

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (BOVINE) | BDBM50062852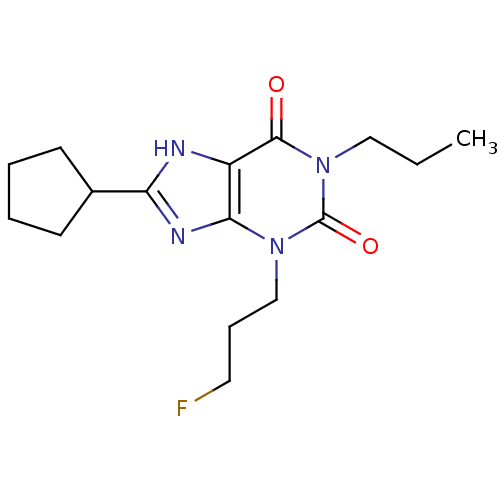 (8-Cyclopentyl-3-(3-fluoro-propyl)-1-propyl-3,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH Curated by ChEMBL | Assay Description In vitro binding affinity for Adenosine A1 receptor of bovine cortex | J Med Chem 45: 5150-6 (2002) BindingDB Entry DOI: 10.7270/Q21J993X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056626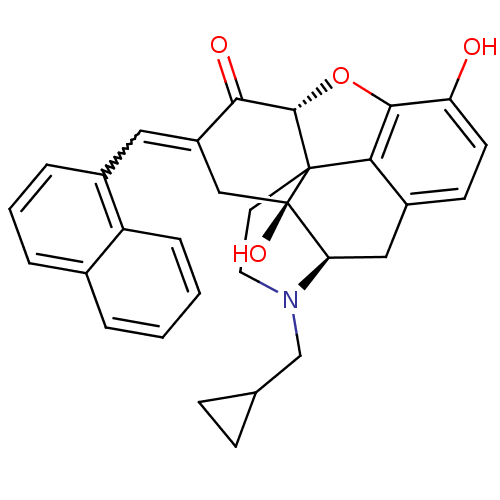 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(1-napht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (RAT) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]U-69593 from Opioid receptor kappa 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056625 (15-[1-(9-anthryl)-(Z)-methylidene]-4-cyclopropylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235385 (APD-811 | Ralinepag) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50450986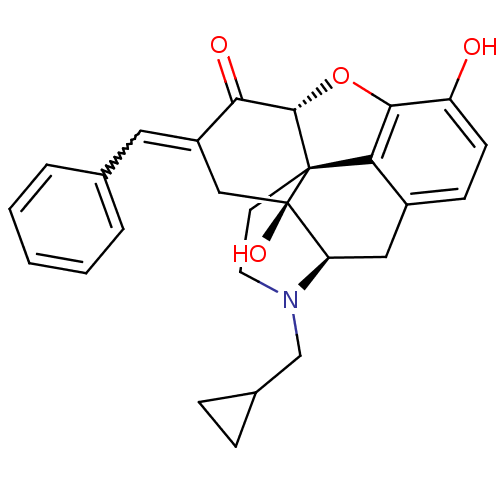 (CHEMBL101519) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056626 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(1-napht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509592 (CHEMBL4460946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50454798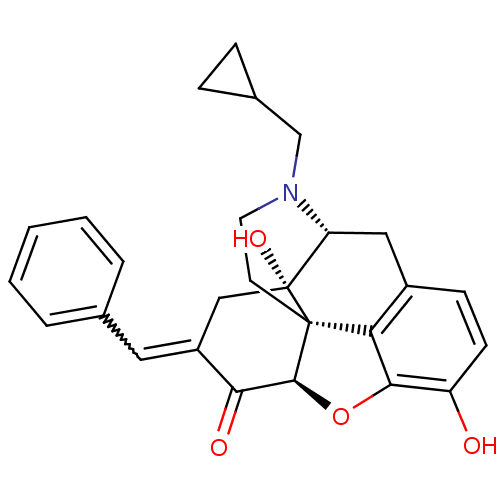 (7-Benzylidenenaltrexone | BNTX) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50235370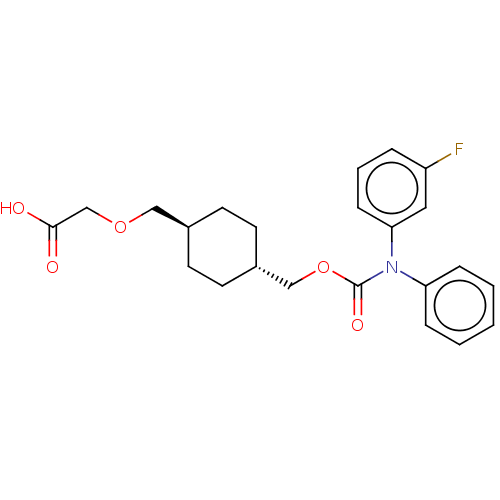 (CHEMBL3933704) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457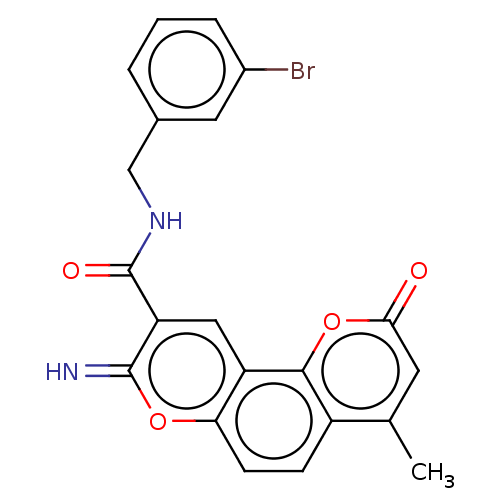 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as free enzyme preincubated for 5 mins followed by su... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50056626 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(1-napht...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50013025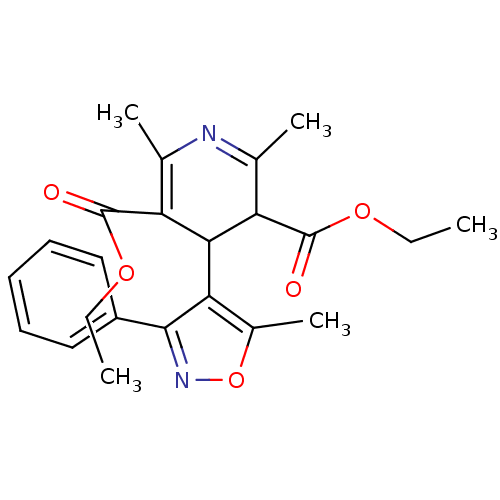 (2,6-Dimethyl-4-(5-methyl-3-phenyl-isoxazol-4-yl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]-PN-200110 binding to Calcium channel in guinea pig heart membrane | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056632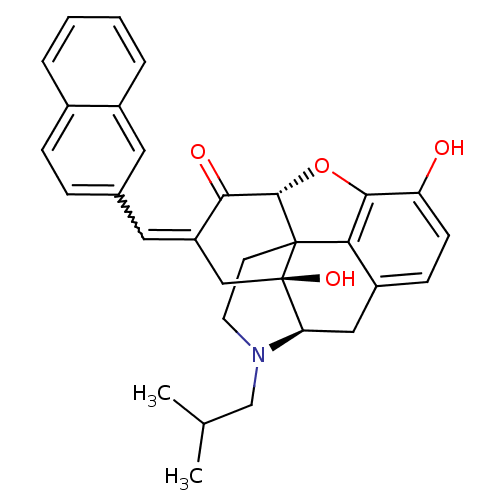 (10,17-dihydroxy-4-isobutyl-15-[1-(2-naphthyl)-(Z)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056625 (15-[1-(9-anthryl)-(Z)-methylidene]-4-cyclopropylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509581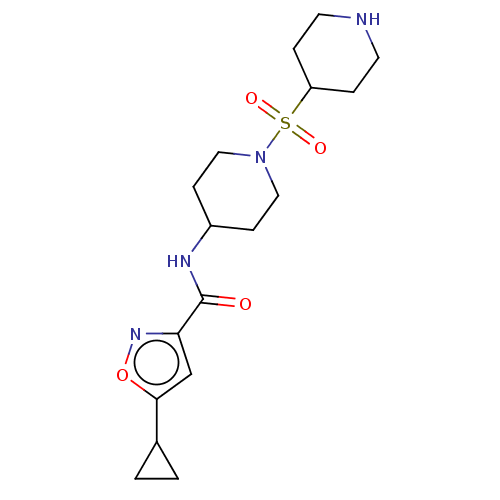 (CHEMBL4535915) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509609 (CHEMBL4476167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Lineweaver-Burk plot analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of PTP1B | Eur J Med Chem 44: 3147-57 (2009) Article DOI: 10.1016/j.ejmech.2009.03.009 BindingDB Entry DOI: 10.7270/Q2FJ2GTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50326165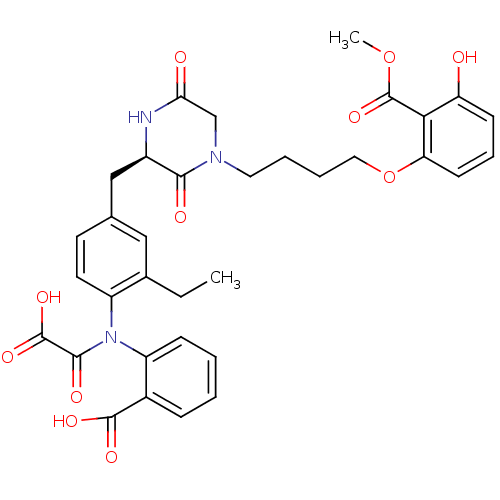 (CHEMBL1241315 | oxalylaminobenzoic acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins by spectrophotometry | Eur J Med Chem 45: 3709-18 (2010) Article DOI: 10.1016/j.ejmech.2010.05.020 BindingDB Entry DOI: 10.7270/Q24Q7V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509609 (CHEMBL4476167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate complex preincubated for 5 mins f... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50013025 (2,6-Dimethyl-4-(5-methyl-3-phenyl-isoxazol-4-yl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]-PN-200110 binding to Calcium channel in guinea pig heart membrane. | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50056627 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(2-napht...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50454798 (7-Benzylidenenaltrexone | BNTX) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50013022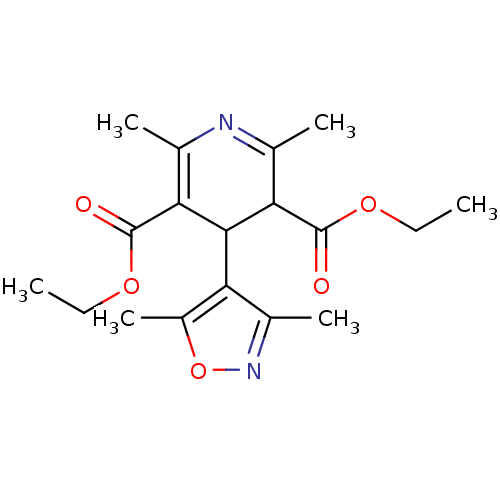 (4-(3,5-Dimethyl-isoxazol-4-yl)-2,6-dimethyl-1,4-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]-PN-200110 binding to Calcium channel in guinea pig heart membrane | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50013022 (4-(3,5-Dimethyl-isoxazol-4-yl)-2,6-dimethyl-1,4-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]-PN-200110 binding to Calcium channel in guinea pig heart membrane. | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50013022 (4-(3,5-Dimethyl-isoxazol-4-yl)-2,6-dimethyl-1,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]PN-200110 binding to Calcium channel in guinea pig ileum membrane. | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50013022 (4-(3,5-Dimethyl-isoxazol-4-yl)-2,6-dimethyl-1,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]PN-200110 binding to Calcium channel in guinea pig ileum membrane | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50450986 (CHEMBL101519) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50056625 (15-[1-(9-anthryl)-(Z)-methylidene]-4-cyclopropylme...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50450986 (CHEMBL101519) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]U-69593 from Opioid receptor kappa 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50056626 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(1-napht...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50056626 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(1-napht...