 Found 175 hits with Last Name = 'podolin' and Initial = 'pl'
Found 175 hits with Last Name = 'podolin' and Initial = 'pl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin S
(Homo sapiens (Human)) | BDBM50349203
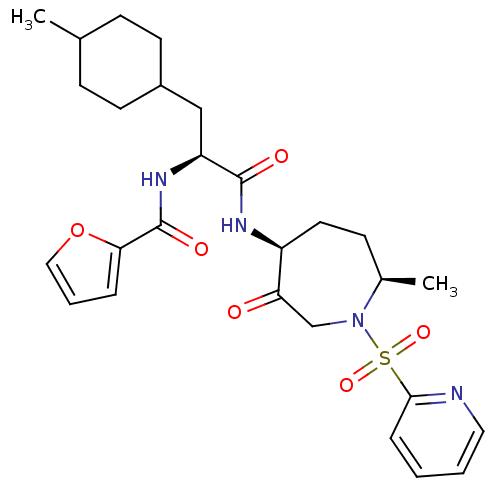
(CHEMBL1807698)Show SMILES CC1CCC(C[C@H](NC(=O)c2ccco2)C(=O)N[C@H]2CC[C@@H](C)N(CC2=O)S(=O)(=O)c2ccccn2)CC1 |r,wU:6.6,wD:21.22,18.18,(28.97,-10.04,;27.63,-10.81,;27.63,-12.35,;26.31,-13.13,;24.98,-12.36,;23.65,-13.13,;23.65,-14.69,;22.31,-15.43,;20.99,-14.64,;21.01,-13.11,;19.65,-15.4,;18.26,-14.75,;17.2,-15.88,;17.95,-17.22,;19.46,-16.92,;24.98,-15.47,;24.96,-17.01,;26.33,-14.72,;27.64,-15.51,;27.51,-17.06,;28.62,-18.12,;30.15,-17.9,;31,-19.19,;30.94,-16.59,;30.39,-15.15,;28.92,-14.67,;28.91,-13.13,;32.47,-16.72,;33.12,-18.11,;31.95,-18.17,;33.36,-15.46,;32.7,-14.06,;33.57,-12.8,;35.12,-12.92,;35.77,-14.33,;34.89,-15.59,;24.96,-10.82,;26.3,-10.04,)| Show InChI InChI=1S/C27H36N4O6S/c1-18-8-11-20(12-9-18)16-22(30-27(34)24-6-5-15-37-24)26(33)29-21-13-10-19(2)31(17-23(21)32)38(35,36)25-7-3-4-14-28-25/h3-7,14-15,18-22H,8-13,16-17H2,1-2H3,(H,29,33)(H,30,34)/t18?,19-,20?,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349197

(CHEMBL1807647)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-12-13-20(22(31)17-30(18)37(34,35)24-11-5-6-14-27-24)28-25(32)21(16-19-8-3-2-4-9-19)29-26(33)23-10-7-15-36-23/h5-7,10-11,14-15,18-21H,2-4,8-9,12-13,16-17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349198

(CHEMBL1807649)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C23H30N4O6S/c1-15(2)13-18(26-23(30)20-7-6-12-33-20)22(29)25-17-10-9-16(3)27(14-19(17)28)34(31,32)21-8-4-5-11-24-21/h4-8,11-12,15-18H,9-10,13-14H2,1-3H3,(H,25,29)(H,26,30)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349205
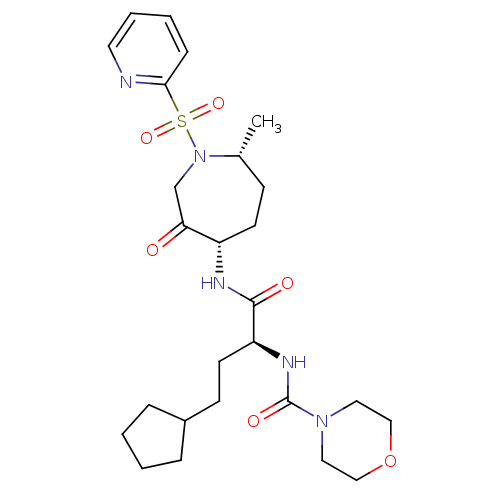
(CHEMBL1807700)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CCC2CCCC2)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H39N5O6S/c1-19-9-11-21(23(32)18-31(19)38(35,36)24-8-4-5-13-27-24)28-25(33)22(12-10-20-6-2-3-7-20)29-26(34)30-14-16-37-17-15-30/h4-5,8,13,19-22H,2-3,6-7,9-12,14-18H2,1H3,(H,28,33)(H,29,34)/t19-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349196

