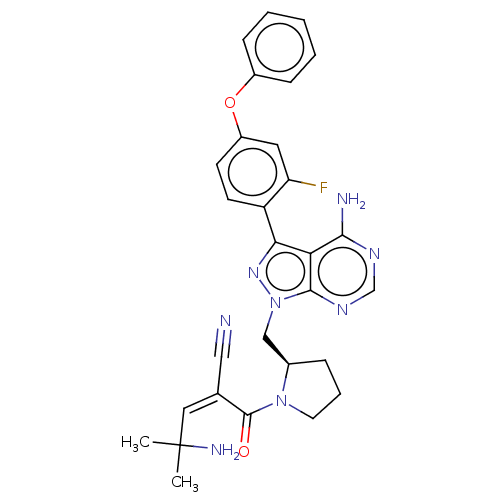 (CHEMBL5174021) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01170 BindingDB Entry DOI: 10.7270/Q2W099WG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384541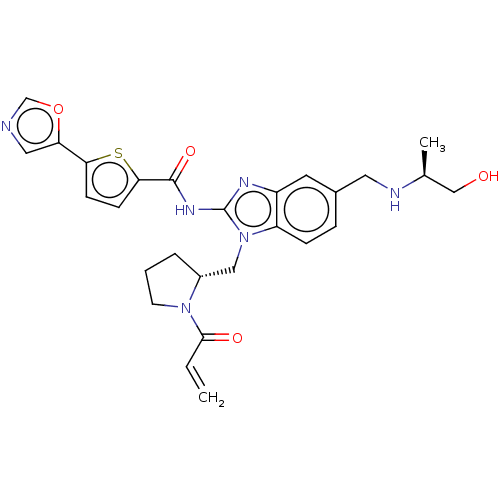 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384539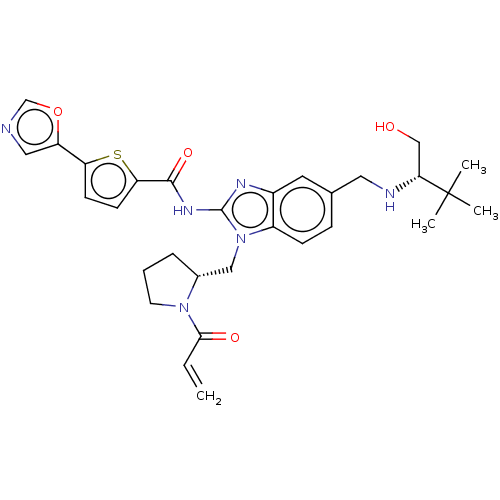 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384538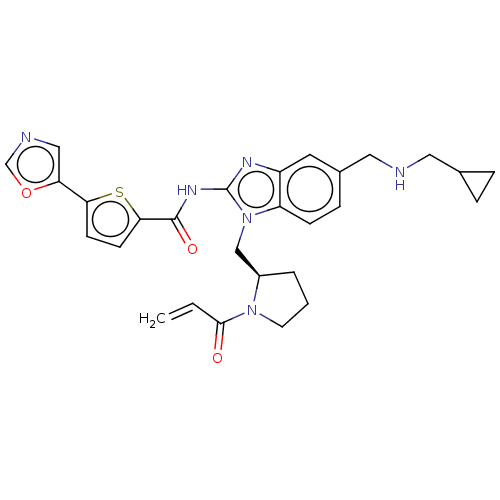 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384543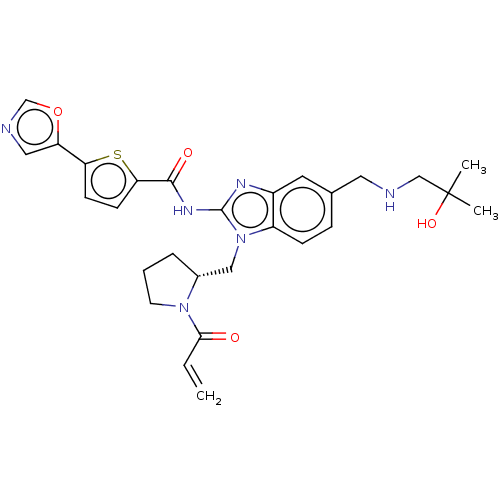 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122417 (US8729078, I-27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384542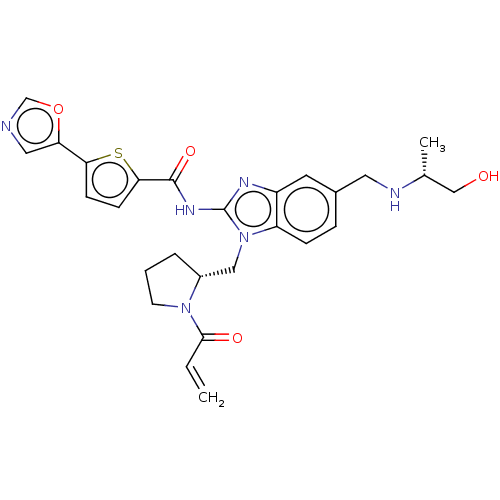 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122415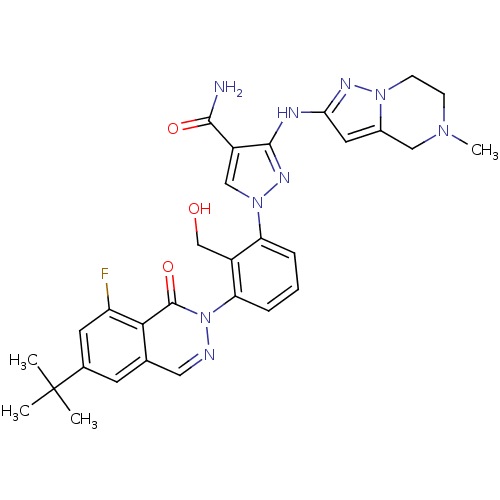 (US8729078, I-25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384540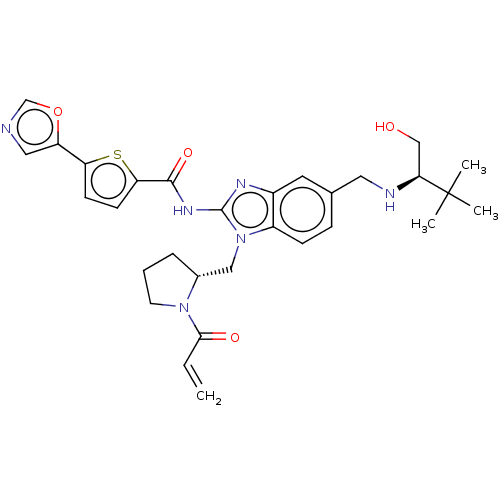 