 Found 1722 hits with Last Name = 'qi' and Initial = 'n'
Found 1722 hits with Last Name = 'qi' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50188619
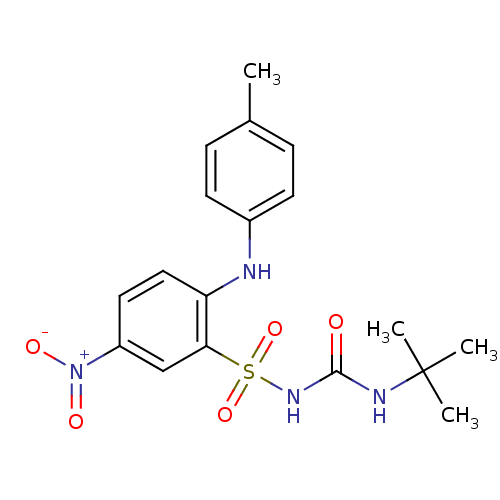
(1-(2-(p-toluidino)-5-nitrophenylsulfonyl)-3-tert-b...)Show SMILES Cc1ccc(Nc2ccc(cc2S(=O)(=O)NC(=O)NC(C)(C)C)[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22N4O5S/c1-12-5-7-13(8-6-12)19-15-10-9-14(22(24)25)11-16(15)28(26,27)21-17(23)20-18(2,3)4/h5-11,19H,1-4H3,(H2,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50188619

(1-(2-(p-toluidino)-5-nitrophenylsulfonyl)-3-tert-b...)Show SMILES Cc1ccc(Nc2ccc(cc2S(=O)(=O)NC(=O)NC(C)(C)C)[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22N4O5S/c1-12-5-7-13(8-6-12)19-15-10-9-14(22(24)25)11-16(15)28(26,27)21-17(23)20-18(2,3)4/h5-11,19H,1-4H3,(H2,20,21,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50188619

(1-(2-(p-toluidino)-5-nitrophenylsulfonyl)-3-tert-b...)Show SMILES Cc1ccc(Nc2ccc(cc2S(=O)(=O)NC(=O)NC(C)(C)C)[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22N4O5S/c1-12-5-7-13(8-6-12)19-15-10-9-14(22(24)25)11-16(15)28(26,27)21-17(23)20-18(2,3)4/h5-11,19H,1-4H3,(H2,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167939
(3,4-DNH | 5-Hydroxy Tryptamine | BM 613 | Benzen |...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50167939

(3,4-DNH | 5-Hydroxy Tryptamine | BM 613 | Benzen |...) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167939

(3,4-DNH | 5-Hydroxy Tryptamine | BM 613 | Benzen |...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086717

