 Found 200 hits with Last Name = 'pausch' and Initial = 'mh'
Found 200 hits with Last Name = 'pausch' and Initial = 'mh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(RAT) | BDBM85011
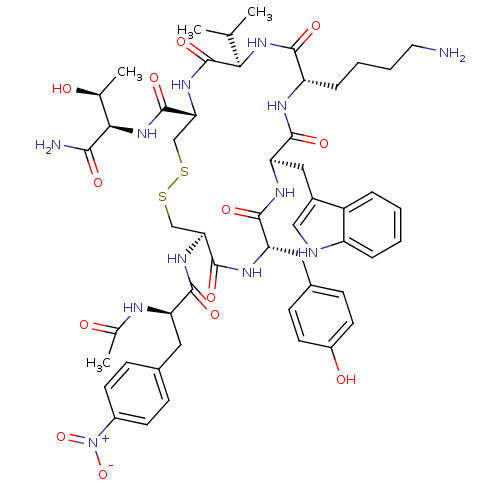
(AcNH-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-NH...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O Show InChI InChI=1S/C52H68N12O13S2/c1-27(2)43-52(75)61-42(51(74)63-44(28(3)65)45(54)68)26-79-78-25-41(60-47(70)38(56-29(4)66)21-30-12-16-33(17-13-30)64(76)77)50(73)58-39(22-31-14-18-34(67)19-15-31)48(71)59-40(23-32-24-55-36-10-6-5-9-35(32)36)49(72)57-37(46(69)62-43)11-7-8-20-53/h5-6,9-10,12-19,24,27-28,37-44,55,65,67H,7-8,11,20-23,25-26,53H2,1-4H3,(H2,54,68)(H,56,66)(H,57,72)(H,58,73)(H,59,71)(H,60,70)(H,61,75)(H,62,69)(H,63,74)/t28-,37-,38+,39-,40+,41-,42+,43+,44+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM50019568
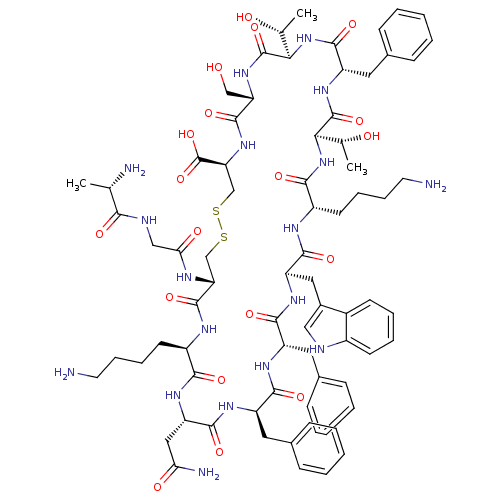
(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM85009
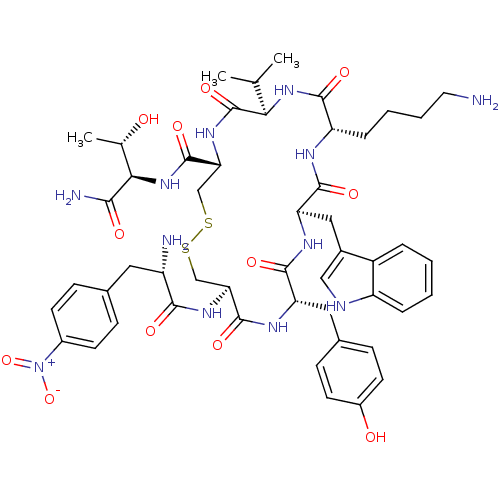
(H2N-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Val-Cys]-Tyr-Nh2)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](N)Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C50H66N12O12S2/c1-26(2)41-50(72)59-40(49(71)61-42(27(3)63)43(53)65)25-76-75-24-39(58-44(66)34(52)20-28-11-15-31(16-12-28)62(73)74)48(70)56-37(21-29-13-17-32(64)18-14-29)46(68)57-38(22-30-23-54-35-9-5-4-8-33(30)35)47(69)55-36(45(67)60-41)10-6-7-19-51/h4-5,8-9,11-18,23,26-27,34,36-42,54,63-64H,6-7,10,19-22,24-25,51-52H2,1-3H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,66)(H,59,72)(H,60,67)(H,61,71)/t27-,34-,36-,37-,38+,39-,40+,41+,42+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084384
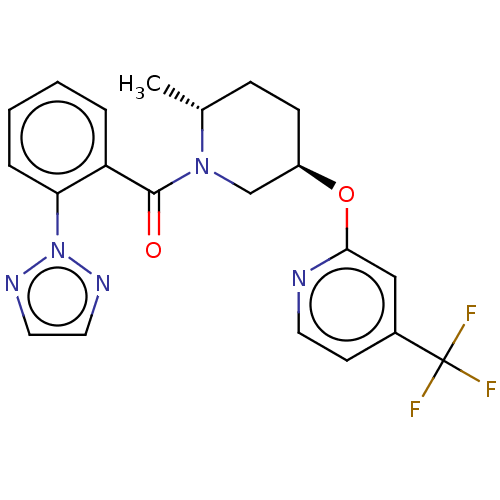
(CHEMBL3426135)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N5O2/c1-14-6-7-16(31-19-12-15(8-9-25-19)21(22,23)24)13-28(14)20(30)17-4-2-3-5-18(17)29-26-10-11-27-29/h2-5,8-12,14,16H,6-7,13H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM85009