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122442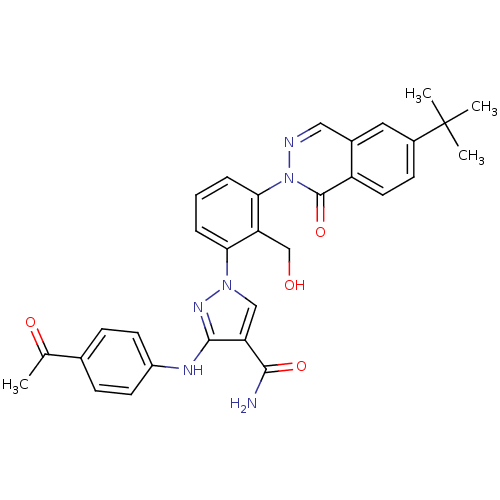 (US8729078, I-53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122428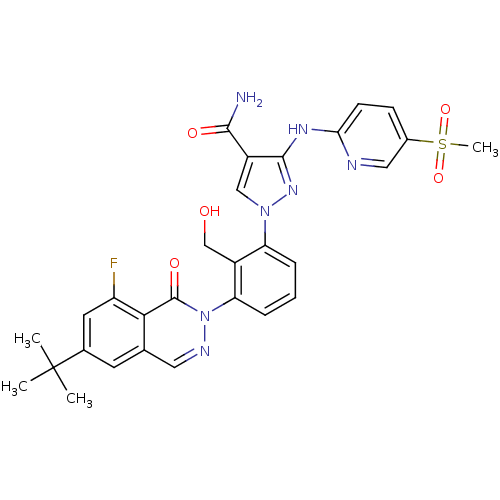 (US8729078, I-38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122429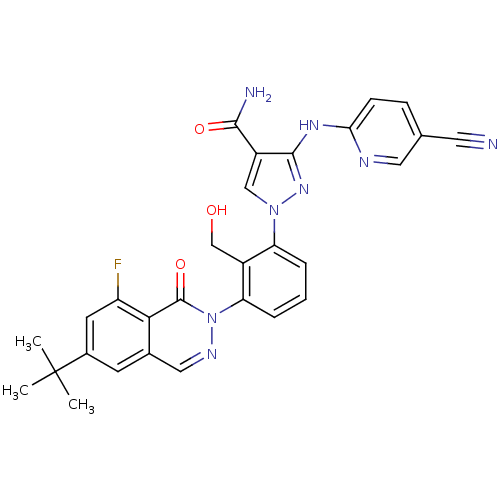 (US8729078, I-39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122414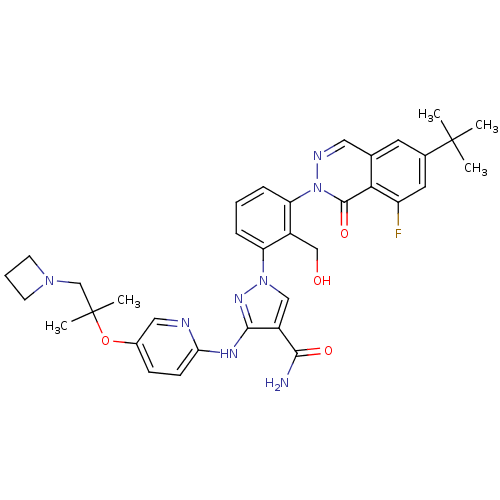 (US8729078, I-24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM521835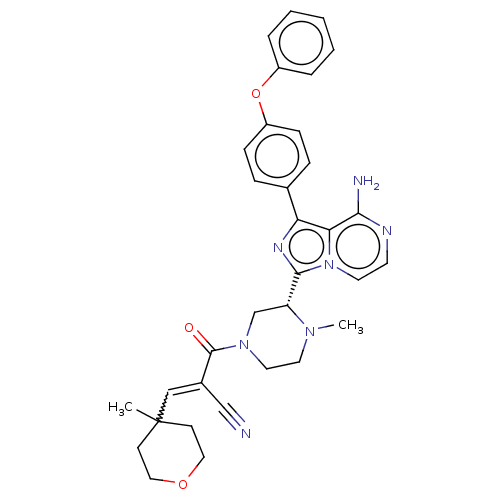 (US11155544, Compound 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... | Citation and Details BindingDB Entry DOI: 10.7270/Q27P92J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384539 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122418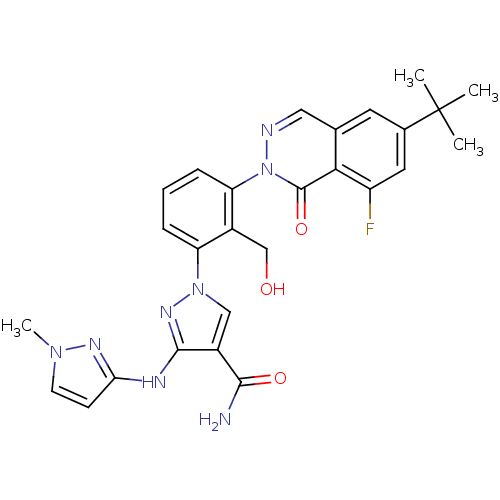 (US8729078, I-28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122423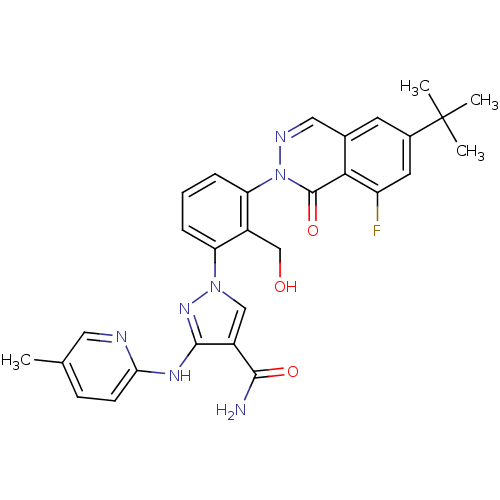 (US8729078, I-33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122420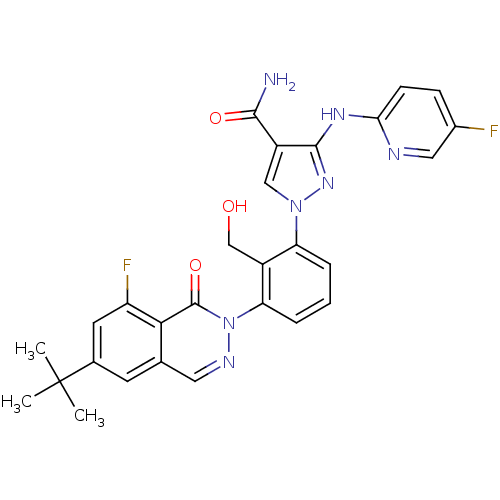 (US8729078, I-30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122413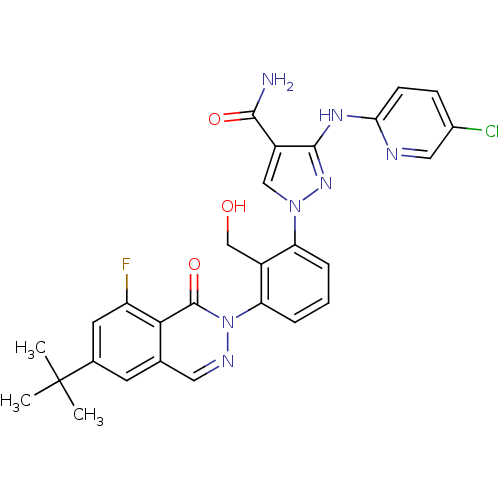 (US8729078, I-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122421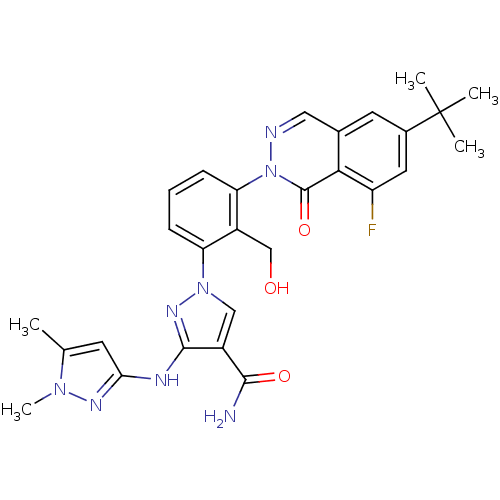 (US8729078, I-31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM143179 (US8940744, 4 | US9266895, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc. US Patent | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | US Patent US8940744 (2015) BindingDB Entry DOI: 10.7270/Q2348J3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50388189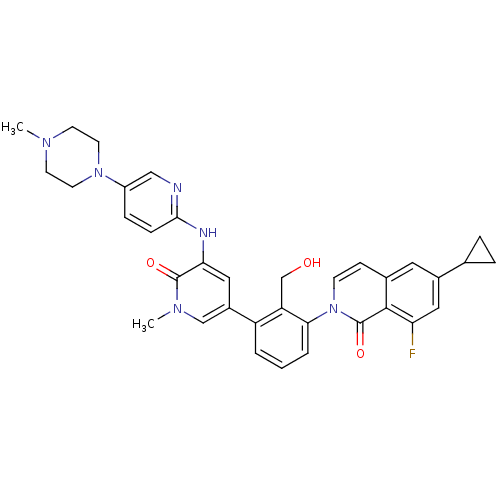 (CHEMBL2057918) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Competitive inhibition of BTK by TR-FRET based competitive assay | J Med Chem 55: 4539-50 (2012) Article DOI: 10.1021/jm300035p BindingDB Entry DOI: 10.7270/Q27H1KMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM143179 (US8940744, 4 | US9266895, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc. US Patent | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | US Patent US9266895 (2016) BindingDB Entry DOI: 10.7270/Q2WD3ZDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM521834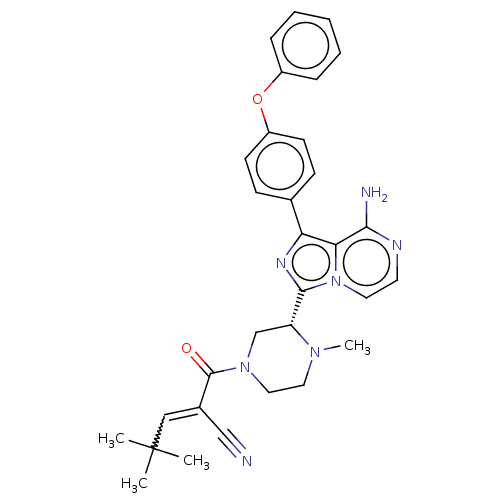 (US11155544, Compound 66) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... | Citation and Details BindingDB Entry DOI: 10.7270/Q27P92J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM397994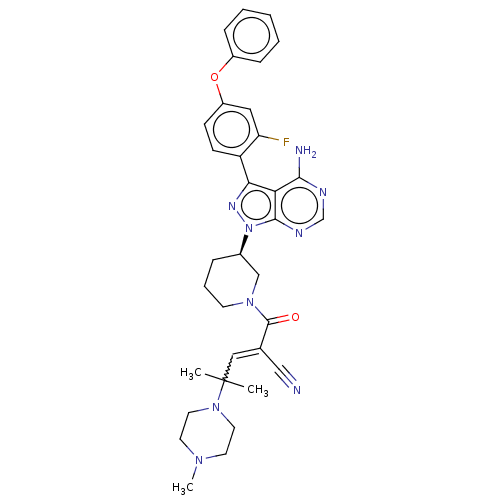 (2-[[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | J Med Chem 52: 3300-7 (2009) BindingDB Entry DOI: 10.7270/Q2057J86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384544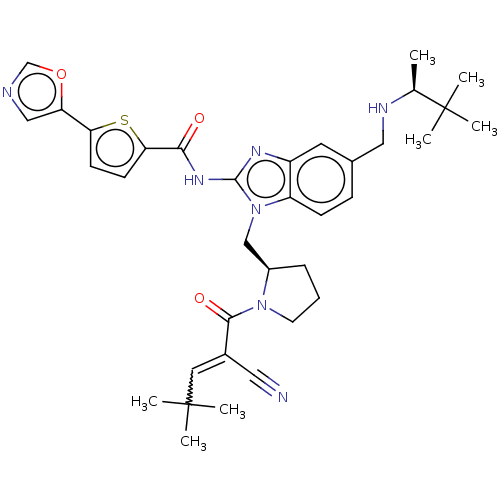 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384543 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384542 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384541 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384538 