(JNJ-39439335 | Mavatrep)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RTX from human TRPV1 transfected in HEK293 cells after 60 mins by scintillation spectroscopic analysis |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM86670
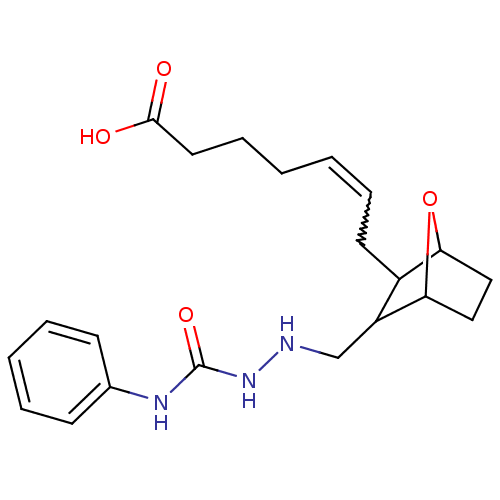
(CAS_98299-61-7 | NSC_104927 | SQ 29548)Show SMILES OC(=O)CCCC=CCC1C2CCC(O2)C1CNNC(=O)Nc1ccccc1 |w:7.7,TLB:16:15:11.12:14,THB:8:9:11.12:14| Show InChI InChI=1S/C21H29N3O4/c25-20(26)11-7-2-1-6-10-16-17(19-13-12-18(16)28-19)14-22-24-21(27)23-15-8-4-3-5-9-15/h1,3-6,8-9,16-19,22H,2,7,10-14H2,(H,25,26)(H2,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, adipocyte
(Rattus norvegicus) | BDBM50212873
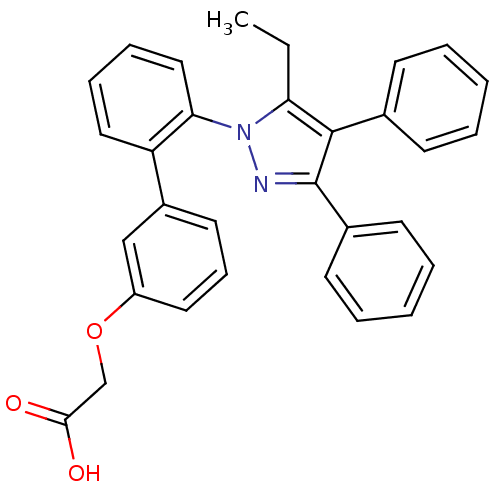
(((2'-(5-ETHYL-3,4-DIPHENYL-1H-PYRAZOL-1-YL)-3-BIPH...)Show SMILES CCc1c(c(nn1-c1ccccc1-c1cccc(OCC(O)=O)c1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H26N2O3/c1-2-27-30(22-12-5-3-6-13-22)31(23-14-7-4-8-15-23)32-33(27)28-19-10-9-18-26(28)24-16-11-17-25(20-24)36-21-29(34)35/h3-20H,2,21H2,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of rat ap2 by fluorescent 1,8-anilino-8-naphthalene sulfonate assay |
Eur J Med Chem 52: 70-81 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.006
BindingDB Entry DOI: 10.7270/Q26H4JD5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty acid-binding protein, adipocyte
(Rattus norvegicus) | BDBM50381857
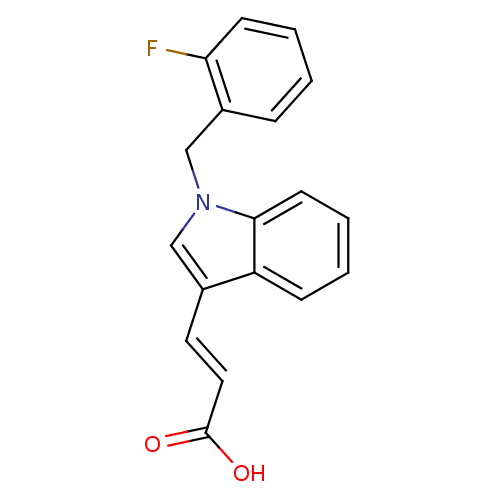
(CHEMBL2023268)Show InChI InChI=1S/C18H14FNO2/c19-16-7-3-1-5-14(16)12-20-11-13(9-10-18(21)22)15-6-2-4-8-17(15)20/h1-11H,12H2,(H,21,22)/b10-9+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of rat ap2 by fluorescent 1,8-anilino-8-naphthalene sulfonate assay |
Eur J Med Chem 52: 70-81 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.006
BindingDB Entry DOI: 10.7270/Q26H4JD5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by ThermoFluor method |
J Med Chem 52: 7528-36 (2009)
Article DOI: 10.1021/jm801432r
BindingDB Entry DOI: 10.7270/Q21J9BQD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167939

