

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50243699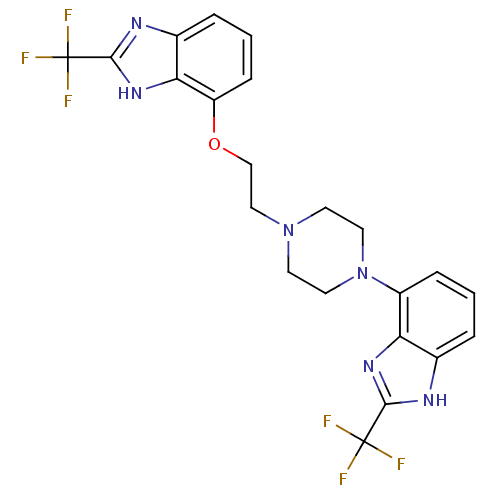 (2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1D receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50243700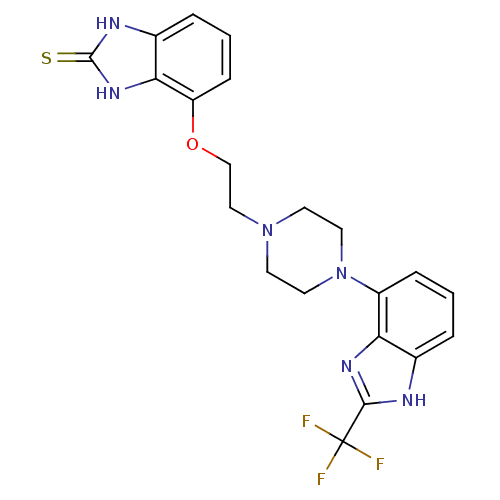 (4-(2-(4-(2-(trifluoromethyl)-1H-benzo[d]imidazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1676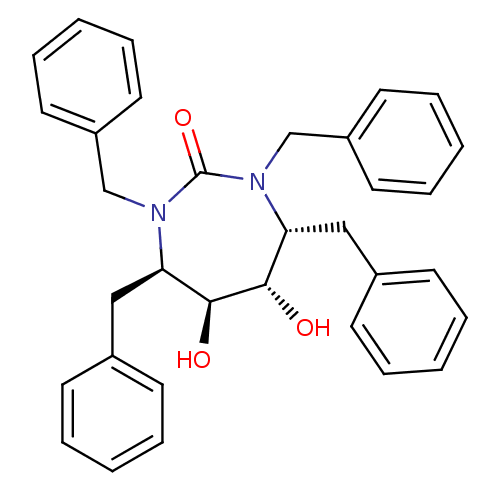 ((4R,5S,6S,7R)-1,3,4,7-tetrabenzyl-5,6-dihydroxy-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1682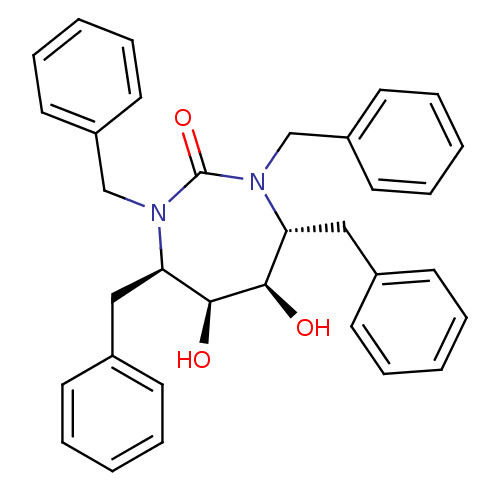 ((4R,5S,6R,7R)-1,3,4,7-tetrabenzyl-5,6-dihydroxy-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073327 ((1S,2R,3S,4R,8R,9R)-4-Benzyl-9-benzyloxy-2,3-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50243699 (2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM50537592 (CHEMBL4632881) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50243699 (2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1B receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447108 (CHEMBL3112881) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A LC assessed as cleavage of SNAP-25 (141 to 206) after 30 mins by LC-MS analysis | J Med Chem 57: 669-76 (2014) Article DOI: 10.1021/jm4012164 BindingDB Entry DOI: 10.7270/Q2GH9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521018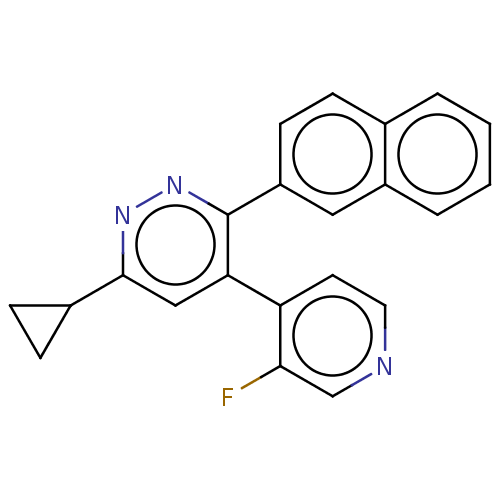 (US11149020, Compound 10 (MW-167)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521021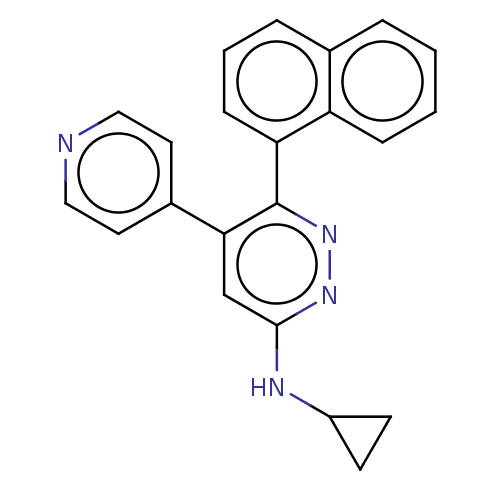 (US11149020, Compound 13 (MW-107)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521019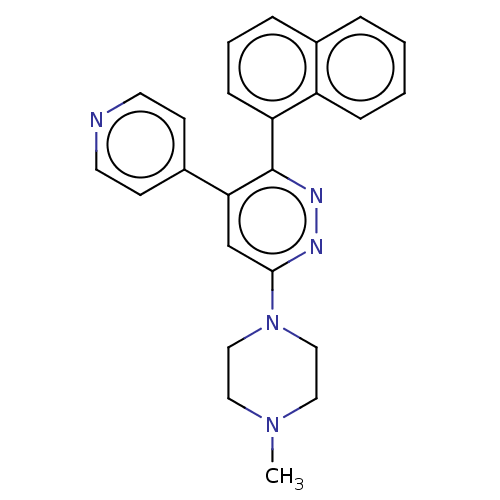 (US11149020, Compound 11 (MW-122)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521025 (US11149020, Compound 16 (MW-200)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537600 (CHEMBL4129018 | US11149020, Compound 27 (MW-150)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537600 (CHEMBL4129018 | US11149020, Compound 27 (MW-150)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521045 (US11149020, Compound 36 (MW-164)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537599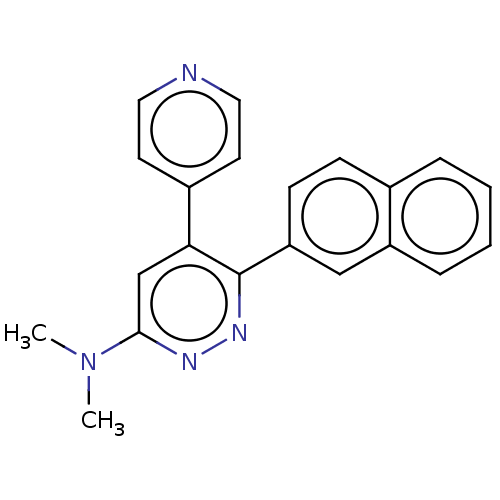 (CHEMBL4648060 | US11149020, Compound 2 (MW-108)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537599 (CHEMBL4648060 | US11149020, Compound 2 (MW-108)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521017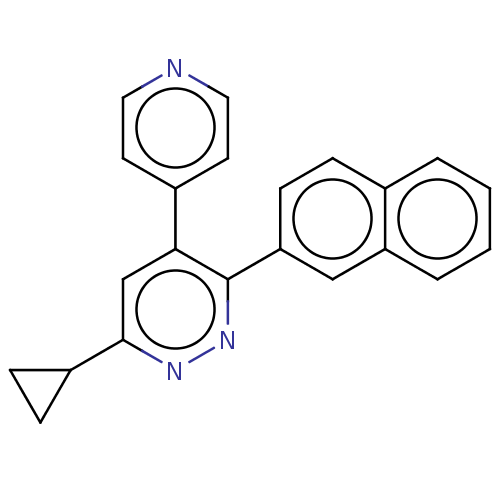 (US11149020, Compound 9 (MW-125)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521016 (US11149020, Compound 7 (MW-077)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50244213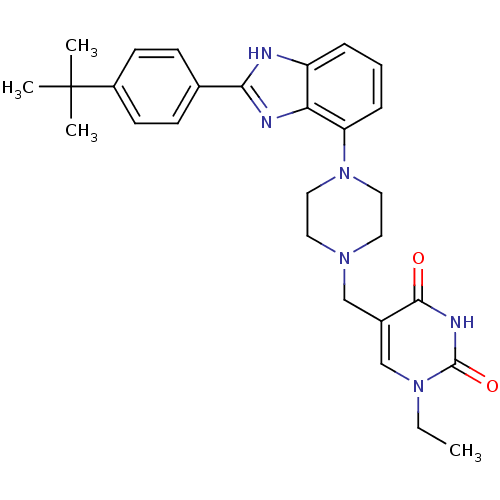 (5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to 5HT2A receptor (unknown origin) | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521024 (US11149020, Compound 15 (MW-156)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50256882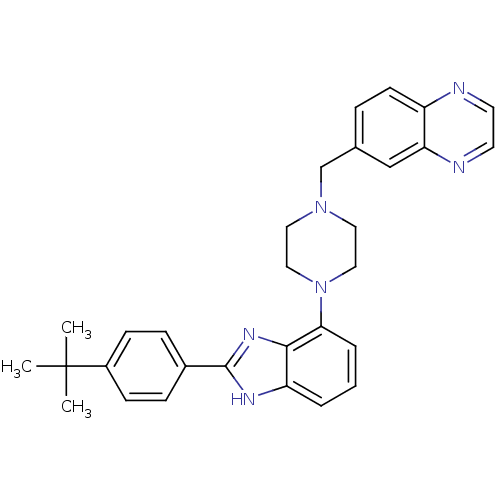 (6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human neurokinin NK2 receptor | J Med Chem 52: 2148-52 (2009) Article DOI: 10.1021/jm801572m BindingDB Entry DOI: 10.7270/Q2ZC82RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521047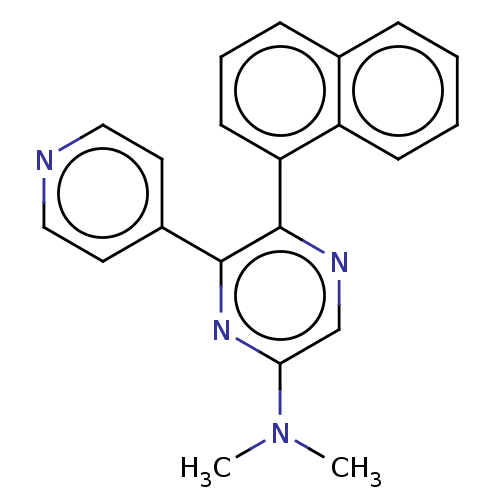 (N,N-dimethyl-5-(naphthalen-1-yl)-6-(pyridin-4-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537592 (CHEMBL4632881) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537597 (CHEMBL4645737 | US11149020, Compound 6 (MW-105)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 657 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073328 ((1S,2R,3S,4R,8R,9R)-4,5-Dibenzyl-9-benzyloxy-2,3-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50256882 (6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human histamine H2 receptor | J Med Chem 52: 2148-52 (2009) Article DOI: 10.1021/jm801572m BindingDB Entry DOI: 10.7270/Q2ZC82RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1678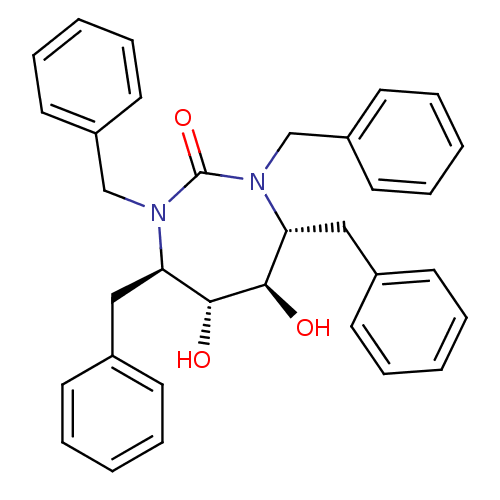 ((4R,5R,6R,7R)-1,3,4,7-tetrabenzyl-5,6-dihydroxy-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073329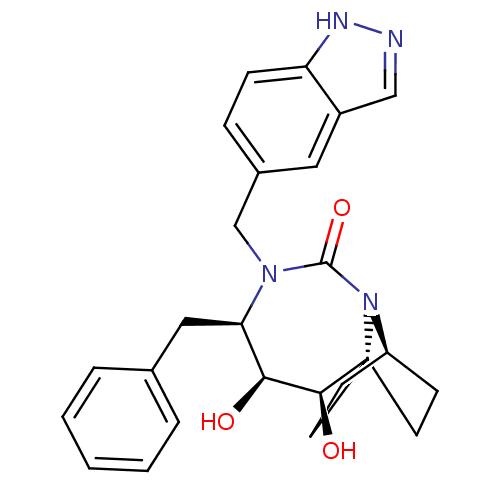 ((2R,3S,4R)-4-Benzyl-2,3-dihydroxy-5-(1H-indazol-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073330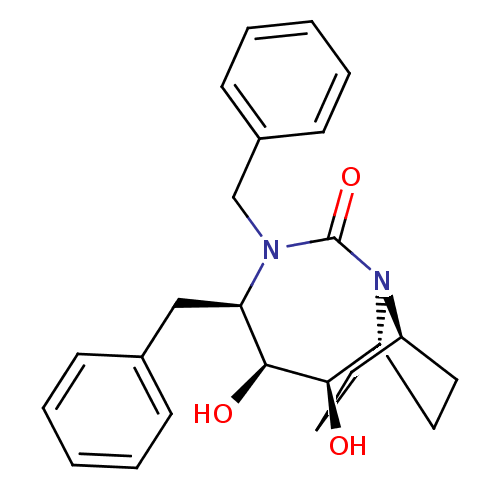 ((2R,3S,4R)-4,5-Dibenzyl-2,3-dihydroxy-5,7-diaza-tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073325 ((2R,3R,4R)-4,5-Dibenzyl-2,3-dihydroxy-5,7-diaza-tr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073326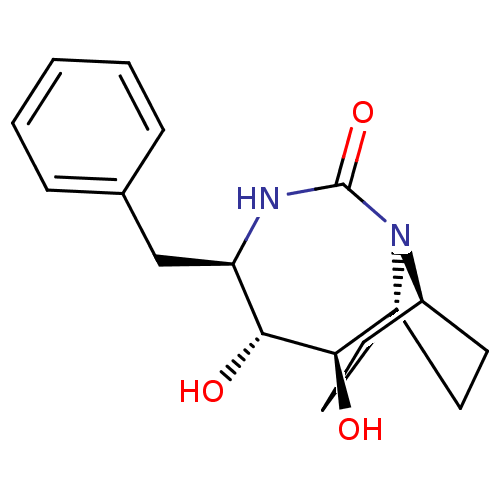 ((2R,3R,4R)-4-Benzyl-2,3-dihydroxy-5,7-diaza-tricyc...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC | Bioorg Med Chem Lett 8: 3615-20 (1999) BindingDB Entry DOI: 10.7270/Q2VX0FP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244213 (5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [125I]-(D-Trp6)-GnRH from human GnRH receptor | Bioorg Med Chem Lett 19: 1986-90 (2009) Article DOI: 10.1016/j.bmcl.2009.02.043 BindingDB