(CHEMBL1807646)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C25H32N4O6S/c1-17-11-12-19(21(30)16-29(17)36(33,34)23-10-4-5-13-26-23)27-24(31)20(15-18-7-2-3-8-18)28-25(32)22-9-6-14-35-22/h4-6,9-10,13-14,17-20H,2-3,7-8,11-12,15-16H2,1H3,(H,27,31)(H,28,32)/t17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349203

(CHEMBL1807698)Show SMILES CC1CCC(C[C@H](NC(=O)c2ccco2)C(=O)N[C@H]2CC[C@@H](C)N(CC2=O)S(=O)(=O)c2ccccn2)CC1 |r,wU:6.6,wD:21.22,18.18,(28.97,-10.04,;27.63,-10.81,;27.63,-12.35,;26.31,-13.13,;24.98,-12.36,;23.65,-13.13,;23.65,-14.69,;22.31,-15.43,;20.99,-14.64,;21.01,-13.11,;19.65,-15.4,;18.26,-14.75,;17.2,-15.88,;17.95,-17.22,;19.46,-16.92,;24.98,-15.47,;24.96,-17.01,;26.33,-14.72,;27.64,-15.51,;27.51,-17.06,;28.62,-18.12,;30.15,-17.9,;31,-19.19,;30.94,-16.59,;30.39,-15.15,;28.92,-14.67,;28.91,-13.13,;32.47,-16.72,;33.12,-18.11,;31.95,-18.17,;33.36,-15.46,;32.7,-14.06,;33.57,-12.8,;35.12,-12.92,;35.77,-14.33,;34.89,-15.59,;24.96,-10.82,;26.3,-10.04,)| Show InChI InChI=1S/C27H36N4O6S/c1-18-8-11-20(12-9-18)16-22(30-27(34)24-6-5-15-37-24)26(33)29-21-13-10-19(2)31(17-23(21)32)38(35,36)25-7-3-4-14-28-25/h3-7,14-15,18-22H,8-13,16-17H2,1-2H3,(H,29,33)(H,30,34)/t18?,19-,20?,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349198

(CHEMBL1807649)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C23H30N4O6S/c1-15(2)13-18(26-23(30)20-7-6-12-33-20)22(29)25-17-10-9-16(3)27(14-19(17)28)34(31,32)21-8-4-5-11-24-21/h4-8,11-12,15-18H,9-10,13-14H2,1-3H3,(H,25,29)(H,26,30)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349197

(CHEMBL1807647)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-12-13-20(22(31)17-30(18)37(34,35)24-11-5-6-14-27-24)28-25(32)21(16-19-8-3-2-4-9-19)29-26(33)23-10-7-15-36-23/h5-7,10-11,14-15,18-21H,2-4,8-9,12-13,16-17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349204

(CHEMBL1807699)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCC(C)(C)CC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C28H38N4O6S/c1-19-9-10-21(23(33)18-32(19)39(36,37)25-8-4-5-15-29-25)30-26(34)22(31-27(35)24-7-6-16-38-24)17-20-11-13-28(2,3)14-12-20/h4-8,15-16,19-22H,9-14,17-18H2,1-3H3,(H,30,34)(H,31,35)/t19-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349199