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM521782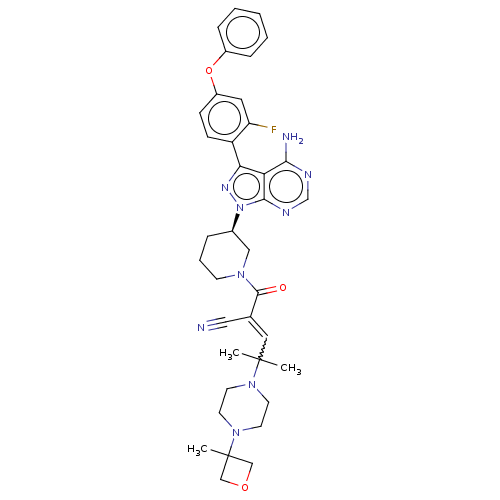 (US11155544, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... | Citation and Details BindingDB Entry DOI: 10.7270/Q27P92J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM504409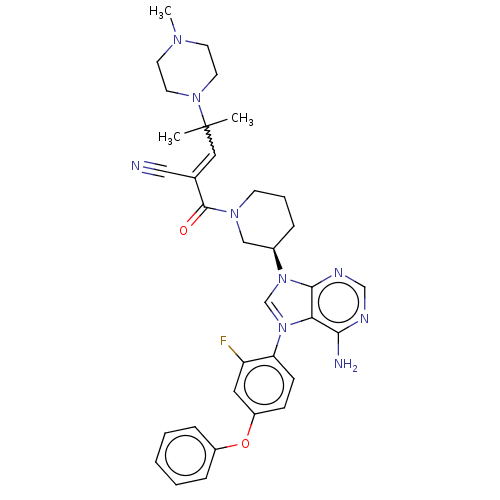 (2-[[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KD2212 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50589186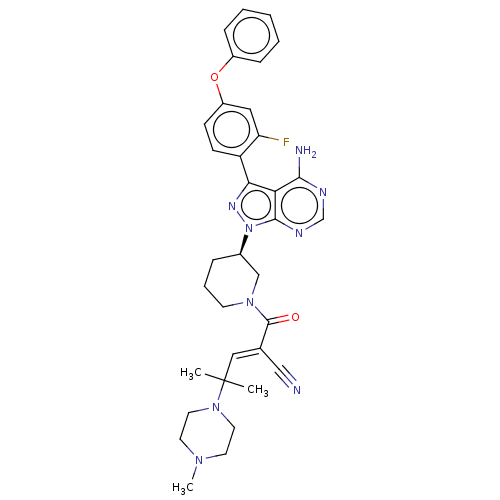 (CHEMBL5189379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01170 BindingDB Entry DOI: 10.7270/Q2W099WG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122416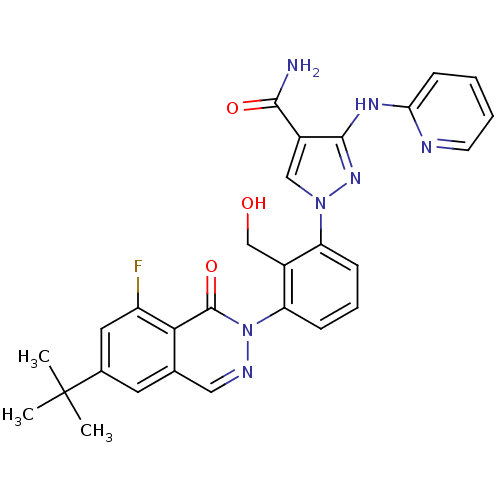 (US8729078, I-26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122444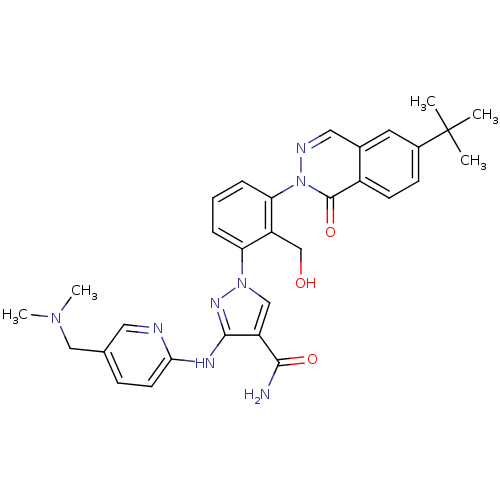 (US8729078, I-55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM287008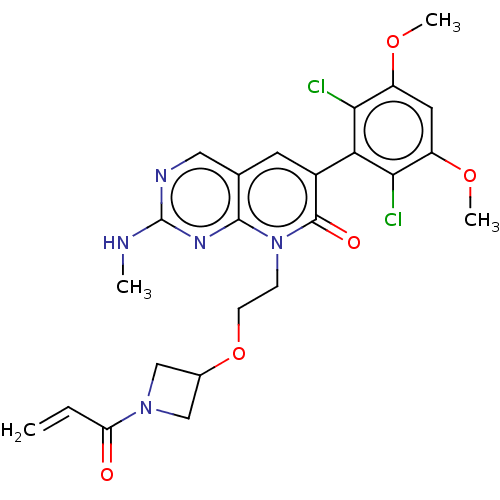 (8-(2-((1-acryloylazetidin-3-yl)oxy)ethyl)-6-(2,6-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description