 Found 178 hits with Last Name = 'pansare' and Initial = 'n'
Found 178 hits with Last Name = 'pansare' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402859
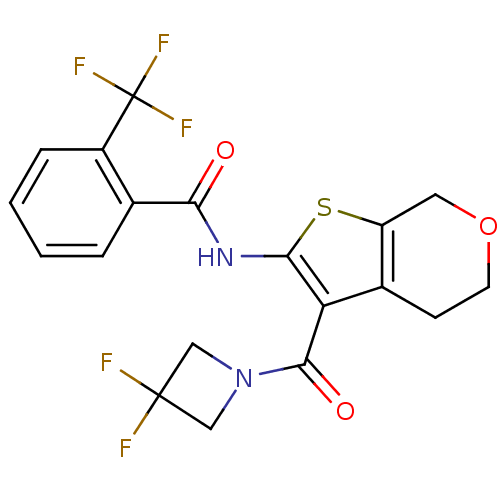
(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402864
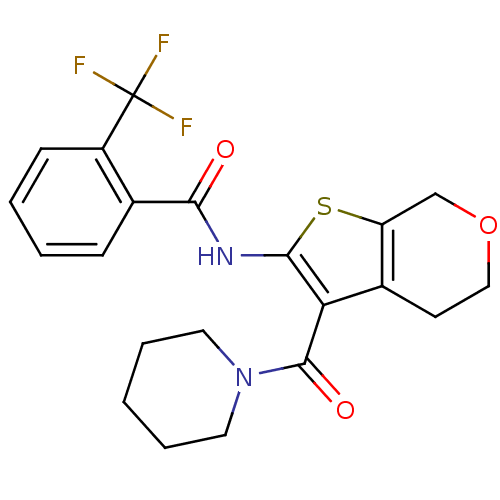
(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402863
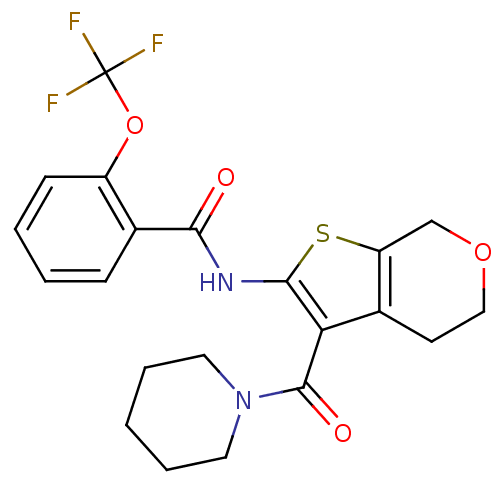
(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 899 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402861
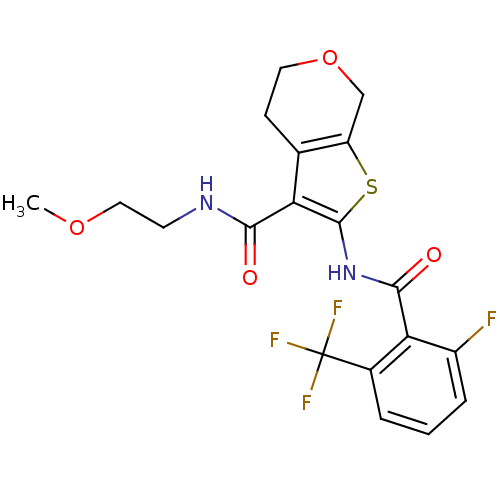
(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 904 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402864

(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402861

(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402863

(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402864

(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402864

(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402863

(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402863

(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402861

(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402861

(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50600442
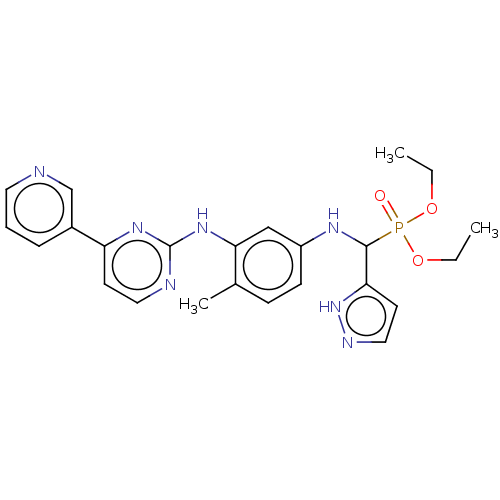
(CHEMBL5200042)Show SMILES CCOP(=O)(OCC)C(Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1)c1ccn[nH]1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50600441
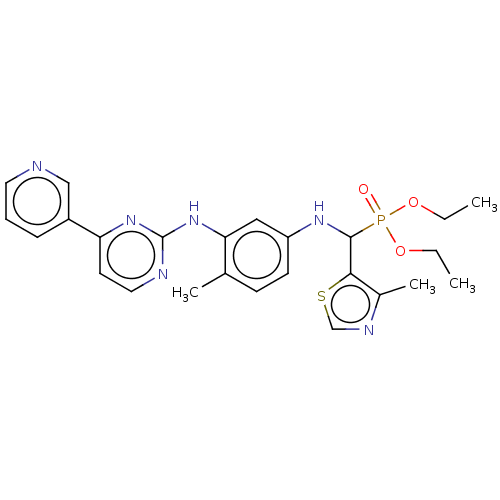
(CHEMBL5180210)Show SMILES CCOP(=O)(OCC)C(Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1)c1scnc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50600442