(CHEMBL1807650)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC(C)(C)C)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H32N4O6S/c1-16-10-11-17(19(29)15-28(16)35(32,33)21-9-5-6-12-25-21)26-22(30)18(14-24(2,3)4)27-23(31)20-8-7-13-34-20/h5-9,12-13,16-18H,10-11,14-15H2,1-4H3,(H,26,30)(H,27,31)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349207
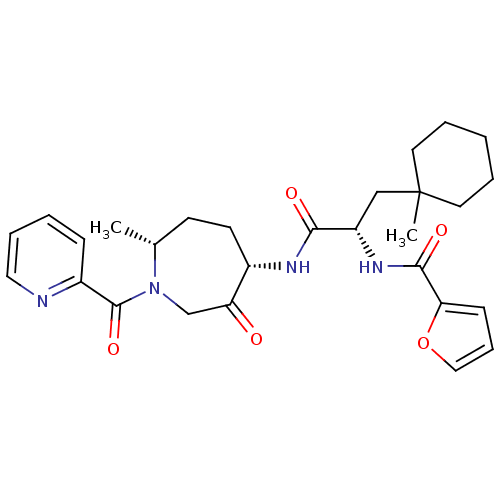
(CHEMBL1807703)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1C(=O)c1ccccn1 |r| Show InChI InChI=1S/C28H36N4O5/c1-19-11-12-20(23(33)18-32(19)27(36)21-9-4-7-15-29-21)30-25(34)22(17-28(2)13-5-3-6-14-28)31-26(35)24-10-8-16-37-24/h4,7-10,15-16,19-20,22H,3,5-6,11-14,17-18H2,1-2H3,(H,30,34)(H,31,35)/t19-,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349206
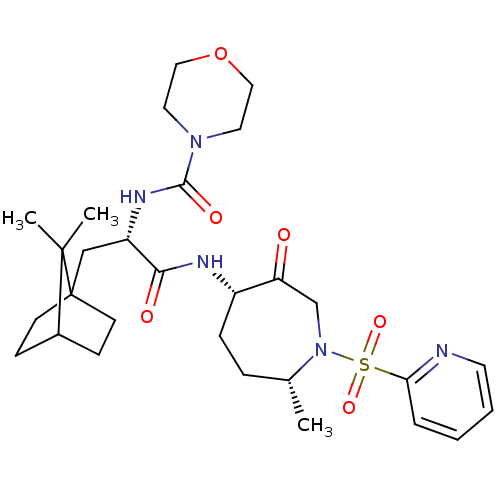
(CHEMBL1807702)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC23CCC(CC2)C3(C)C)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C29H43N5O6S/c1-20-7-8-22(24(35)19-34(20)41(38,39)25-6-4-5-13-30-25)31-26(36)23(32-27(37)33-14-16-40-17-15-33)18-29-11-9-21(10-12-29)28(29,2)3/h4-6,13,20-23H,7-12,14-19H2,1-3H3,(H,31,36)(H,32,37)/t20-,21?,22+,23+,29?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349202
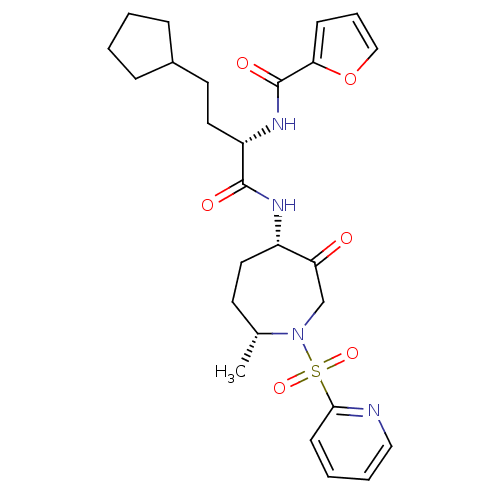
(CHEMBL1807697)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CCC2CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-11-13-20(22(31)17-30(18)37(34,35)24-10-4-5-15-27-24)28-25(32)21(14-12-19-7-2-3-8-19)29-26(33)23-9-6-16-36-23/h4-6,9-10,15-16,18-21H,2-3,7-8,11-14,17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349198

(CHEMBL1807649)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C23H30N4O6S/c1-15(2)13-18(26-23(30)20-7-6-12-33-20)22(29)25-17-10-9-16(3)27(14-19(17)28)34(31,32)21-8-4-5-11-24-21/h4-8,11-12,15-18H,9-10,13-14H2,1-3H3,(H,25,29)(H,26,30)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349195

(CHEMBL1807645)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2=CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r,t:10| Show InChI InChI=1S/C25H30N4O6S/c1-17-11-12-19(21(30)16-29(17)36(33,34)23-10-4-5-13-26-23)27-24(31)20(15-18-7-2-3-8-18)28-25(32)22-9-6-14-35-22/h4-7,9-10,13-14,17,19-20H,2-3,8,11-12,15-16H2,1H3,(H,27,31)(H,28,32)/t17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349199