(H2N-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Val-Cys]-Tyr-Nh2)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](N)Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C50H66N12O12S2/c1-26(2)41-50(72)59-40(49(71)61-42(27(3)63)43(53)65)25-76-75-24-39(58-44(66)34(52)20-28-11-15-31(16-12-28)62(73)74)48(70)56-37(21-29-13-17-32(64)18-14-29)46(68)57-38(22-30-23-54-35-9-5-4-8-33(30)35)47(69)55-36(45(67)60-41)10-6-7-19-51/h4-5,8-9,11-18,23,26-27,34,36-42,54,63-64H,6-7,10,19-22,24-25,51-52H2,1-3H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,66)(H,59,72)(H,60,67)(H,61,71)/t27-,34-,36-,37-,38+,39-,40+,41+,42+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084394
(CHEMBL3426145)Show SMILES CSc1ccnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c1 |r| Show InChI InChI=1S/C21H23N5O2S/c1-15-7-8-16(28-20-13-17(29-2)9-10-22-20)14-25(15)21(27)18-5-3-4-6-19(18)26-23-11-12-24-26/h3-6,9-13,15-16H,7-8,14H2,1-2H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50316949
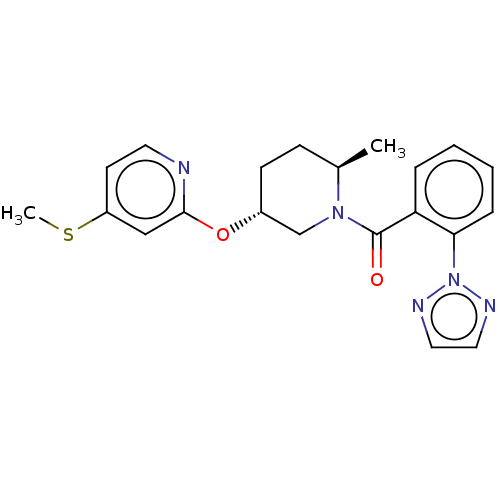
((3R)-3-((3-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl...)Show SMILES O=C1Cc2c(N1)ccc1O[C@H](CN3C4CCC3CC(Cc3ccc5CCC(=O)Nc5c3)C4)COc21 |r| Show InChI InChI=1S/C28H31N3O4/c32-26-8-3-18-2-1-16(12-24(18)30-26)9-17-10-19-4-5-20(11-17)31(19)14-21-15-34-28-22-13-27(33)29-23(22)6-7-25(28)35-21/h1-2,6-7,12,17,19-21H,3-5,8-11,13-15H2,(H,29,33)(H,30,32)/t17?,19?,20?,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM85012
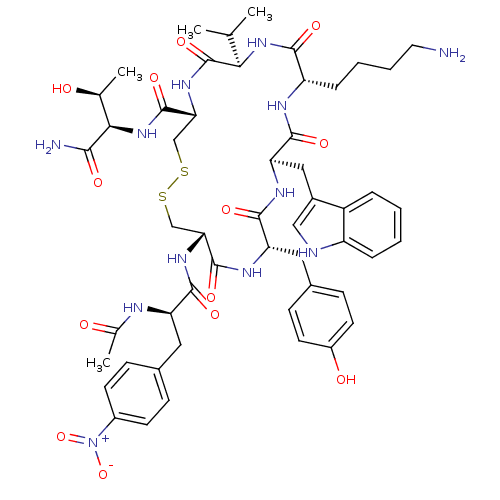
(AcNH-4-NO2-Phe-c[D-Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O Show InChI InChI=1S/C52H68N12O13S2/c1-27(2)43-52(75)61-42(51(74)63-44(28(3)65)45(54)68)26-79-78-25-41(60-47(70)38(56-29(4)66)21-30-12-16-33(17-13-30)64(76)77)50(73)58-39(22-31-14-18-34(67)19-15-31)48(71)59-40(23-32-24-55-36-10-6-5-9-35(32)36)49(72)57-37(46(69)62-43)11-7-8-20-53/h5-6,9-10,12-19,24,27-28,37-44,55,65,67H,7-8,11,20-23,25-26,53H2,1-4H3,(H2,54,68)(H,56,66)(H,57,72)(H,58,73)(H,59,71)(H,60,70)(H,61,75)(H,62,69)(H,63,74)/t28-,37-,38+,39-,40+,41+,42+,43+,44+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084396
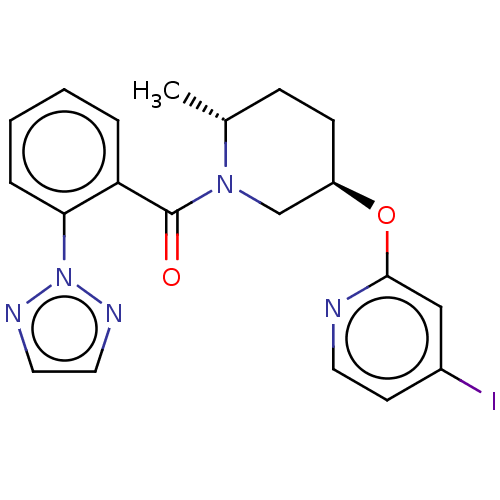
(CHEMBL3426143)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(I)ccn1 |r| Show InChI InChI=1S/C20H20IN5O2/c1-14-6-7-16(28-19-12-15(21)8-9-22-19)13-25(14)20(27)17-4-2-3-5-18(17)26-23-10-11-24-26/h2-5,8-12,14,16H,6-7,13H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302220
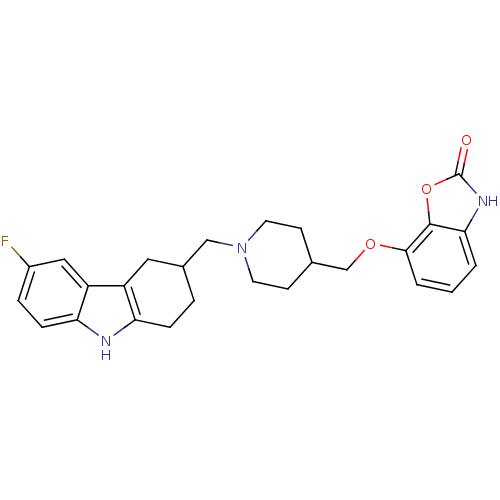
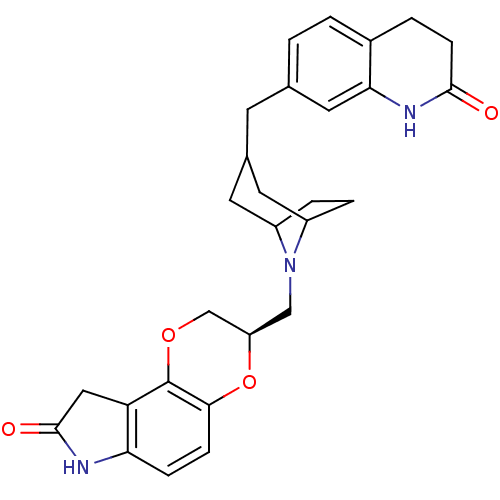
(7-((1-((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-...)Show SMILES Fc1ccc2[nH]c3CCC(CN4CCC(COc5cccc6[nH]c(=O)oc56)CC4)Cc3c2c1 Show InChI InChI=1S/C26H28FN3O3/c27-18-5-7-22-20(13-18)19-12-17(4-6-21(19)28-22)14-30-10-8-16(9-11-30)15-32-24-3-1-2-23-25(24)33-26(31)29-23/h1-3,5,7,13,16-17,28H,4,6,8-12,14-15H2,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM85009

