

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343682 (((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-ylox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343681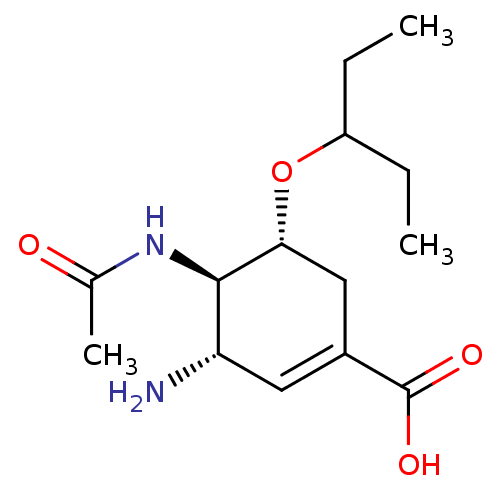 ((3S,4R,5R)-4-Acetamido-3-amino-5-(1-ethylpropoxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327504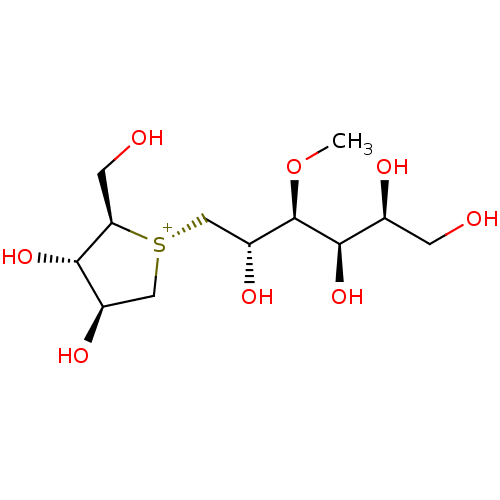 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327503 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330961 ((2R,3S,4S)-1-((2S,3S,4R,5R,6S)-2,3,4,5,6,7-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330954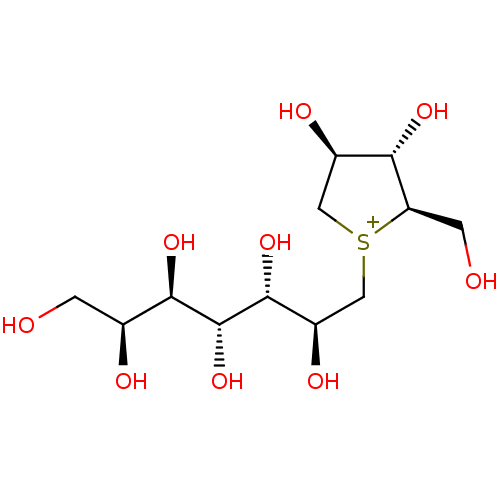 (CHEMBL1276973 | de-O-sulfonated kotalanol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327502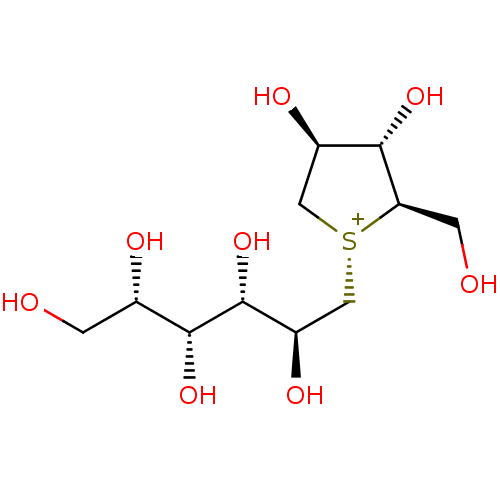 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human N-terminal maltase-glucoamylase | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327502 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330960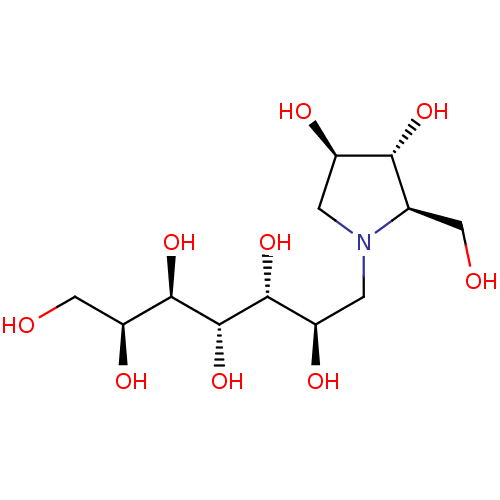 ((2R,3R,4R)-1-((2R,3R,4S,5R,6S)-2,3,4,5,6,7-hexahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343684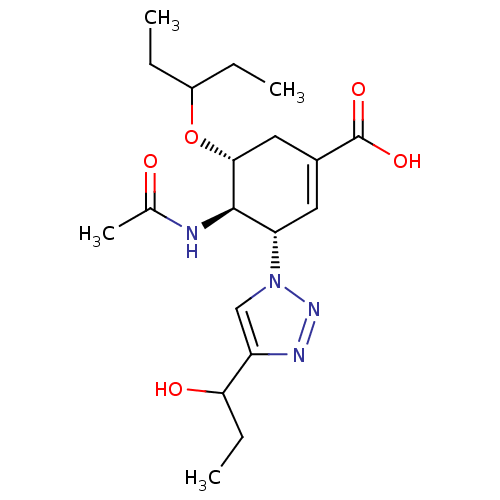 ((3S,4R,5R)-4-Acetamido-5-(1-ethyl-propoxy)-3-[4-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330959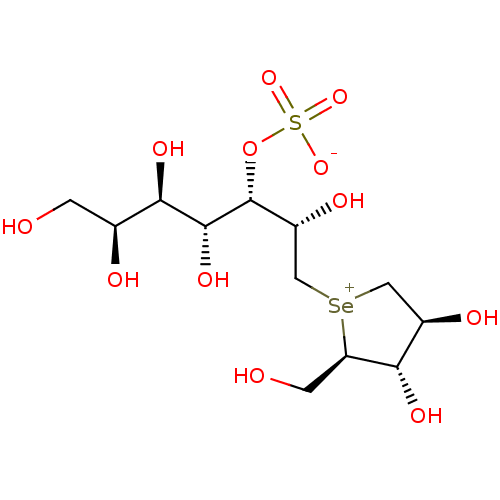 (CHEMBL1277153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316180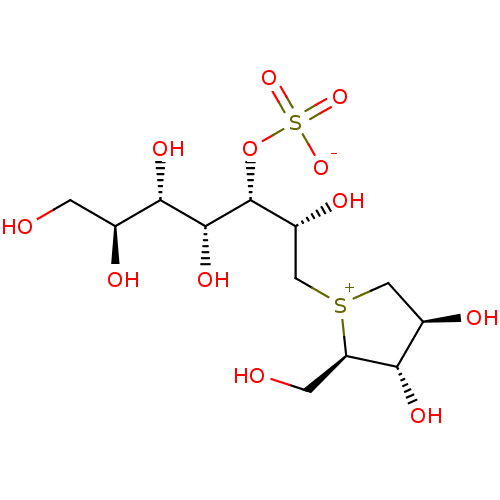 ((1S,2R,3S,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327502 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316181 ((1S,2R,3S,4R)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343685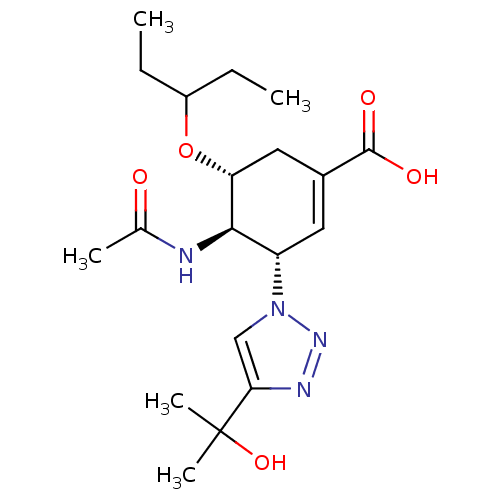 ((3S,4R,5R)-4-Acetamido-5-(1-ethylpropoxy)-3-[4-(1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327501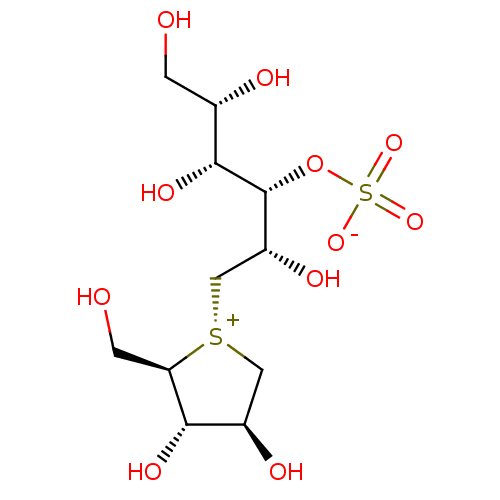 (CHEMBL1258528 | ponkoranol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316179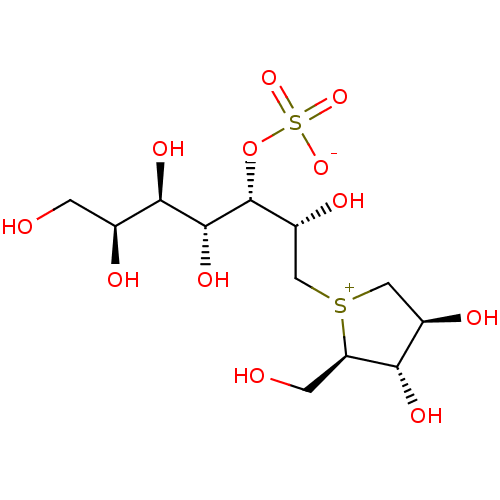 ((1S,2R,3R,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316179 ((1S,2R,3R,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316178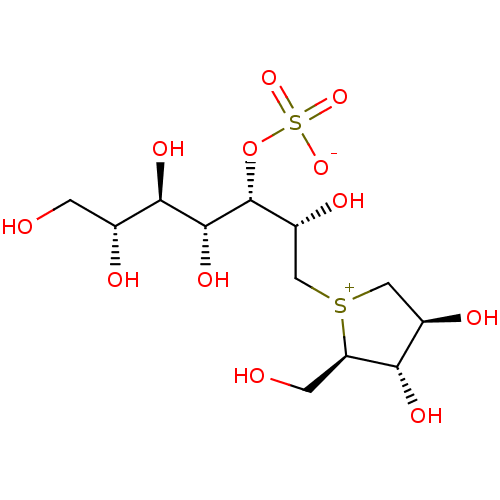 (1,4-Dideoxy-1,4-[[2S,3S,4R,5R,6R-2,4,5,6,7-pentahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50327502 ((1R,2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-1-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343683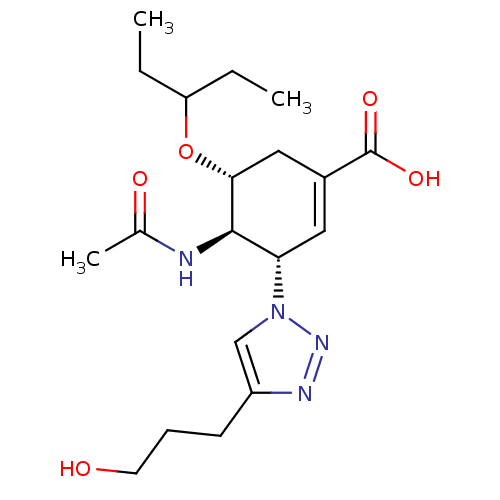 ((3S,4R,5R)-4-Acetamido-5-(1-ethylpropoxy)-3-[4-(3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human N-terminal maltase-glucoamylase | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem Lett 20: 5686-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.020 BindingDB Entry DOI: 10.7270/Q2WQ0411 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50327504 (1,4-Dideoxy-1,4-[[2S,3S,4R,5S]-2,4,5,6-tetrahydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal maltase-glucoamylase by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 6491-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.069 BindingDB Entry DOI: 10.7270/Q2Q81DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031612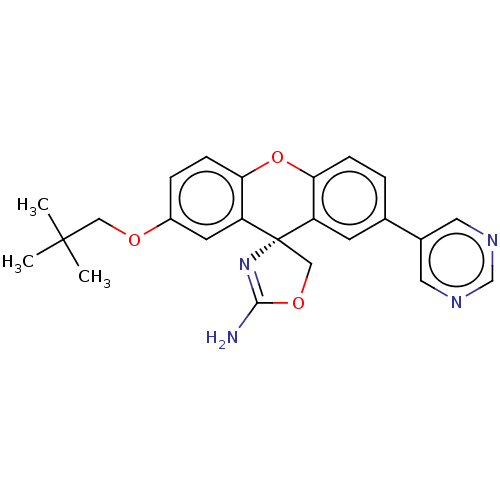 (CHEMBL3354688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113085 BindingDB Entry DOI: 10.7270/Q2BC43MB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50242271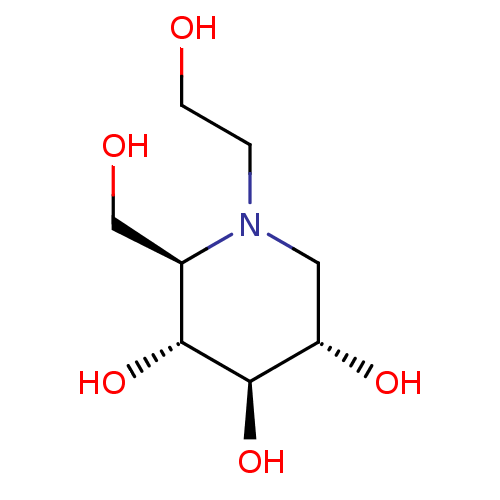 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330955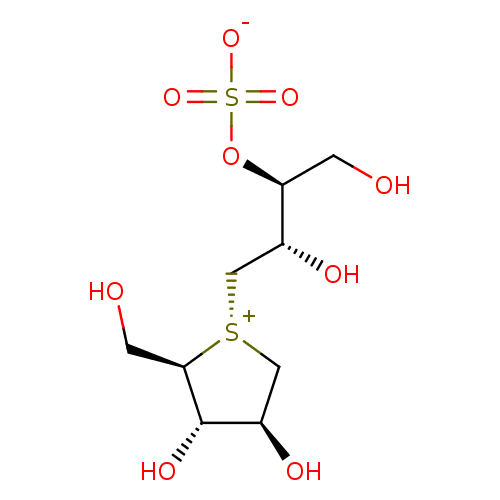 ((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343686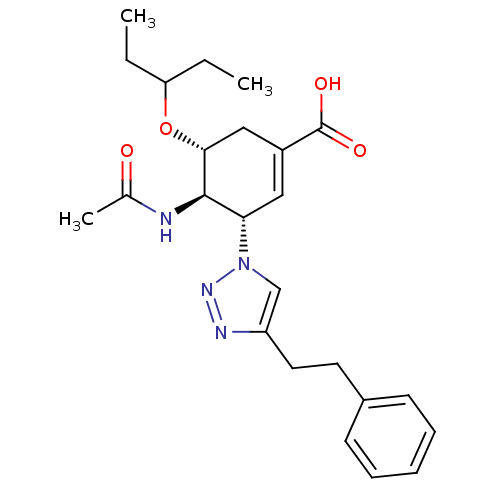 ((3S,4R,5R)-4-acetamido-5-(pentan-3-yloxy)-3-(4-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330957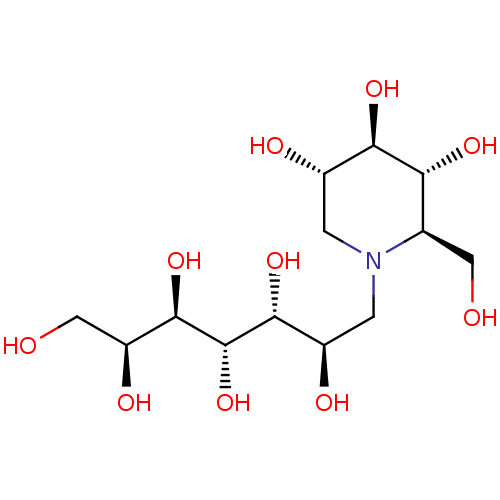 (7'-[(1,5-Dideoxy-1,5-imino-D-glucitol)-5-N-ammoniu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330956 (7'-[(1,5-Dideoxy-1,5-imino-D-glucitol)-5-N-ammoniu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343689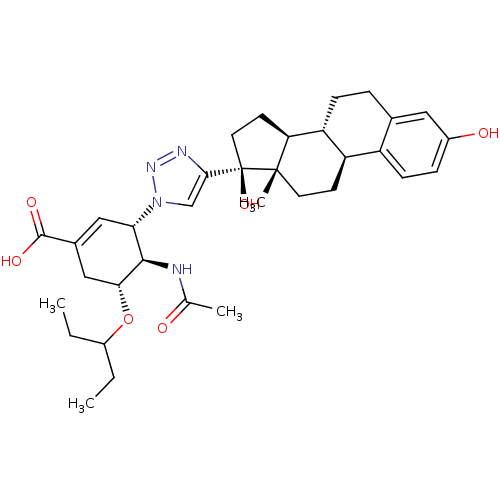 ((3S,4R,5R)-4-acetamido-3-(4-((8R,9S,13S,14S,17S)-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343690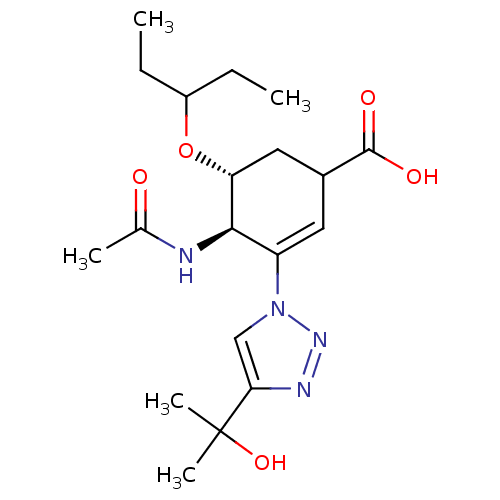 ((4R,5R)-4-acetamido-3-(4-(2-hydroxypropan-2-yl)-1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50343680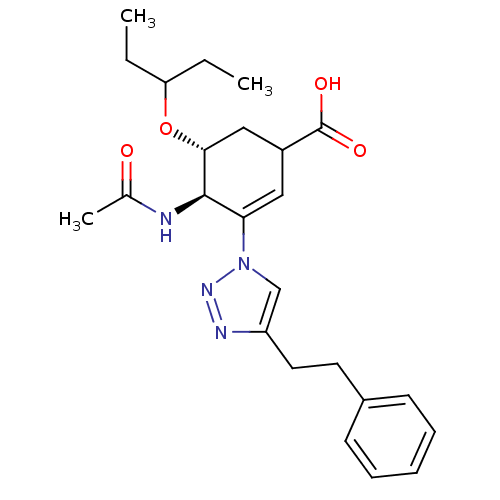 ((4R,5R)-4-acetamido-5-(pentan-3-yloxy)-3-(4-phenet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of influenza A nuraminidase N1 | J Med Chem 53: 7377-91 (2010) Article DOI: 10.1021/jm100822f BindingDB Entry DOI: 10.7270/Q2S75K5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330958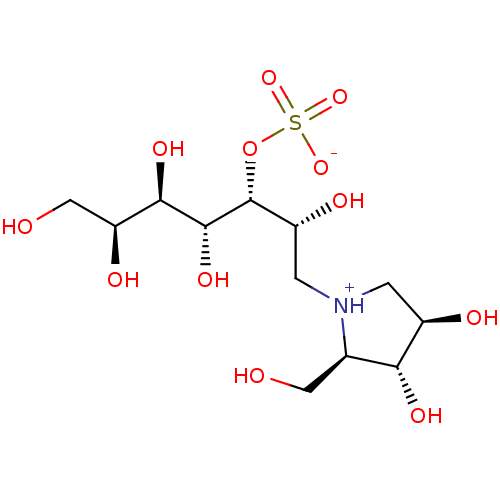 ((2R,3R,4R,5R,6S)-1-((2R,3R,4R)-3,4-dihydroxy-2-(hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assay | Bioorg Med Chem 18: 7794-8 (2010) Article DOI: 10.1016/j.bmc.2010.09.059 BindingDB Entry DOI: 10.7270/Q2TT4RZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50601401 (CHEMBL5201184) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113085 BindingDB Entry DOI: 10.7270/Q2BC43MB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50451624 (CHEMBL331897) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113085 BindingDB Entry DOI: 10.7270/Q2BC43MB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031612 (CHEMBL3354688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113085 BindingDB Entry DOI: 10.7270/Q2BC43MB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098641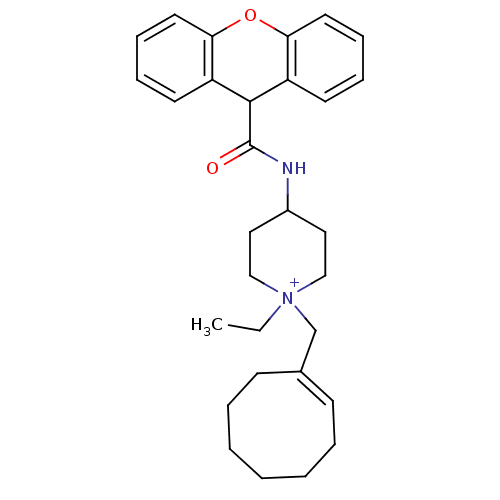 (1-Cyclooct-1-enylmethyl-1-ethyl-4-[(9H-xanthene-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113085 BindingDB Entry DOI: 10.7270/Q2BC43MB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840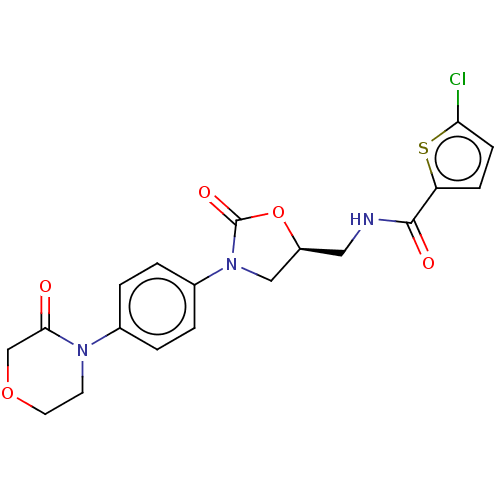 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto (CEQUIMED-UP) Curated by ChEMBL | Assay Description Inhibition of human factor 10a assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysis | J Med Chem 54: 5373-84 (2011) Article DOI: 10.1021/jm2006589 BindingDB Entry DOI: 10.7270/Q27P90HT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50554438 (CHEMBL4796244) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length recombinant human POP expressed in Escherichia coli BL21 cells using Z-Gly-Pro-AMC as substrate measured for 5 mins | Citation and Details Article DOI: 10.1021/acs.jnatprod.0c00648 BindingDB Entry DOI: 10.7270/Q2JS9V3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50438994 (CHEMBL2420683) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438994 (CHEMBL2420683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098634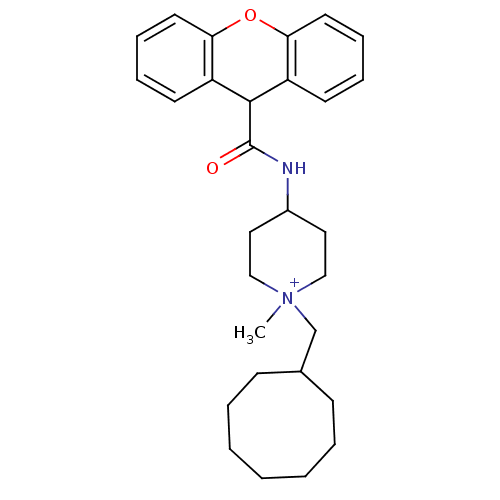 (1-Cyclooctylmethyl-1-methyl-4-[(9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113085 BindingDB Entry DOI: 10.7270/Q2BC43MB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438995 (CHEMBL2420682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50438993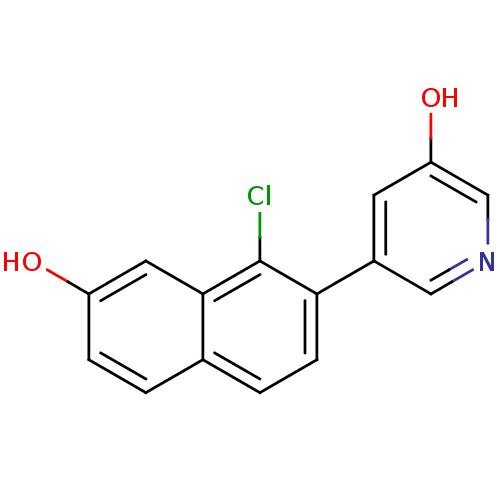 (CHEMBL2420684) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 352 total ) | Next | Last >> |