(CHEMBL1807650)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC(C)(C)C)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H32N4O6S/c1-16-10-11-17(19(29)15-28(16)35(32,33)21-9-5-6-12-25-21)26-22(30)18(14-24(2,3)4)27-23(31)20-8-7-13-34-20/h5-9,12-13,16-18H,10-11,14-15H2,1-4H3,(H,26,30)(H,27,31)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349196

(CHEMBL1807646)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C25H32N4O6S/c1-17-11-12-19(21(30)16-29(17)36(33,34)23-10-4-5-13-26-23)27-24(31)20(15-18-7-2-3-8-18)28-25(32)22-9-6-14-35-22/h4-6,9-10,13-14,17-20H,2-3,7-8,11-12,15-16H2,1H3,(H,27,31)(H,28,32)/t17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349195

(CHEMBL1807645)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2=CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r,t:10| Show InChI InChI=1S/C25H30N4O6S/c1-17-11-12-19(21(30)16-29(17)36(33,34)23-10-4-5-13-26-23)27-24(31)20(15-18-7-2-3-8-18)28-25(32)22-9-6-14-35-22/h4-7,9-10,13-14,17,19-20H,2-3,8,11-12,15-16H2,1H3,(H,27,31)(H,28,32)/t17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098576
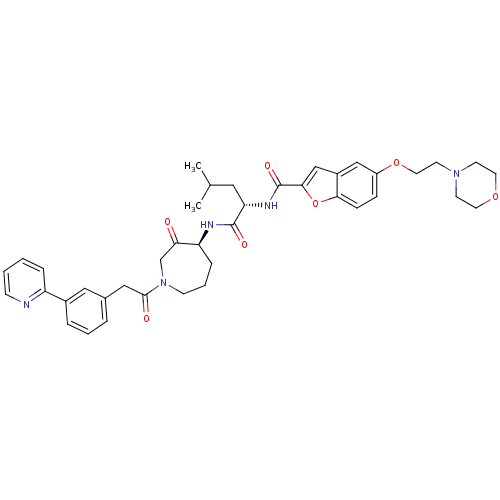
(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349195

(CHEMBL1807645)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2=CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r,t:10| Show InChI InChI=1S/C25H30N4O6S/c1-17-11-12-19(21(30)16-29(17)36(33,34)23-10-4-5-13-26-23)27-24(31)20(15-18-7-2-3-8-18)28-25(32)22-9-6-14-35-22/h4-7,9-10,13-14,17,19-20H,2-3,8,11-12,15-16H2,1H3,(H,27,31)(H,28,32)/t17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349208

(CHEMBL1807704)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)C(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H30N4O5/c1-15(2)13-19(27-23(31)21-8-6-12-33-21)22(30)26-17-10-9-16(3)28(14-20(17)29)24(32)18-7-4-5-11-25-18/h4-8,11-12,15-17,19H,9-10,13-14H2,1-3H3,(H,26,30)(H,27,31)/t16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349208

(CHEMBL1807704)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)C(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H30N4O5/c1-15(2)13-19(27-23(31)21-8-6-12-33-21)22(30)26-17-10-9-16(3)28(14-20(17)29)24(32)18-7-4-5-11-25-18/h4-8,11-12,15-17,19H,9-10,13-14H2,1-3H3,(H,26,30)(H,27,31)/t16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349202

(CHEMBL1807697)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CCC2CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-11-13-20(22(31)17-30(18)37(34,35)24-10-4-5-15-27-24)28-25(32)21(14-12-19-7-2-3-8-19)29-26(33)23-9-6-16-36-23/h4-6,9-10,15-16,18-21H,2-3,7-8,11-14,17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349205

(CHEMBL1807700)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CCC2CCCC2)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H39N5O6S/c1-19-9-11-21(23(32)18-31(19)38(35,36)24-8-4-5-13-27-24)28-25(33)22(12-10-20-6-2-3-7-20)29-26(34)30-14-16-37-17-15-30/h4-5,8,13,19-22H,2-3,6-7,9-12,14-18H2,1H3,(H,28,33)(H,29,34)/t19-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349210
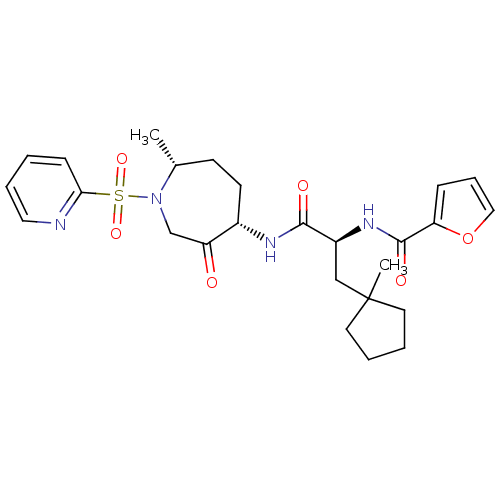
(CHEMBL1807651)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-10-11-19(21(31)17-30(18)37(34,35)23-9-3-6-14-27-23)28-24(32)20(16-26(2)12-4-5-13-26)29-25(33)22-8-7-15-36-22/h3,6-9,14-15,18-20H,4-5,10-13,16-17H2,1-2H3,(H,28,32)(H,29,33)/t18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349199

