 Found 54 hits with Last Name = 'preobrazhenskaya' and Initial = 'mn'
Found 54 hits with Last Name = 'preobrazhenskaya' and Initial = 'mn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50012948
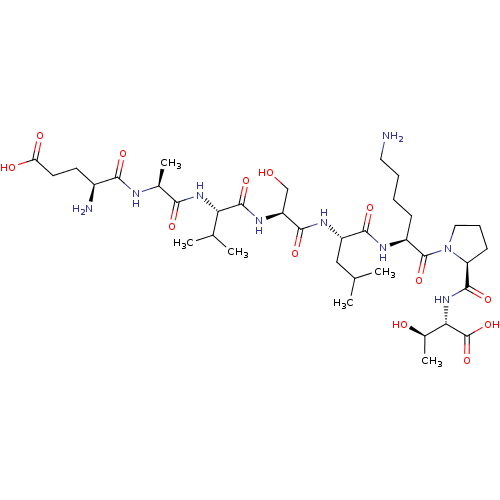
(CHEMBL3261358)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C37H65N9O13/c1-18(2)16-24(32(53)41-23(10-7-8-14-38)36(57)46-15-9-11-26(46)34(55)45-29(21(6)48)37(58)59)42-33(54)25(17-47)43-35(56)28(19(3)4)44-30(51)20(5)40-31(52)22(39)12-13-27(49)50/h18-26,28-29,47-48H,7-17,38-39H2,1-6H3,(H,40,52)(H,41,53)(H,42,54)(H,43,56)(H,44,51)(H,45,55)(H,49,50)(H,58,59)/t20-,21+,22-,23-,24-,25-,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Innsbruck Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PKCepsilon (unknown origin)-MBP-tagged RACK2 interaction after 1 hr by ELISA-based assay relative to untreated control |
J Med Chem 57: 3235-46 (2014)
Article DOI: 10.1021/jm401605c
BindingDB Entry DOI: 10.7270/Q2028T25 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50012920
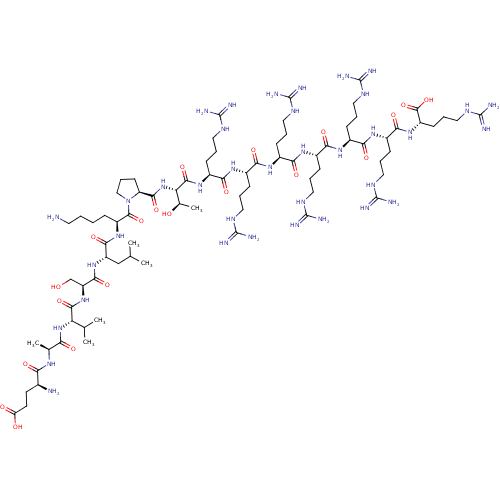
(CHEMBL3261356)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C79H149N37O20/c1-39(2)37-52(112-67(130)53(38-117)113-69(132)56(40(3)4)114-58(121)41(5)103-59(122)43(81)26-27-55(119)120)66(129)110-50(17-7-8-28-80)71(134)116-36-16-25-54(116)68(131)115-57(42(6)118)70(133)109-49(23-14-34-101-78(92)93)64(127)107-47(21-12-32-99-76(88)89)62(125)105-45(19-10-30-97-74(84)85)60(123)104-44(18-9-29-96-73(82)83)61(124)106-46(20-11-31-98-75(86)87)63(126)108-48(22-13-33-100-77(90)91)65(128)111-51(72(135)136)24-15-35-102-79(94)95/h39-54,56-57,117-118H,7-38,80-81H2,1-6H3,(H,103,122)(H,104,123)(H,105,125)(H,106,124)(H,107,127)(H,108,126)(H,109,133)(H,110,129)(H,111,128)(H,112,130)(H,113,132)(H,114,121)(H,115,131)(H,119,120)(H,135,136)(H4,82,83,96)(H4,84,85,97)(H4,86,87,98)(H4,88,89,99)(H4,90,91,100)(H4,92,93,101)(H4,94,95,102)/t41-,42+,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,56-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Innsbruck Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PKCepsilon (unknown origin)-MBP-tagged RACK2 interaction after 1 hr by ELISA-based assay relative to untreated control |
J Med Chem 57: 3235-46 (2014)
Article DOI: 10.1021/jm401605c
BindingDB Entry DOI: 10.7270/Q2028T25 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50246815
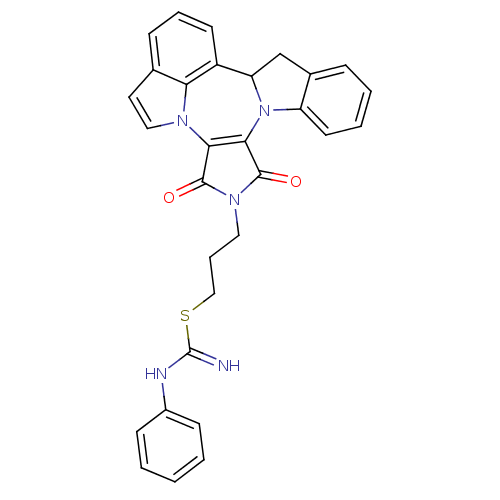
(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50246774
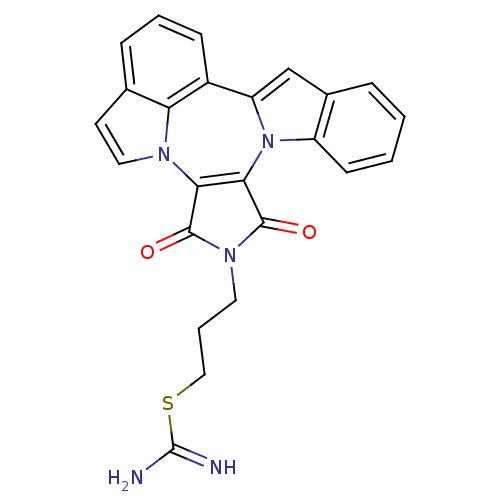
(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human INSR |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/CycD1 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human MET |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human SRC |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50246776
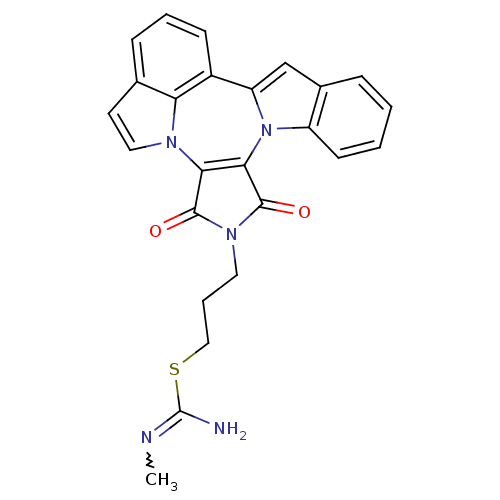
(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-1
(Homo sapiens (Human)) | BDBM50135227
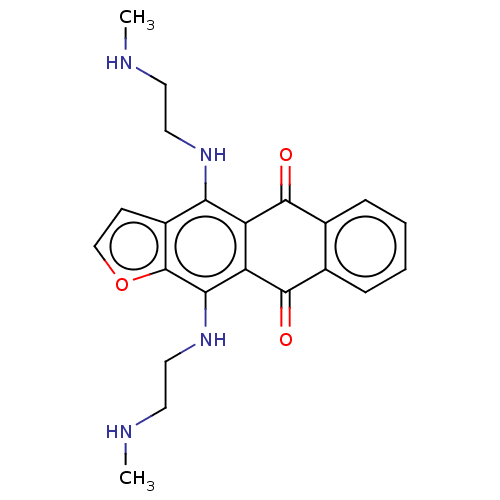
(CHEMBL3747698)Show SMILES CNCCNc1c2C(=O)c3ccccc3C(=O)c2c(NCCNC)c2occc12 Show InChI InChI=1S/C22H24N4O3/c1-23-8-10-25-18-15-7-12-29-22(15)19(26-11-9-24-2)17-16(18)20(27)13-5-3-4-6-14(13)21(17)28/h3-7,12,23-26H,8-11H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gause Institute of New Antibiotics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SIRT1 deacetylase activity using Arg-His-Lys-Lys(epsilon-acetyl)-AMC as substrate incubated for 45 mins by fluorescen... |
J Med Chem 58: 9522-34 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00859
BindingDB Entry DOI: 10.7270/Q21Z467Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFRbeta |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50246815