(CHEMBL5200042)Show SMILES CCOP(=O)(OCC)C(Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1)c1ccn[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50600443

(CHEMBL5173220)Show SMILES CCOP(=O)(OCC)C(Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1)c1ccc[nH]1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50600441

(CHEMBL5180210)Show SMILES CCOP(=O)(OCC)C(Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1)c1scnc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50600443

(CHEMBL5173220)Show SMILES CCOP(=O)(OCC)C(Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1)c1ccc[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128747
BindingDB Entry DOI: 10.7270/Q2FF3XDT |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50543963
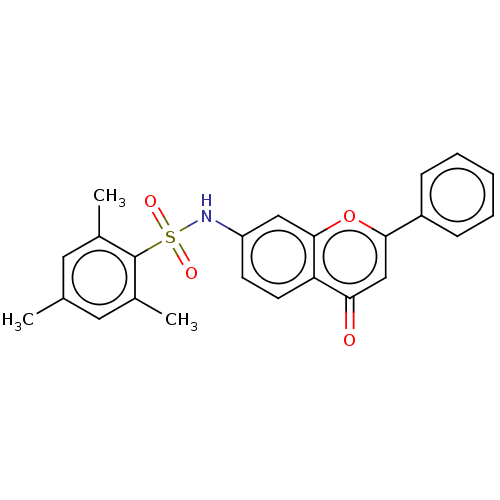
(CHEMBL4636680)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C24H21NO4S/c1-15-11-16(2)24(17(3)12-15)30(27,28)25-19-9-10-20-21(26)14-22(29-23(20)13-19)18-7-5-4-6-8-18/h4-14,25H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50543962
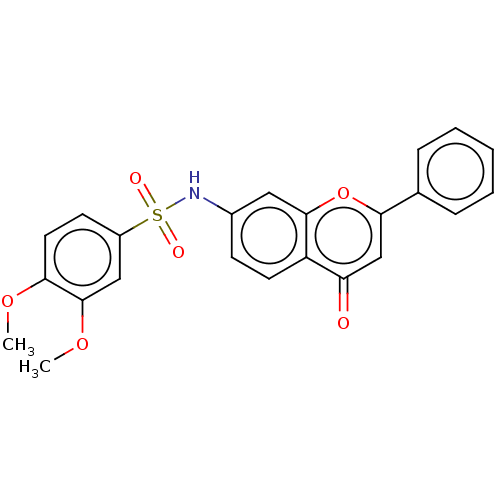
(CHEMBL4642846)Show SMILES COc1ccc(cc1OC)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C23H19NO6S/c1-28-20-11-9-17(13-23(20)29-2)31(26,27)24-16-8-10-18-19(25)14-21(30-22(18)12-16)15-6-4-3-5-7-15/h3-14,24H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50543960
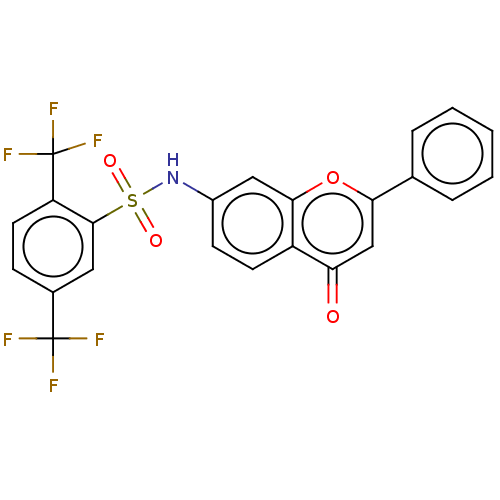
(CHEMBL4633308)Show SMILES FC(F)(F)c1ccc(c(c1)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C23H13F6NO4S/c24-22(25,26)14-6-9-17(23(27,28)29)21(10-14)35(32,33)30-15-7-8-16-18(31)12-19(34-20(16)11-15)13-4-2-1-3-5-13/h1-12,30H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50543961
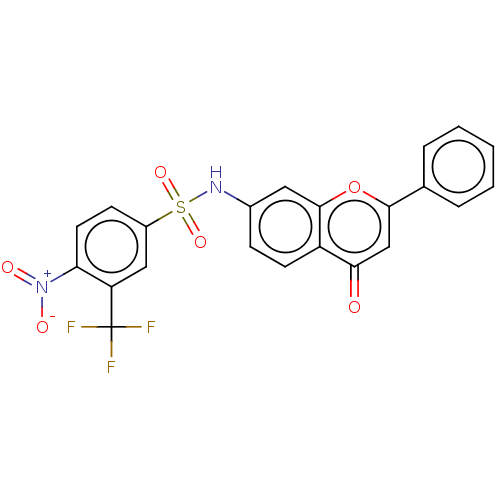
(CHEMBL4647427)Show SMILES [O-][N+](=O)c1ccc(cc1C(F)(F)F)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C22H13F3N2O6S/c23-22(24,25)17-11-15(7-9-18(17)27(29)30)34(31,32)26-14-6-8-16-19(28)12-20(33-21(16)10-14)13-4-2-1-3-5-13/h1-12,26H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50543958