(CHEMBL1807650)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC(C)(C)C)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H32N4O6S/c1-16-10-11-17(19(29)15-28(16)35(32,33)21-9-5-6-12-25-21)26-22(30)18(14-24(2,3)4)27-23(31)20-8-7-13-34-20/h5-9,12-13,16-18H,10-11,14-15H2,1-4H3,(H,26,30)(H,27,31)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349210

(CHEMBL1807651)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-10-11-19(21(31)17-30(18)37(34,35)23-9-3-6-14-27-23)28-24(32)20(16-26(2)12-4-5-13-26)29-25(33)22-8-7-15-36-22/h3,6-9,14-15,18-20H,4-5,10-13,16-17H2,1-2H3,(H,28,32)(H,29,33)/t18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349196

(CHEMBL1807646)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C25H32N4O6S/c1-17-11-12-19(21(30)16-29(17)36(33,34)23-10-4-5-13-26-23)27-24(31)20(15-18-7-2-3-8-18)28-25(32)22-9-6-14-35-22/h4-6,9-10,13-14,17-20H,2-3,7-8,11-12,15-16H2,1H3,(H,27,31)(H,28,32)/t17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27.7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349209

(CHEMBL1807648)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N4O6S/c1-19-13-14-21(23(32)18-31(19)38(35,36)25-12-6-7-15-28-25)29-26(33)22(17-20-9-4-2-3-5-10-20)30-27(34)24-11-8-16-37-24/h6-8,11-12,15-16,19-22H,2-5,9-10,13-14,17-18H2,1H3,(H,29,33)(H,30,34)/t19-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349208

(CHEMBL1807704)Show SMILES CC(C)C[C@H](NC(=O)c1ccco1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)C(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H30N4O5/c1-15(2)13-19(27-23(31)21-8-6-12-33-21)22(30)26-17-10-9-16(3)28(14-20(17)29)24(32)18-7-4-5-11-25-18/h4-8,11-12,15-17,19H,9-10,13-14H2,1-3H3,(H,26,30)(H,27,31)/t16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349211
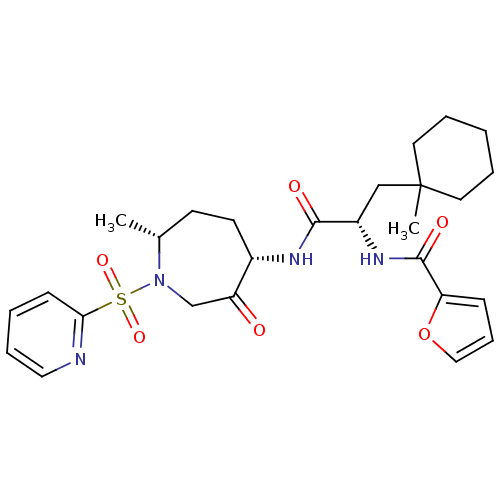
(CHEMBL1807652)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N4O6S/c1-19-11-12-20(22(32)18-31(19)38(35,36)24-10-4-7-15-28-24)29-25(33)21(17-27(2)13-5-3-6-14-27)30-26(34)23-9-8-16-37-23/h4,7-10,15-16,19-21H,3,5-6,11-14,17-18H2,1-2H3,(H,29,33)(H,30,34)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349201
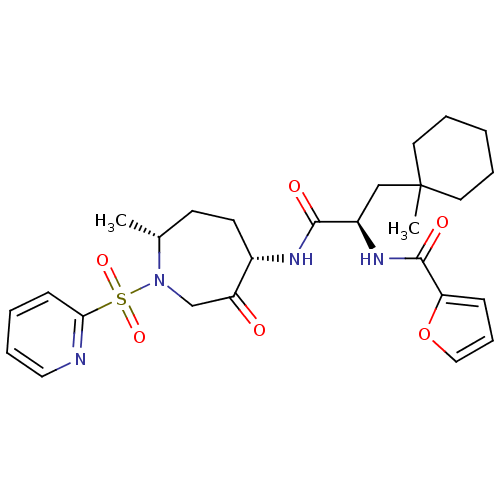
(CHEMBL1807696)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N4O6S/c1-19-11-12-20(22(32)18-31(19)38(35,36)24-10-4-7-15-28-24)29-25(33)21(17-27(2)13-5-3-6-14-27)30-26(34)23-9-8-16-37-23/h4,7-10,15-16,19-21H,3,5-6,11-14,17-18H2,1-2H3,(H,29,33)(H,30,34)/t19-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349197