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246730
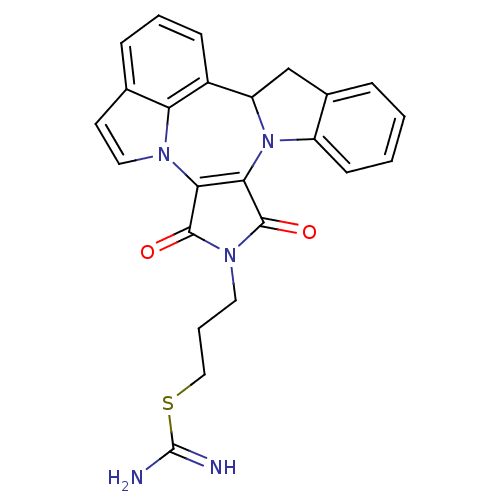
(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES NC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:10| Show InChI InChI=1S/C24H21N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,18H,4,10,12-13H2,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human FAK |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human AXL |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR3 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Dual serine/threonine and tyrosine protein kinase
(Homo sapiens (Human)) | BDBM50154291
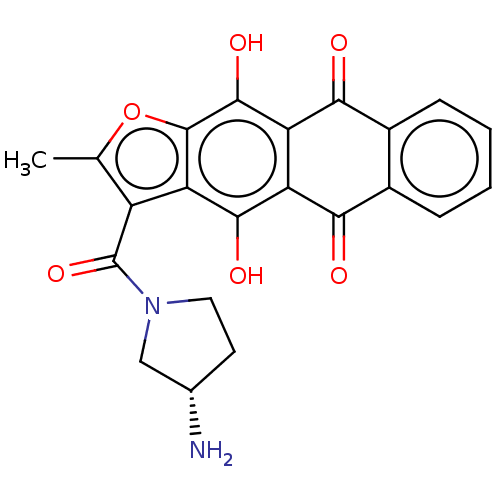
(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human RIPK5 by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase tousled-like 1
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG, C-terminal HIS8-tagged TLK1 expressed in sf9 cells by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal GST-HIS6-tagged PIM3 expressed in sf9 cells by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal GST-HIS6-tagged PIM1 expressed in sf9 cells by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human MKNK1 by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human MAP3K7 by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal GST-HIS6-tagged Aurora C expressed in sf9 cells by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50154291