(3,4-DNH | 5-Hydroxy Tryptamine | BM 613 | Benzen |...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li&eagrove;ge
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 293-301 (2005)
Article DOI: 10.1124/jpet.104.079301
BindingDB Entry DOI: 10.7270/Q2NV9GVJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587720
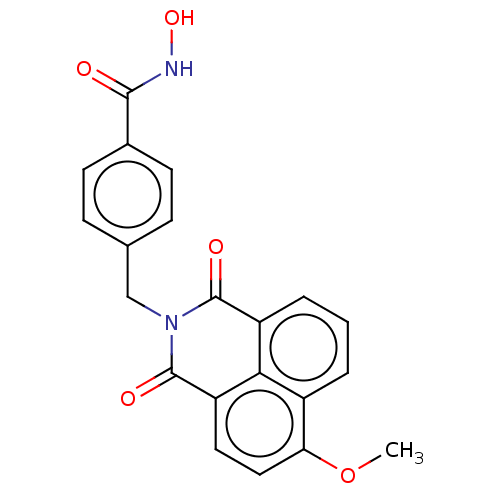
(CHEMBL5195946)Show SMILES COc1ccc2C(=O)N(Cc3ccc(cc3)C(=O)NO)C(=O)c3cccc1c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM248583
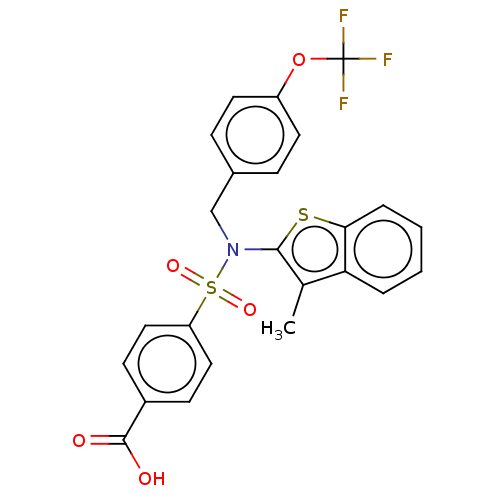
(US9434711, 496)Show SMILES Cc1c(sc2ccccc12)N(Cc1ccc(OC(F)(F)F)cc1)S(=O)(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H18F3NO5S2/c1-15-20-4-2-3-5-21(20)34-22(15)28(14-16-6-10-18(11-7-16)33-24(25,26)27)35(31,32)19-12-8-17(9-13-19)23(29)30/h2-13H,14H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica, N.V.
US Patent
| Assay Description
For patch clamp experiments, HEK293 cells are stably transfected with canine TRPM8 and cultured in DMEM supplemented with 10% fetal bovine serum, 100... |
US Patent US9434711 (2016)
BindingDB Entry DOI: 10.7270/Q2H41QB6 |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Homo sapiens (Human)) | BDBM50147315
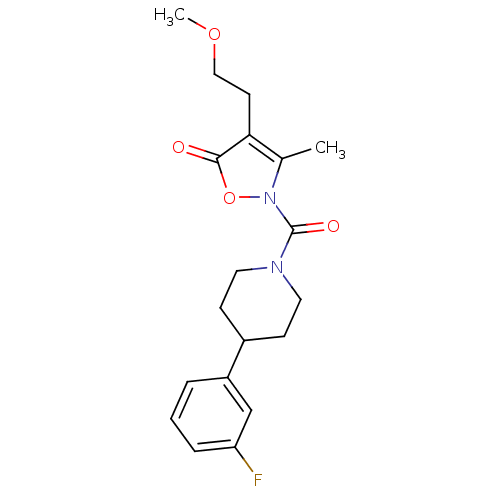
(2-(4-(3-fluorophenyl)piperidine-1-carbonyl)-4-(2-m...)Show SMILES COCCc1c(C)n(oc1=O)C(=O)N1CCC(CC1)c1cccc(F)c1 Show InChI InChI=1S/C19H23FN2O4/c1-13-17(8-11-25-2)18(23)26-22(13)19(24)21-9-6-14(7-10-21)15-4-3-5-16(20)12-15/h3-5,12,14H,6-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant hormone sensitive lipase (HSL) |
Bioorg Med Chem Lett 14: 3155-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.015
BindingDB Entry DOI: 10.7270/Q2NV9HQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM50335165

(3-[2-(1-Oxa-2-aza-spiro[4.5]dec-2-en-3-yl)-6-(2-tr...)Show SMILES OCCCc1cc(cc2[nH]c(nc12)C1=NOC2(C1)CCCCC2)-c1ccccc1C(F)(F)F |t:15| Show InChI InChI=1S/C25H26F3N3O2/c26-25(27,28)19-9-3-2-8-18(19)17-13-16(7-6-12-32)22-20(14-17)29-23(30-22)21-15-24(33-31-21)10-4-1-5-11-24/h2-3,8-9,13-14,32H,1,4-7,10-12,15H2,(H,29,30) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at canine TRPM8 expressed in HEK293 cells assessed as inhibition of intracellular calcium accumulation by FLIPR assay |
J Med Chem 54: 233-47 (2011)
Article DOI: 10.1021/jm101075v
BindingDB Entry DOI: 10.7270/Q20C4WRJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040437
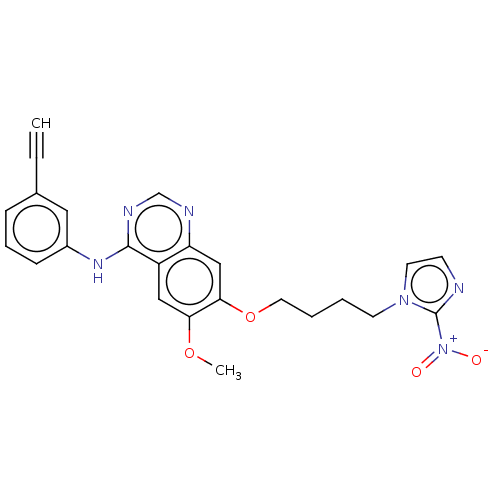
(CHEMBL3360608)Show SMILES COc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C24H22N6O4/c1-3-17-7-6-8-18(13-17)28-23-19-14-21(33-2)22(15-20(19)26-16-27-23)34-12-5-4-10-29-11-9-25-24(29)30(31)32/h1,6-9,11,13-16H,4-5,10,12H2,2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040432
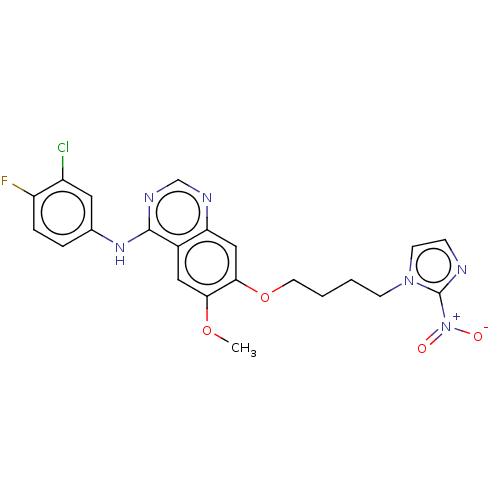
(CHEMBL3360603)Show SMILES COc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1OCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C22H20ClFN6O4/c1-33-19-11-15-18(26-13-27-21(15)28-14-4-5-17(24)16(23)10-14)12-20(19)34-9-3-2-7-29-8-6-25-22(29)30(31)32/h4-6,8,10-13H,2-3,7,9H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039330