(CHEMBL1807647)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-12-13-20(22(31)17-30(18)37(34,35)24-11-5-6-14-27-24)28-25(32)21(16-19-8-3-2-4-9-19)29-26(33)23-10-7-15-36-23/h5-7,10-11,14-15,18-21H,2-4,8-9,12-13,16-17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349211

(CHEMBL1807652)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N4O6S/c1-19-11-12-20(22(32)18-31(19)38(35,36)24-10-4-7-15-28-24)29-25(33)21(17-27(2)13-5-3-6-14-27)30-26(34)23-9-8-16-37-23/h4,7-10,15-16,19-21H,3,5-6,11-14,17-18H2,1-2H3,(H,29,33)(H,30,34)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349212
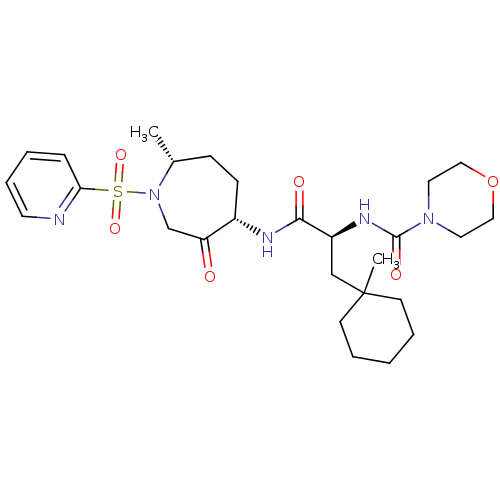
(CHEMBL1807701)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H41N5O6S/c1-20-9-10-21(23(33)19-32(20)39(36,37)24-8-4-7-13-28-24)29-25(34)22(18-27(2)11-5-3-6-12-27)30-26(35)31-14-16-38-17-15-31/h4,7-8,13,20-22H,3,5-6,9-12,14-19H2,1-2H3,(H,29,34)(H,30,35)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48.7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349204

(CHEMBL1807699)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2CCC(C)(C)CC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C28H38N4O6S/c1-19-9-10-21(23(33)18-32(19)39(36,37)25-8-4-5-15-29-25)30-26(34)22(31-27(35)24-7-6-16-38-24)17-20-11-13-28(2,3)14-12-20/h4-8,15-16,19-22H,9-14,17-18H2,1-3H3,(H,30,34)(H,31,35)/t19-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349200
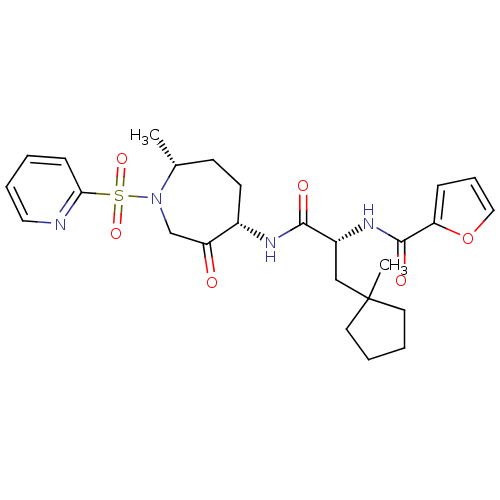
(CHEMBL1807695)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@@H](CC2(C)CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-10-11-19(21(31)17-30(18)37(34,35)23-9-3-6-14-27-23)28-24(32)20(16-26(2)12-4-5-13-26)29-25(33)22-8-7-15-36-22/h3,6-9,14-15,18-20H,4-5,10-13,16-17H2,1-2H3,(H,28,32)(H,29,33)/t18-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S using Ac-KQLR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349212