(H2N-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Val-Cys]-Tyr-Nh2)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](N)Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C50H66N12O12S2/c1-26(2)41-50(72)59-40(49(71)61-42(27(3)63)43(53)65)25-76-75-24-39(58-44(66)34(52)20-28-11-15-31(16-12-28)62(73)74)48(70)56-37(21-29-13-17-32(64)18-14-29)46(68)57-38(22-30-23-54-35-9-5-4-8-33(30)35)47(69)55-36(45(67)60-41)10-6-7-19-51/h4-5,8-9,11-18,23,26-27,34,36-42,54,63-64H,6-7,10,19-22,24-25,51-52H2,1-3H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,66)(H,59,72)(H,60,67)(H,61,71)/t27-,34-,36-,37-,38+,39-,40+,41+,42+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084383
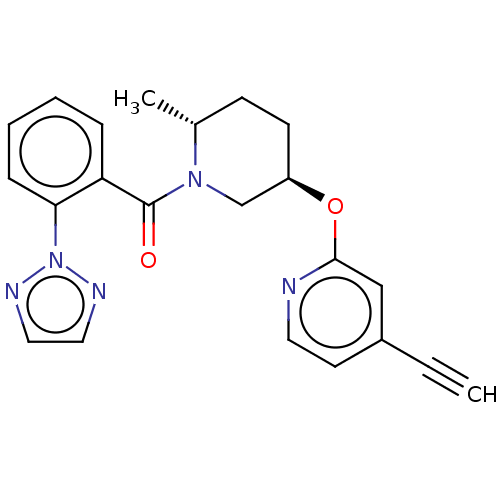
(CHEMBL3426136)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)C#C |r| Show InChI InChI=1S/C22H21N5O2/c1-3-17-10-11-23-21(14-17)29-18-9-8-16(2)26(15-18)22(28)19-6-4-5-7-20(19)27-24-12-13-25-27/h1,4-7,10-14,16,18H,8-9,15H2,2H3/t16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084385

(CHEMBL3426134)Show SMILES CCc1ccnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c1 |r| Show InChI InChI=1S/C22H25N5O2/c1-3-17-10-11-23-21(14-17)29-18-9-8-16(2)26(15-18)22(28)19-6-4-5-7-20(19)27-24-12-13-25-27/h4-7,10-14,16,18H,3,8-9,15H2,1-2H3/t16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084393