(CHEMBL3775181)Show SMILES CS(O)(=O)=O.Cc1oc2c(O)c3C(=O)c4ccccc4C(=O)c3c(O)c2c1C(=O)N1CC[C@H](N)C1 |r| Show InChI InChI=1S/C22H18N2O6.CH4O3S/c1-9-13(22(29)24-7-6-10(23)8-24)16-19(27)14-15(20(28)21(16)30-9)18(26)12-5-3-2-4-11(12)17(14)25;1-5(2,3)4/h2-5,10,27-28H,6-8,23H2,1H3;1H3,(H,2,3,4)/t10-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Russia; Mendeleyev University of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal GST-HIS6-tagged Aurora B expressed in sf9 cells by flashplate based radiometric 33pan-quinase assay |
Eur J Med Chem 112: 114-29 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.050
BindingDB Entry DOI: 10.7270/Q2VQ34K1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246815

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50246732
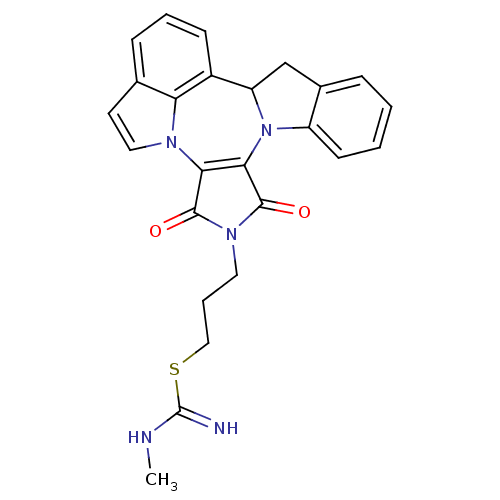
(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES CNC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:11| Show InChI InChI=1S/C25H23N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,19H,5,11,13-14H2,1H3,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human INSR |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246732

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES CNC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:11| Show InChI InChI=1S/C25H23N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,19H,5,11,13-14H2,1H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50246732

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES CNC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:11| Show InChI InChI=1S/C25H23N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,19H,5,11,13-14H2,1H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/CycD1 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50012919

(CHEMBL3261343)Show SMILES CC(=O)c1cccc(NC(=O)c2sc3nc4cc5OCCOc5cc4cc3c2N)c1 Show InChI InChI=1S/C22H17N3O4S/c1-11(26)12-3-2-4-14(7-12)24-21(27)20-19(23)15-8-13-9-17-18(29-6-5-28-17)10-16(13)25-22(15)30-20/h2-4,7-10H,5-6,23H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Innsbruck Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PKCepsilon (unknown origin)-MBP-tagged RACK2 interaction after 1 hr by ELISA-based assay relative to untreated control |
J Med Chem 57: 3235-46 (2014)
Article DOI: 10.1021/jm401605c
BindingDB Entry DOI: 10.7270/Q2028T25 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/CycD1 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human INSR |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50246732