(CHEMBL1807701)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H41N5O6S/c1-20-9-10-21(23(33)19-32(20)39(36,37)24-8-4-7-13-28-24)29-25(34)22(18-27(2)11-5-3-6-12-27)30-26(35)31-14-16-38-17-15-31/h4,7-8,13,20-22H,3,5-6,9-12,14-19H2,1-2H3,(H,29,34)(H,30,35)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349206

(CHEMBL1807702)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC23CCC(CC2)C3(C)C)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C29H43N5O6S/c1-20-7-8-22(24(35)19-34(20)41(38,39)25-6-4-5-13-30-25)31-26(36)23(32-27(37)33-14-16-40-17-15-33)18-29-11-9-21(10-12-29)28(29,2)3/h4-6,13,20-23H,7-12,14-19H2,1-3H3,(H,31,36)(H,32,37)/t20-,21?,22+,23+,29?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349206

(CHEMBL1807702)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC23CCC(CC2)C3(C)C)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C29H43N5O6S/c1-20-7-8-22(24(35)19-34(20)41(38,39)25-6-4-5-13-30-25)31-26(36)23(32-27(37)33-14-16-40-17-15-33)18-29-11-9-21(10-12-29)28(29,2)3/h4-6,13,20-23H,7-12,14-19H2,1-3H3,(H,31,36)(H,32,37)/t20-,21?,22+,23+,29?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349207

(CHEMBL1807703)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1C(=O)c1ccccn1 |r| Show InChI InChI=1S/C28H36N4O5/c1-19-11-12-20(23(33)18-32(19)27(36)21-9-4-7-15-29-21)30-25(34)22(17-28(2)13-5-3-6-14-28)31-26(35)24-10-8-16-37-24/h4,7-10,15-16,19-20,22H,3,5-6,11-14,17-18H2,1-2H3,(H,30,34)(H,31,35)/t19-,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50180851
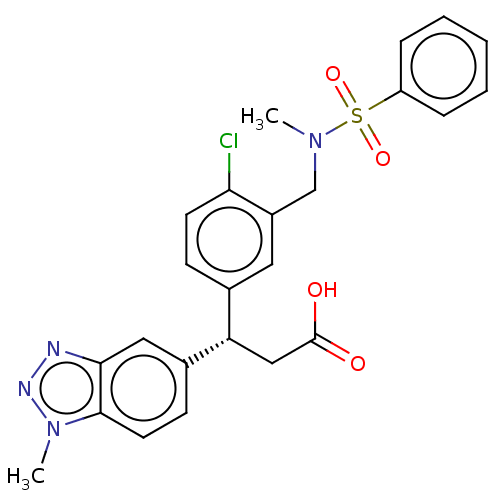
(CHEMBL3818085 | US10144731, Example 54)Show SMILES CN(Cc1cc(ccc1Cl)[C@H](CC(O)=O)c1ccc2n(C)nnc2c1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C24H23ClN4O4S/c1-28(34(32,33)19-6-4-3-5-7-19)15-18-12-16(8-10-21(18)25)20(14-24(30)31)17-9-11-23-22(13-17)26-27-29(23)2/h3-13,20H,14-15H2,1-2H3,(H,30,31)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 5'-TAMRA-NRF2 peptide from human recombinant N-terminal His6 and Avi-tagged KEAP1 (321 to 609 residues) expressed in baculovirus infe... |
J Med Chem 59: 3991-4006 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00228
BindingDB Entry DOI: 10.7270/Q25T3NDT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349207

(CHEMBL1807703)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1C(=O)c1ccccn1 |r| Show InChI InChI=1S/C28H36N4O5/c1-19-11-12-20(23(33)18-32(19)27(36)21-9-4-7-15-29-21)30-25(34)22(17-28(2)13-5-3-6-14-28)31-26(35)24-10-8-16-37-24/h4,7-10,15-16,19-20,22H,3,5-6,11-14,17-18H2,1-2H3,(H,30,34)(H,31,35)/t19-,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 429 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349203