(CHEMBL3355877)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C24H24ClFN6O4/c1-35-21-14-20-17(23(29-15-28-20)30-16-6-7-19(26)18(25)12-16)13-22(21)36-11-5-3-2-4-9-31-10-8-27-24(31)32(33)34/h6-8,10,12-15H,2-5,9,11H2,1H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040435

(CHEMBL3360606)Show SMILES COc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1OCCOCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C22H20ClFN6O5/c1-33-19-11-15-18(26-13-27-21(15)28-14-2-3-17(24)16(23)10-14)12-20(19)35-9-8-34-7-6-29-5-4-25-22(29)30(31)32/h2-5,10-13H,6-9H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040449

(CHEMBL3360619)Show SMILES COc1cc2c(Nc3cccc(Br)c3)ncnc2cc1OCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C23H23BrN6O4/c1-33-20-13-18-19(26-15-27-22(18)28-17-7-5-6-16(24)12-17)14-21(20)34-11-4-2-3-9-29-10-8-25-23(29)30(31)32/h5-8,10,12-15H,2-4,9,11H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 1
(Mus musculus) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of mouse TSSK1 by luminescent ADP-Glo assay kit |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040448
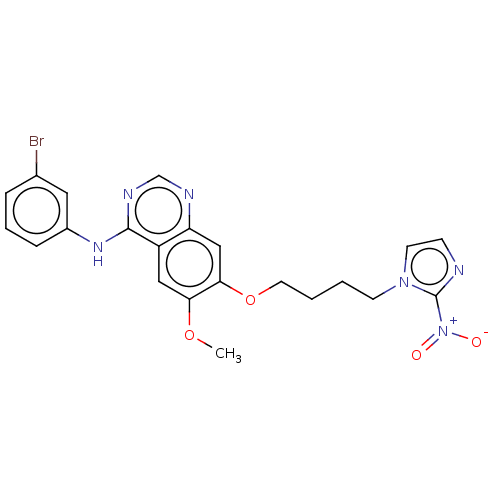
(CHEMBL3360618)Show SMILES COc1cc2c(Nc3cccc(Br)c3)ncnc2cc1OCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C22H21BrN6O4/c1-32-19-12-17-18(25-14-26-21(17)27-16-6-4-5-15(23)11-16)13-20(19)33-10-3-2-8-28-9-7-24-22(28)29(30)31/h4-7,9,11-14H,2-3,8,10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587716
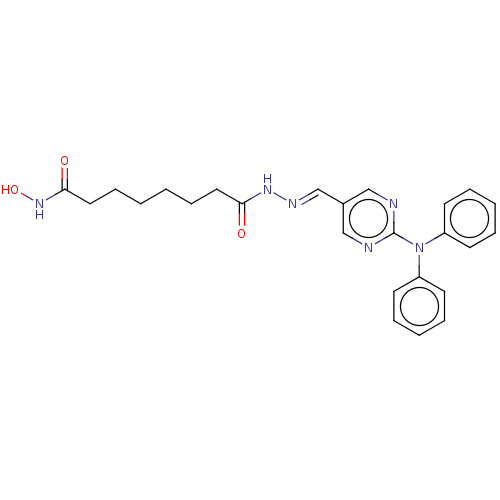
(CHEMBL5188297)Show SMILES ONC(=O)CCCCCCC(=O)N\N=C\c1cnc(nc1)N(c1ccccc1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM50036555