Entry DOI: 10.7270/Q28S4PS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244213 (5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50256836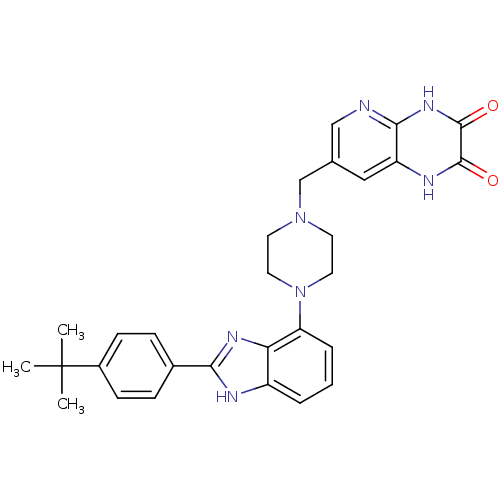 (7-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptor | J Med Chem 52: 2148-52 (2009) Article DOI: 10.1021/jm801572m BindingDB Entry DOI: 10.7270/Q2ZC82RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50243853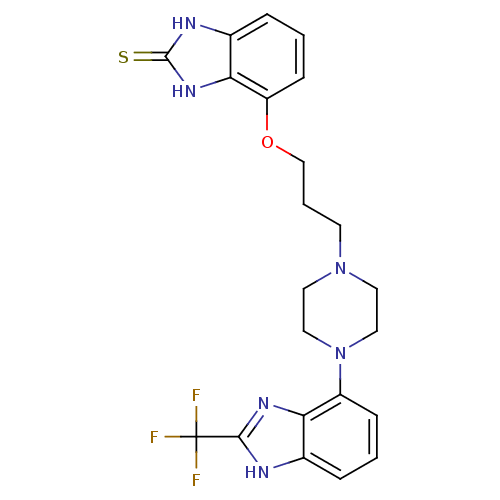 (4-(3-(4-(2-(Trifluoromethyl)-1H-benzo[d]imidazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50315277 (5-((4-(2-(4-tert-butylphenyl)imidazo[1,2-a]pyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-GnRH from human recombinant GNRH receptor by scintillation counting | Bioorg Med Chem Lett 20: 2512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.02.099 BindingDB Entry DOI: 10.7270/Q2W0962G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50256834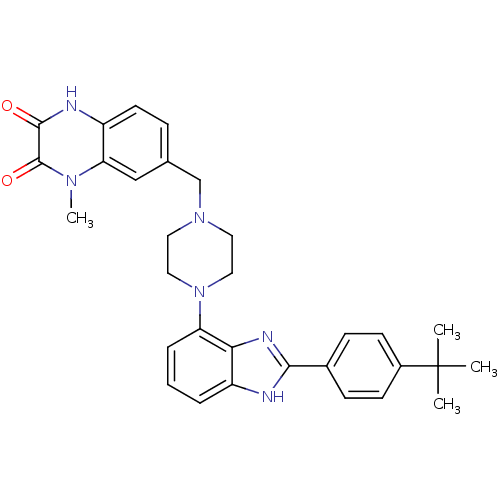 (7-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptor | J Med Chem 52: 2148-52 (2009) Article DOI: 10.1021/jm801572m BindingDB Entry DOI: 10.7270/Q2ZC82RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244213 (5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant GnRH receptor expressed in HEK293 cells assessed as reduction in (D-Trp6)-GnRH-stimulated IP production by w... | Bioorg Med Chem Lett 19: 1986-90 (2009) Article DOI: 10.1016/j.bmcl.2009.02.043 BindingDB Entry DOI: 10.7270/Q28S4PS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244213 (5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant GnRH receptor assessed as reduction in (D-Trp6)LHRH-induced