In vitro antagonist activity against rat prostatic androgen receptor (AR) | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM397998 (2-((R)-3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KD2212 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM397998 (2-((R)-3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | J Med Chem 52: 3300-7 (2009) BindingDB Entry DOI: 10.7270/Q2057J86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50589207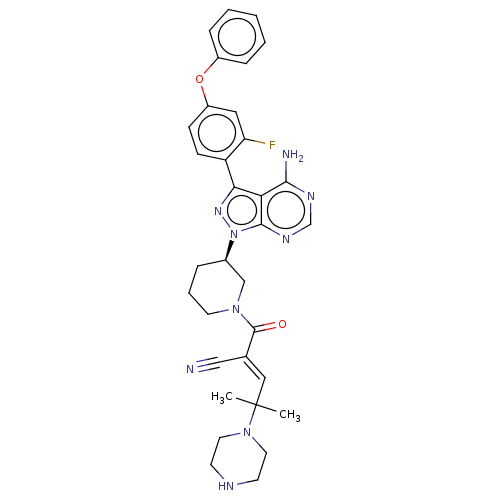 (CHEMBL5169419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01170 BindingDB Entry DOI: 10.7270/Q2W099WG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM143181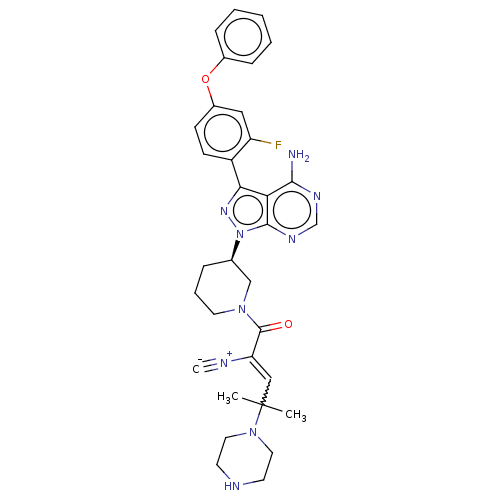 (US8940744, 6 | US9266895, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc. US Patent | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | US Patent US8940744 (2015) BindingDB Entry DOI: 