(CHEMBL1807698)Show SMILES CC1CCC(C[C@H](NC(=O)c2ccco2)C(=O)N[C@H]2CC[C@@H](C)N(CC2=O)S(=O)(=O)c2ccccn2)CC1 |r,wU:6.6,wD:21.22,18.18,(28.97,-10.04,;27.63,-10.81,;27.63,-12.35,;26.31,-13.13,;24.98,-12.36,;23.65,-13.13,;23.65,-14.69,;22.31,-15.43,;20.99,-14.64,;21.01,-13.11,;19.65,-15.4,;18.26,-14.75,;17.2,-15.88,;17.95,-17.22,;19.46,-16.92,;24.98,-15.47,;24.96,-17.01,;26.33,-14.72,;27.64,-15.51,;27.51,-17.06,;28.62,-18.12,;30.15,-17.9,;31,-19.19,;30.94,-16.59,;30.39,-15.15,;28.92,-14.67,;28.91,-13.13,;32.47,-16.72,;33.12,-18.11,;31.95,-18.17,;33.36,-15.46,;32.7,-14.06,;33.57,-12.8,;35.12,-12.92,;35.77,-14.33,;34.89,-15.59,;24.96,-10.82,;26.3,-10.04,)| Show InChI InChI=1S/C27H36N4O6S/c1-18-8-11-20(12-9-18)16-22(30-27(34)24-6-5-15-37-24)26(33)29-21-13-10-19(2)31(17-23(21)32)38(35,36)25-7-3-4-14-28-25/h3-7,14-15,18-22H,8-13,16-17H2,1-2H3,(H,29,33)(H,30,34)/t18?,19-,20?,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349212

(CHEMBL1807701)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H41N5O6S/c1-20-9-10-21(23(33)19-32(20)39(36,37)24-8-4-7-13-28-24)29-25(34)22(18-27(2)11-5-3-6-12-27)30-26(35)31-14-16-38-17-15-31/h4,7-8,13,20-22H,3,5-6,9-12,14-19H2,1-2H3,(H,29,34)(H,30,35)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human Raji cells assessed as decrease in cell surface expression of MHC class 2/CLIP by flow cytometric analysis |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349202

(CHEMBL1807697)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CCC2CCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-18-11-13-20(22(31)17-30(18)37(34,35)24-10-4-5-15-27-24)28-25(32)21(14-12-19-7-2-3-8-19)29-26(33)23-9-6-16-36-23/h4-6,9-10,15-16,18-21H,2-3,7-8,11-14,17H2,1H3,(H,28,32)(H,29,33)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human Raji cells assessed as decrease in cell surface expression of MHC class 2/CLIP by flow cytometric analysis |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50349205

(CHEMBL1807700)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CCC2CCCC2)NC(=O)N2CCOCC2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H39N5O6S/c1-19-9-11-21(23(32)18-31(19)38(35,36)24-8-4-5-13-27-24)28-25(33)22(12-10-20-6-2-3-7-20)29-26(34)30-14-16-37-17-15-30/h4-5,8,13,19-22H,2-3,6-7,9-12,14-18H2,1H3,(H,28,33)(H,29,34)/t19-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K using z-LR-AFC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50349211

(CHEMBL1807652)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N4O6S/c1-19-11-12-20(22(32)18-31(19)38(35,36)24-10-4-7-15-28-24)29-25(33)21(17-27(2)13-5-3-6-14-27)30-26(34)23-9-8-16-37-23/h4,7-10,15-16,19-21H,3,5-6,11-14,17-18H2,1-2H3,(H,29,33)(H,30,34)/t19-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S in human Raji cells assessed as decrease in cell surface expression of MHC class 2/CLIP by flow cytometric analysis |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50349201

(CHEMBL1807696)Show SMILES C[C@@H]1CC[C@H](NC(=O)[C@@H](CC2(C)CCCCC2)NC(=O)c2ccco2)C(=O)CN1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N4O6S/c1-19-11-12-20(22(32)18-31(19)38(35,36)24-10-4-7-15-28-24)29-25(33)21(17-27(2)13-5-3-6-14-27)30-26(34)23-9-8-16-37-23/h4,7-10,15-16,19-21H,3,5-6,11-14,17-18H2,1-2H3,(H,29,33)(H,30,34)/t19-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L using Z-LR-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 21: 4409-15 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.045
BindingDB Entry DOI: 10.7270/Q2TT4R90 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data