(CHEMBL3426146)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H23N5O4S/c1-15-7-8-16(30-20-13-17(9-10-22-20)31(2,28)29)14-25(15)21(27)18-5-3-4-6-19(18)26-23-11-12-24-26/h3-6,9-13,15-16H,7-8,14H2,1-2H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302225
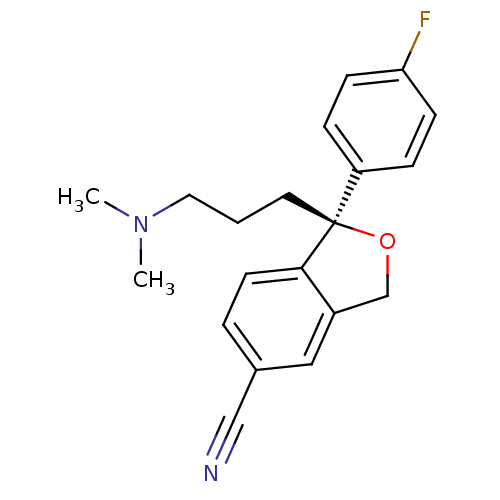
((1S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl...)Show SMILES CN(C)CCC[C@]1(OCc2cc(ccc12)C#N)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302225

((1S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl...)Show SMILES CN(C)CCC[C@]1(OCc2cc(ccc12)C#N)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084395
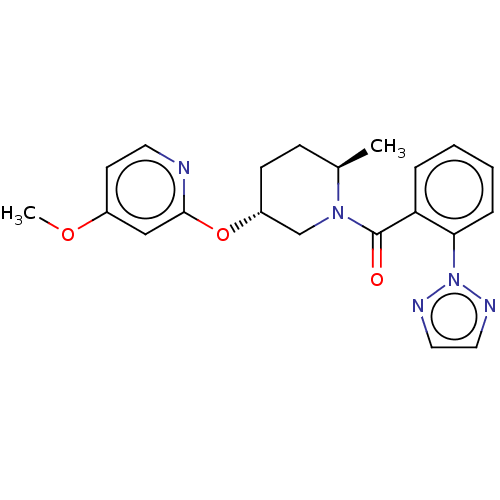
(CHEMBL3426144 | US9745284, 2)Show SMILES COc1ccnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c1 |r| Show InChI InChI=1S/C21H23N5O3/c1-15-7-8-17(29-20-13-16(28-2)9-10-22-20)14-25(15)21(27)18-5-3-4-6-19(18)26-23-11-12-24-26/h3-6,9-13,15,17H,7-8,14H2,1-2H3/t15-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084398
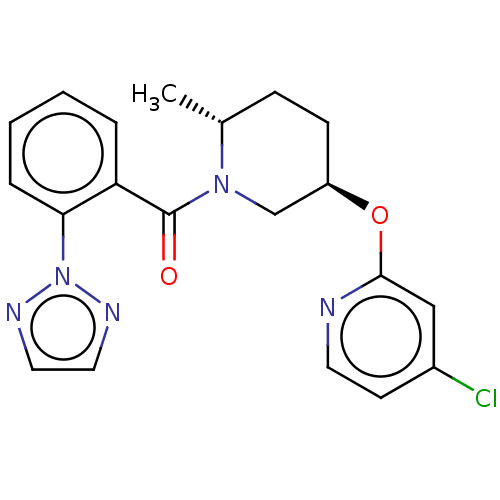
(CHEMBL3426141)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(Cl)ccn1 |r| Show InChI InChI=1S/C20H20ClN5O2/c1-14-6-7-16(28-19-12-15(21)8-9-22-19)13-25(14)20(27)17-4-2-3-5-18(17)26-23-10-11-24-26/h2-5,8-12,14,16H,6-7,13H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50316948
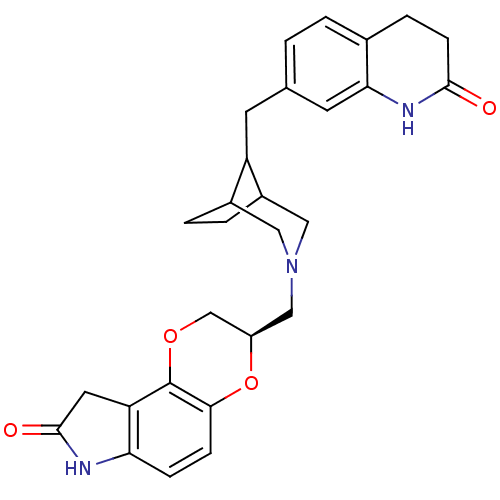
((R)-7-[8-(2-Oxo-1,2,3,4-tetrahydro-quinolin-7-ylme...)Show SMILES O=C1Cc2c(N1)ccc1O[C@H](CN3CC4CCC(C3)C4Cc3ccc4CCC(=O)Nc4c3)COc21 |r| Show InChI InChI=1S/C28H31N3O4/c32-26-8-5-17-2-1-16(10-24(17)30-26)9-21-18-3-4-19(21)13-31(12-18)14-20-15-34-28-22-11-27(33)29-23(22)6-7-25(28)35-20/h1-2,6-7,10,18-21H,3-5,8-9,11-15H2,(H,29,33)(H,30,32)/t18?,19?,20-,21?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM97406