myo-(1,2)-[3H]inositol production | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50315276 (5-((4-(2-(4-tert-butylphenyl)imidazo[1,2-a]pyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-GnRH from human recombinant GNRH receptor by scintillation counting | Bioorg Med Chem Lett 20: 2512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.02.099 BindingDB Entry DOI: 10.7270/Q2W0962G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244211 (6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptor | J Med Chem 52: 2148-52 (2009) Article DOI: 10.1021/jm801572m BindingDB Entry DOI: 10.7270/Q2ZC82RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244211 (6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50315277 (5-((4-(2-(4-tert-butylphenyl)imidazo[1,2-a]pyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant GNRH receptor assessed as inhibition of D-Trp6-GNRH-induced IP accumulation after 1 hr by rapid filtration a... | Bioorg Med Chem Lett 20: 2512-5 (2010) Article DOI: 10.1016/j.bmcl.2010.02.099 BindingDB Entry DOI: 10.7270/Q2W0962G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50244212 (5-((4-(2-(4-tert-Butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptor | Bioorg Med Chem 16: 6617-40 (2008) Article DOI: 10.1016/j.bmc.2008.05.024 BindingDB Entry DOI: 10.7270/Q2FB52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50256835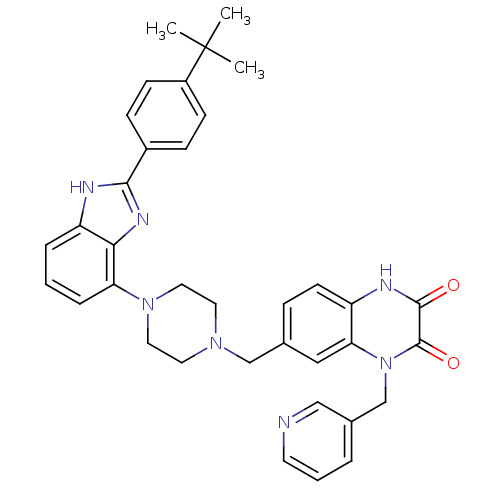 (7-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptor | J Med Chem 52: 2148-52 (2009) Article DOI: 10.1021/jm801572m BindingDB Entry DOI: 10.7270/Q2ZC82RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 509 total ) | Next | Last >> |