(CHEMBL4643603)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C22H14F3NO4S/c23-22(24,25)15-7-4-8-17(11-15)31(28,29)26-16-9-10-18-19(27)13-20(30-21(18)12-16)14-5-2-1-3-6-14/h1-13,26H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50543959
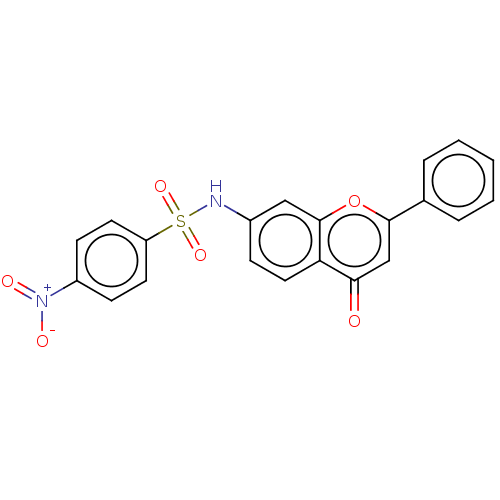
(CHEMBL4636706)Show SMILES [O-][N+](=O)c1ccc(cc1)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C21H14N2O6S/c24-19-13-20(14-4-2-1-3-5-14)29-21-12-15(6-11-18(19)21)22-30(27,28)17-9-7-16(8-10-17)23(25)26/h1-13,22H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127246
BindingDB Entry DOI: 10.7270/Q2WQ07CR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50402865
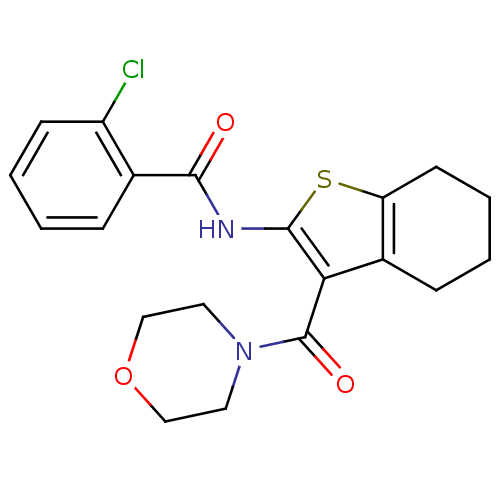
(CHEMBL2205621)Show InChI InChI=1S/C20H21ClN2O3S/c21-15-7-3-1-5-13(15)18(24)22-19-17(14-6-2-4-8-16(14)27-19)20(25)23-9-11-26-12-10-23/h1,3,5,7H,2,4,6,8-12H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at mouse CB2 receptor after 4 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor after 4 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402866
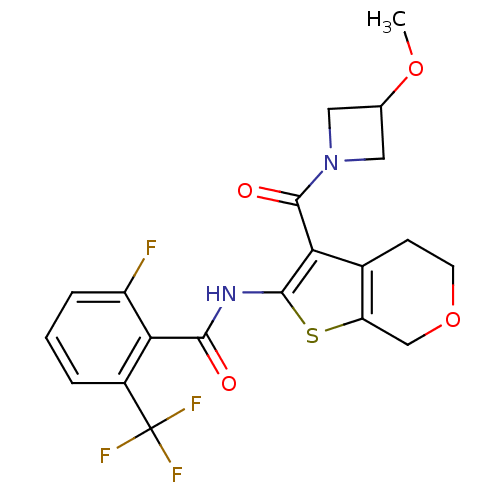
(CHEMBL2205590)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H18F4N2O4S/c1-29-10-7-26(8-10)19(28)15-11-5-6-30-9-14(11)31-18(15)25-17(27)16-12(20(22,23)24)3-2-4-13(16)21/h2-4,10H,5-9H2,1H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor after 4 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data