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]U-69593 from Opioid receptor kappa 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056627 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(2-napht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056629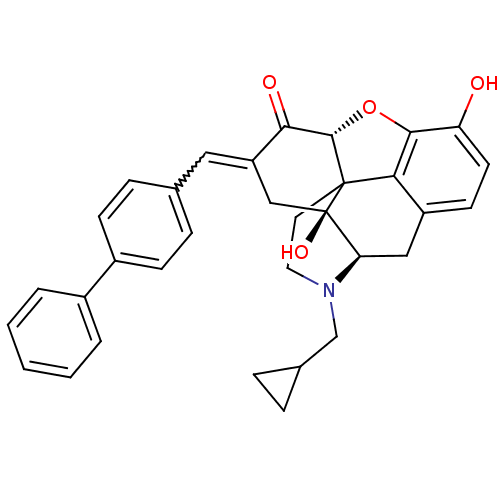 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50454798 (7-Benzylidenenaltrexone | BNTX) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]U-69593 from Opioid receptor kappa 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50056629 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(4-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50056632 (10,17-dihydroxy-4-isobutyl-15-[1-(2-naphthyl)-(Z)-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DAMGO from Opioid receptor mu 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (RAT) | BDBM50235370 (CHEMBL3933704) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50013021 (4-(5-Ethyl-3-methyl-isoxazol-4-yl)-2,6-dimethyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]-PN-200110 binding to Calcium channel in guinea pig heart membrane | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Homo sapiens (Human)) | BDBM50013021 (4-(5-Ethyl-3-methyl-isoxazol-4-yl)-2,6-dimethyl-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibition of [3H]PN-200110 binding to Calcium channel in guinea pig ileum membrane | J Med Chem 33: 2255-9 (1990) BindingDB Entry DOI: 10.7270/Q2KS6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of TCPTP | Eur J Med Chem 44: 3147-57 (2009) Article DOI: 10.1016/j.ejmech.2009.03.009 BindingDB Entry DOI: 10.7270/Q2FJ2GTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50326165 (CHEMBL1241315 | oxalylaminobenzoic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant TCPTP after 30 mins by spectrophotometry | Eur J Med Chem 45: 3709-18 (2010) Article DOI: 10.1016/j.ejmech.2010.05.020 BindingDB Entry DOI: 10.7270/Q24Q7V6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50056627 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(2-napht...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]U-69593 from Opioid receptor kappa 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50056629 (4-cyclopropylmethyl-10,17-dihydroxy-15-[1-(4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Binding affinity was determined in a crude membrane preparation from guinea pig brain by displacement of [3H]DPDPE from Opioid receptor delta 1 | J Med Chem 40: 749-53 (1997) Article DOI: 10.1021/jm960573f BindingDB Entry DOI: 10.7270/Q29887P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (RAT) | BDBM50235385 (APD-811 | Ralinepag) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3026 total ) | Next | Last >> |