(Orexin receptor antagonist 1 | US20130102619, 1)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)C#N |r| Show InChI InChI=1S/C21H20N6O2/c1-15-6-7-17(29-20-12-16(13-22)8-9-23-20)14-26(15)21(28)18-4-2-3-5-19(18)27-24-10-11-25-27/h2-5,8-12,15,17H,6-7,14H2,1H3/t15-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50316947
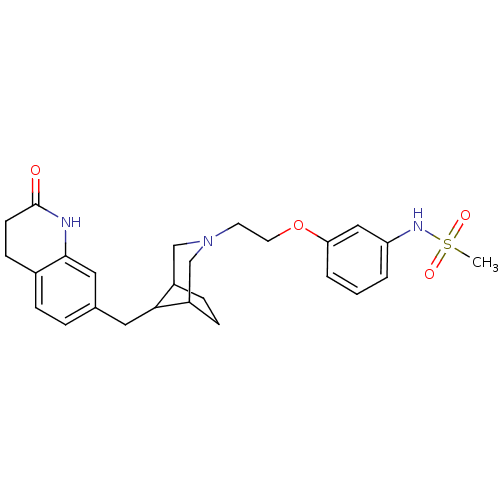
(CHEMBL1087818 | N-(3-(2-(8-((2-oxo-1,2,3,4-tetrahy...)Show SMILES CS(=O)(=O)Nc1cccc(OCCN2CC3CCC(C2)C3Cc2ccc3CCC(=O)Nc3c2)c1 Show InChI InChI=1S/C26H33N3O4S/c1-34(31,32)28-22-3-2-4-23(15-22)33-12-11-29-16-20-7-8-21(17-29)24(20)13-18-5-6-19-9-10-26(30)27-25(19)14-18/h2-6,14-15,20-21,24,28H,7-13,16-17H2,1H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084397

(CHEMBL3426142)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(Br)ccn1 |r| Show InChI InChI=1S/C20H20BrN5O2/c1-14-6-7-16(28-19-12-15(21)8-9-22-19)13-25(14)20(27)17-4-2-3-5-18(17)26-23-10-11-24-26/h2-5,8-12,14,16H,6-7,13H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50316955
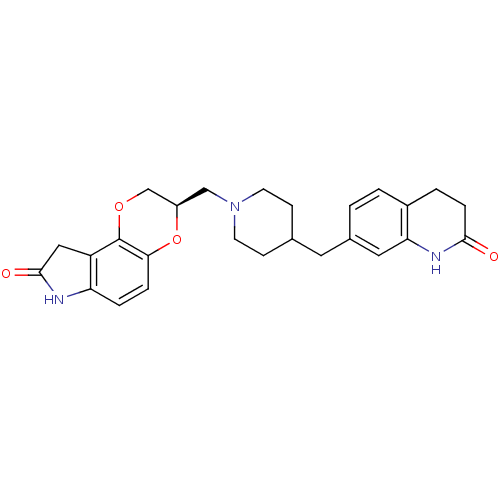
((R)-3-((4-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)...)Show SMILES O=C1Cc2c(N1)ccc1O[C@H](CN3CCC(Cc4ccc5CCC(=O)Nc5c4)CC3)COc21 |r| Show InChI InChI=1S/C26H29N3O4/c30-24-6-3-18-2-1-17(12-22(18)28-24)11-16-7-9-29(10-8-16)14-19-15-32-26-20-13-25(31)27-21(20)4-5-23(26)33-19/h1-2,4-5,12,16,19H,3,6-11,13-15H2,(H,27,31)(H,28,30)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50302213
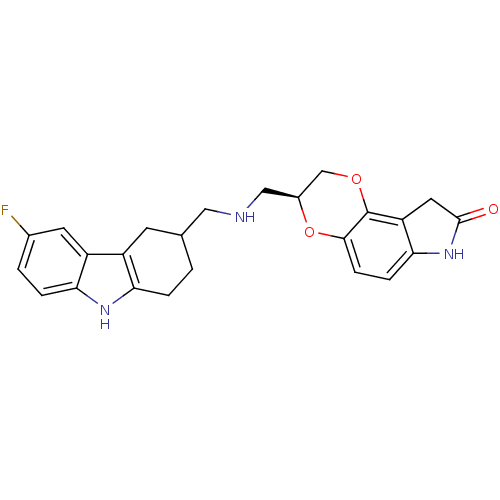
((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM85011