10.7270/Q2348J3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM143181 (US8940744, 6 | US9266895, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc. US Patent | Assay Description A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of the pre... | US Patent US9266895 (2016) BindingDB Entry DOI: 10.7270/Q2WD3ZDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122419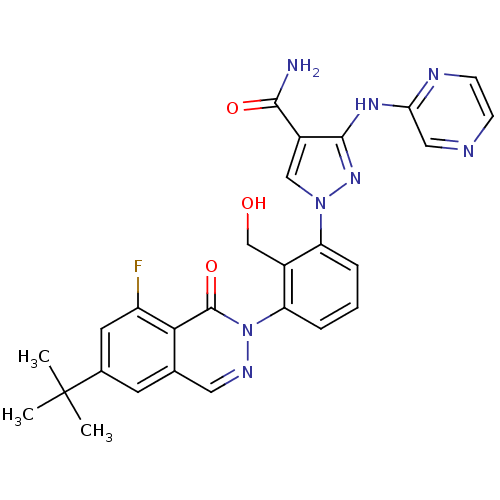 (US8729078, I-29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of BLK | J Med Chem 55: 4539-50 (2012) Article DOI: 10.1021/jm300035p BindingDB Entry DOI: 10.7270/Q27H1KMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50589186 (CHEMBL5189379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01170 BindingDB Entry DOI: 10.7270/Q2W099WG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM50589186 (CHEMBL5189379) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01170 BindingDB Entry DOI: 10.7270/Q2W099WG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 880 total ) | Next | Last >> |