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES CNC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:11| Show InChI InChI=1S/C25H23N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,19H,5,11,13-14H2,1H3,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human MET |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFRbeta |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246731
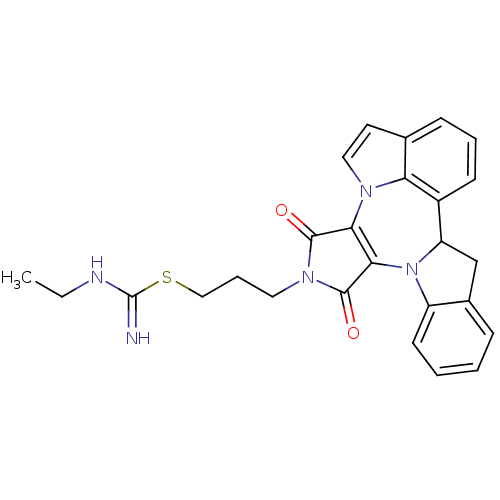
(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES CCNC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:12| Show InChI InChI=1S/C26H25N5O2S/c1-2-28-26(27)34-14-6-12-30-24(32)22-23(25(30)33)31-19-10-4-3-7-17(19)15-20(31)18-9-5-8-16-11-13-29(22)21(16)18/h3-5,7-11,13,20H,2,6,12,14-15H2,1H3,(H2,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50246773
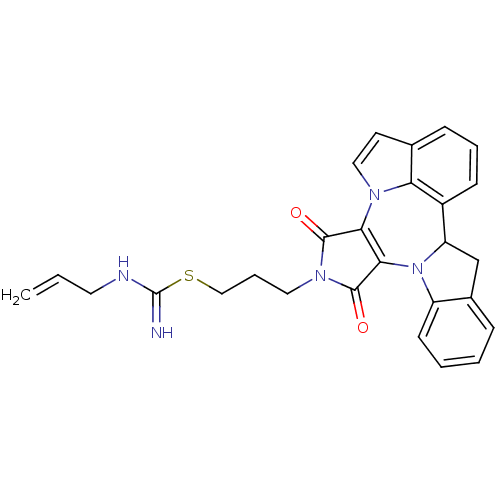
(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES C=CCNC(=N)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:13| Show InChI InChI=1S/C27H25N5O2S/c1-2-12-29-27(28)35-15-6-13-31-25(33)23-24(26(31)34)32-20-10-4-3-7-18(20)16-21(32)19-9-5-8-17-11-14-30(23)22(17)19/h2-5,7-11,14,21H,1,6,12-13,15-16H2,(H2,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/CycD1 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human MET |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human ERBB2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50246775

(2-[3-(4,5-Dihydro-1H-imidazol-2-ylsulfanyl)propyl]...)Show SMILES O=C1N(CCCSC2=NCCN2)C(=O)C2=C1N1C(Cc3ccccc13)c1cccc3ccn2c13 |c:15,t:7| Show InChI InChI=1S/C26H23N5O2S/c32-24-22-23(25(33)30(24)12-4-14-34-26-27-10-11-28-26)31-19-8-2-1-5-17(19)15-20(31)18-7-3-6-16-9-13-29(22)21(16)18/h1-3,5-9,13,20H,4,10-12,14-15H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyc2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50246815

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/CycD1 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50246815

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human TIE2 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50246774

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES NC(=N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O Show InChI InChI=1S/C24H19N5O2S/c25-24(26)32-12-4-10-28-22(30)20-21(23(28)31)29-17-8-2-1-5-15(17)13-18(29)16-7-3-6-14-9-11-27(20)19(14)16/h1-3,5-9,11,13H,4,10,12H2,(H3,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50246815

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50246815

(3-(1,3-Dioxo-1,3,9b,10-tetrahydro-2H-indolo[1',7':...)Show SMILES N=C(Nc1ccccc1)SCCCN1C(=O)C2=C(C1=O)n1ccc3cccc(C4Cc5ccccc5N24)c13 |c:17| Show InChI InChI=1S/C30H25N5O2S/c31-30(32-21-10-2-1-3-11-21)38-17-7-15-34-28(36)26-27(29(34)37)35-23-13-5-4-8-20(23)18-24(35)22-12-6-9-19-14-16-33(26)25(19)22/h1-6,8-14,16,24H,7,15,17-18H2,(H2,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR3 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human ARK5 |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50246776

(3-(1,3-Dioxo-1,3-dihydro-2H-indolo[1',7':4,5,6]pyr...)Show SMILES CN=C(N)SCCCn1c(=O)c2c(n3c(cc4ccccc34)c3cccc4ccn2c34)c1=O |w:1.0| Show InChI InChI=1S/C25H21N5O2S/c1-27-25(26)33-13-5-11-29-23(31)21-22(24(29)32)30-18-9-3-2-6-16(18)14-19(30)17-8-4-7-15-10-12-28(21)20(15)17/h2-4,6-10,12,14H,5,11,13H2,1H3,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of General Genetics
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR |
J Med Chem 51: 7731-6 (2008)
Article DOI: 10.1021/jm800758s
BindingDB Entry DOI: 10.7270/Q2HD7VH4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data