(AcNH-4-NO2-Phe-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-NH...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O Show InChI InChI=1S/C52H68N12O13S2/c1-27(2)43-52(75)61-42(51(74)63-44(28(3)65)45(54)68)26-79-78-25-41(60-47(70)38(56-29(4)66)21-30-12-16-33(17-13-30)64(76)77)50(73)58-39(22-31-14-18-34(67)19-15-31)48(71)59-40(23-32-24-55-36-10-6-5-9-35(32)36)49(72)57-37(46(69)62-43)11-7-8-20-53/h5-6,9-10,12-19,24,27-28,37-44,55,65,67H,7-8,11,20-23,25-26,53H2,1-4H3,(H2,54,68)(H,56,66)(H,57,72)(H,58,73)(H,59,71)(H,60,70)(H,61,75)(H,62,69)(H,63,74)/t28-,37-,38+,39-,40+,41-,42+,43+,44+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM85012

(AcNH-4-NO2-Phe-c[D-Cys-Tyr-D-Trp-Lys-Thr-Cys]-Tyr-...)Show SMILES CC(C)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@@H](NC1=O)C(=O)N[C@H]([C@H](C)O)C(N)=O)NC(=O)[C@@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O Show InChI InChI=1S/C52H68N12O13S2/c1-27(2)43-52(75)61-42(51(74)63-44(28(3)65)45(54)68)26-79-78-25-41(60-47(70)38(56-29(4)66)21-30-12-16-33(17-13-30)64(76)77)50(73)58-39(22-31-14-18-34(67)19-15-31)48(71)59-40(23-32-24-55-36-10-6-5-9-35(32)36)49(72)57-37(46(69)62-43)11-7-8-20-53/h5-6,9-10,12-19,24,27-28,37-44,55,65,67H,7-8,11,20-23,25-26,53H2,1-4H3,(H2,54,68)(H,56,66)(H,57,72)(H,58,73)(H,59,71)(H,60,70)(H,61,75)(H,62,69)(H,63,74)/t28-,37-,38+,39-,40+,41+,42+,43+,44+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50316952
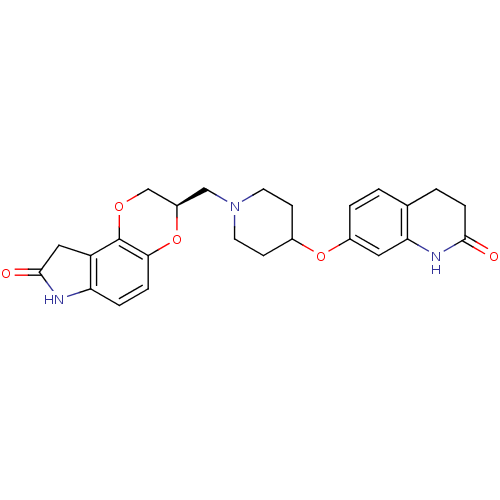
((R)-3-((4-(2-oxo-1,2,3,4-tetrahydroquinolin-7-ylox...)Show SMILES O=C1Cc2c(N1)ccc1O[C@H](CN3CCC(CC3)Oc3ccc4CCC(=O)Nc4c3)COc21 |r| Show InChI InChI=1S/C25H27N3O5/c29-23-6-2-15-1-3-17(11-21(15)27-23)32-16-7-9-28(10-8-16)13-18-14-31-25-19-12-24(30)26-20(19)4-5-22(25)33-18/h1,3-5,11,16,18H,2,6-10,12-14H2,(H,26,30)(H,27,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone form human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084389
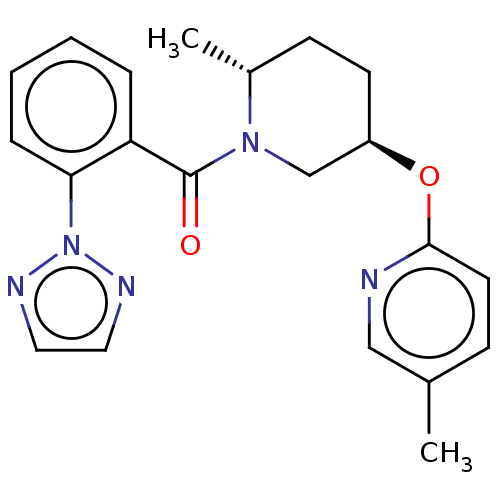
(CHEMBL3426130)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1ccc(C)cn1 |r| Show InChI InChI=1S/C21H23N5O2/c1-15-7-10-20(22-13-15)28-17-9-8-16(2)25(14-17)21(27)18-5-3-4-6-19(18)26-23-11-12-24-26/h3-7,10-13,16-17H,8-9,14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084388

(CHEMBL3426131)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(C)ccn1 |r| Show InChI InChI=1S/C21H23N5O2/c1-15-9-10-22-20(13-15)28-17-8-7-16(2)25(14-17)21(27)18-5-3-4-6-19(18)26-23-11-12-24-26/h3-6,9-13,16-17H,7-8,14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from D2 receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from D2 receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302218
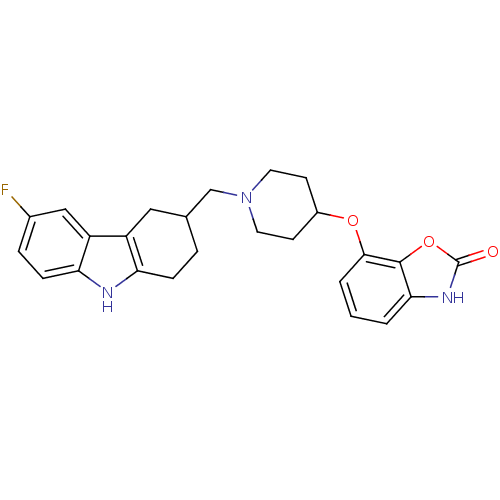
(7-(1-((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-y...)Show SMILES Fc1ccc2[nH]c3CCC(CN4CCC(CC4)Oc4cccc5[nH]c(=O)oc45)Cc3c2c1 Show InChI InChI=1S/C25H26FN3O3/c26-16-5-7-21-19(13-16)18-12-15(4-6-20(18)27-21)14-29-10-8-17(9-11-29)31-23-3-1-2-22-24(23)32-25(30)28-22/h1-3,5,7,13,15,17,27H,4,6,8-12,14H2,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from D2 receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone form human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084391
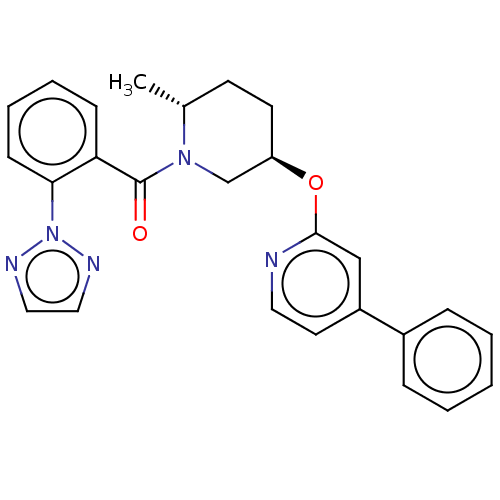
(CHEMBL3426148)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)-c1ccccc1 |r| Show InChI InChI=1S/C26H25N5O2/c1-19-11-12-22(33-25-17-21(13-14-27-25)20-7-3-2-4-8-20)18-30(19)26(32)23-9-5-6-10-24(23)31-28-15-16-29-31/h2-10,13-17,19,22H,11-12,18H2,1H3/t19-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084380
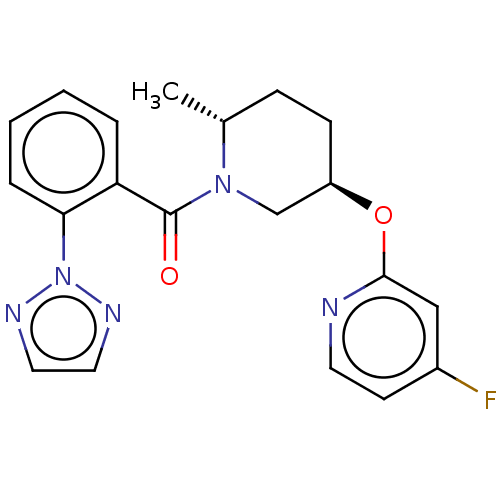
(CHEMBL3426140)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(F)ccn1 |r| Show InChI InChI=1S/C20H20FN5O2/c1-14-6-7-16(28-19-12-15(21)8-9-22-19)13-25(14)20(27)17-4-2-3-5-18(17)26-23-10-11-24-26/h2-5,8-12,14,16H,6-7,13H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084382
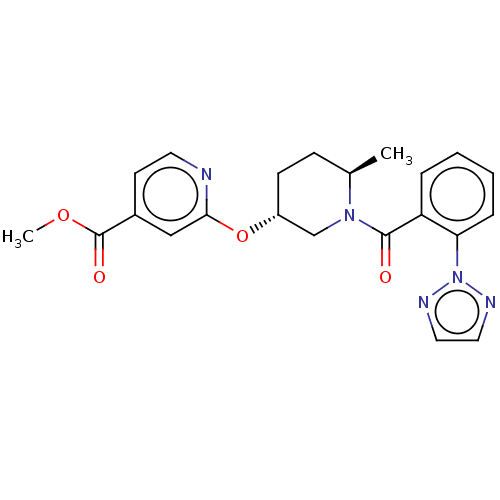
(CHEMBL3426138 | US9556145, example 1)Show SMILES COC(=O)c1ccnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c1 |r| Show InChI InChI=1S/C22H23N5O4/c1-15-7-8-17(31-20-13-16(9-10-23-20)22(29)30-2)14-26(15)21(28)18-5-3-4-6-19(18)27-24-11-12-25-27/h3-6,9-13,15,17H,7-8,14H2,1-2H3/t15-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084392
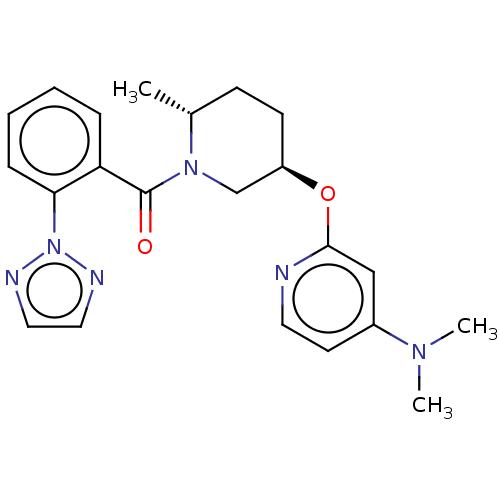
(CHEMBL3426147)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)N(C)C |r| Show InChI InChI=1S/C22H26N6O2/c1-16-8-9-18(30-21-14-17(26(2)3)10-11-23-21)15-27(16)22(29)19-6-4-5-7-20(19)28-24-12-13-25-28/h4-7,10-14,16,18H,8-9,15H2,1-3H3/t16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50316946

(CHEMBL1087585 | N-(2-fluoro-5-(2-(8-((2-oxo-1,2,3,...)Show SMILES CS(=O)(=O)Nc1cc(OCCN2CC3CCC(C2)C3Cc2ccc3CCC(=O)Nc3c2)ccc1F Show InChI InChI=1S/C26H32FN3O4S/c1-35(32,33)29-25-14-21(7-8-23(25)27)34-11-10-30-15-19-4-5-20(16-30)22(19)12-17-2-3-18-6-9-26(31)28-24(18)13-17/h2-3,7-8,13-14,19-20,22,29H,4-6,9-12,15-16H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram form human SRET by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(RAT) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyenamid Agricultural Research Center
Curated by PDSP Ki Database
| |
Mol Pharmacol 50: 709-15 (1996)
BindingDB Entry DOI: 10.7270/Q2QZ28HK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50302212

((3S)-3-((3-( 6-fluoro-2,3,4,9-tetrahydro-1H-carbaz...)Show SMILES Fc1ccc2[nH]c3CC(CCCNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)CCc3c2c1 |r| Show InChI InChI=1S/C26H28FN3O3/c27-16-4-6-21-19(11-16)18-5-3-15(10-23(18)29-21)2-1-9-28-13-17-14-32-26-20-12-25(31)30-22(20)7-8-24(26)33-17/h4,6-8,11,15,17,28-29H,1-3,5,9-10,12-14H2,(H,30,31)/t15?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50316948

((R)-7-[8-(2-Oxo-1,2,3,4-tetrahydro-quinolin-7-ylme...)Show SMILES O=C1Cc2c(N1)ccc1O[C@H](CN3CC4CCC(C3)C4Cc3ccc4CCC(=O)Nc4c3)COc21 |r| Show InChI InChI=1S/C28H31N3O4/c32-26-8-5-17-2-1-16(10-24(17)30-26)9-21-18-3-4-19(21)13-31(12-18)14-20-15-34-28-22-11-27(33)29-23(22)6-7-25(28)35-20/h1-2,6-7,10,18-21H,3-5,8-9,11-15H2,(H,29,33)(H,30,32)/t18?,19?,20-,21?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone form human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084381

(CHEMBL3426139)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)C(=O)N(C)C |r| Show InChI InChI=1S/C23H26N6O3/c1-16-8-9-18(32-21-14-17(10-11-24-21)22(30)27(2)3)15-28(16)23(31)19-6-4-5-7-20(19)29-25-12-13-26-29/h4-7,10-14,16,18H,8-9,15H2,1-3H3/t16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50316946

(CHEMBL1087585 | N-(2-fluoro-5-(2-(8-((2-oxo-1,2,3,...)Show SMILES CS(=O)(=O)Nc1cc(OCCN2CC3CCC(C2)C3Cc2ccc3CCC(=O)Nc3c2)ccc1F Show InChI InChI=1S/C26H32FN3O4S/c1-35(32,33)29-25-14-21(7-8-23(25)27)34-11-10-30-15-19-4-5-20(16-30)22(19)12-17-2-3-18-6-9-26(31)28-24(18)13-17/h2-3,7-8,13-14,19-20,22,29H,4-6,9-12,15-16H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone form human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 20: 2983-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.105
BindingDB Entry DOI: 10.7270/Q2VH5P0Q |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084390
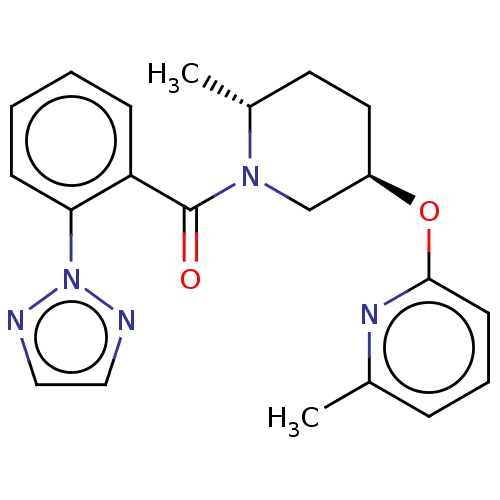
(CHEMBL3426129)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cccc(C)n1 |r| Show InChI InChI=1S/C21H23N5O2/c1-15-6-5-9-20(24-15)28-17-11-10-16(2)25(14-17)21(27)18-7-3-4-8-19(18)26-22-12-13-23-26/h3-9,12-13,16-17H,10-11,14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data