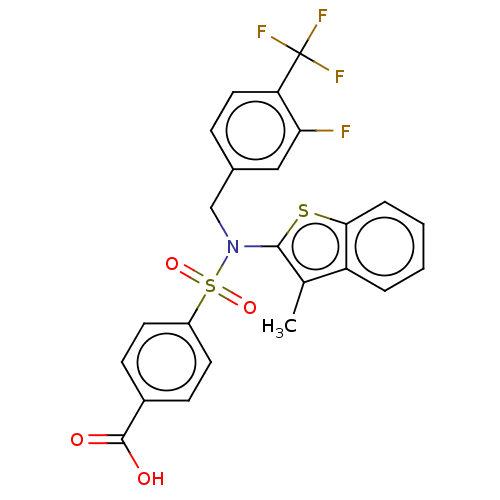
(CHEMBL3353610 | US9434711, 497)Show SMILES Cc1c(sc2ccccc12)N(Cc1ccc(c(F)c1)C(F)(F)F)S(=O)(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H17F4NO4S2/c1-14-18-4-2-3-5-21(18)34-22(14)29(13-15-6-11-19(20(25)12-15)24(26,27)28)35(32,33)17-9-7-16(8-10-17)23(30)31/h2-12H,13H2,1H3,(H,30,31) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V.
US Patent
| Assay Description
The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... |
US Patent US9434711 (2016)
BindingDB Entry DOI: 10.7270/Q2H41QB6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50335157
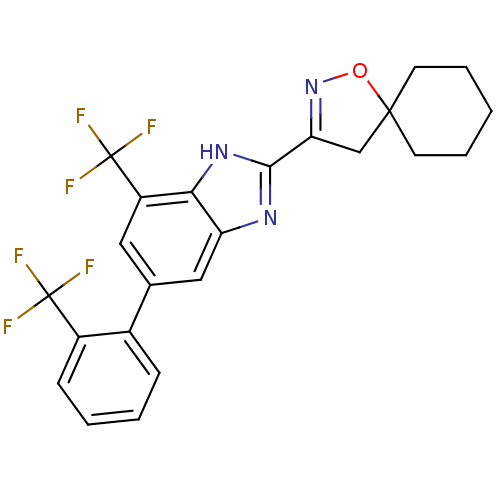
(3-[7-Trifluoromethyl-5-(2-trifluoromethyl-phenyl)-...)Show SMILES FC(F)(F)c1ccccc1-c1cc(c2[nH]c(nc2c1)C1=NOC2(C1)CCCCC2)C(F)(F)F |t:22| Show InChI InChI=1S/C23H19F6N3O/c24-22(25,26)15-7-3-2-6-14(15)13-10-16(23(27,28)29)19-17(11-13)30-20(31-19)18-12-21(33-32-18)8-4-1-5-9-21/h2-3,6-7,10-11H,1,4-5,8-9,12H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.413 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human TRPM8 expressed in HEK293 cells assessed as inhibition of cold-induced channel current by whole cell electrophysiology |
J Med Chem 54: 233-47 (2011)
Article DOI: 10.1021/jm101075v
BindingDB Entry DOI: 10.7270/Q20C4WRJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50188618
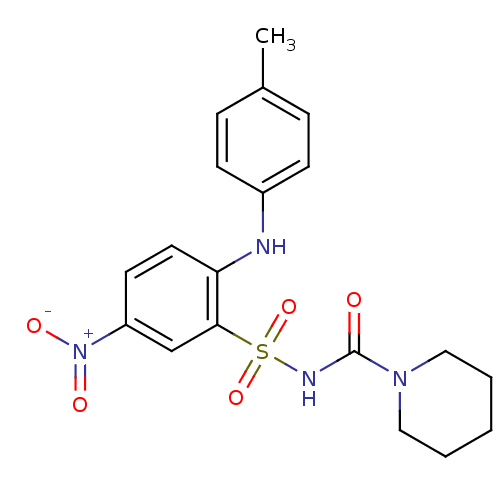
(CHEMBL209168 | N-[2-(4-methylphenylamino)-5-nitrob...)Show SMILES Cc1ccc(Nc2ccc(cc2S(=O)(=O)NC(=O)N2CCCCC2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C19H22N4O5S/c1-14-5-7-15(8-6-14)20-17-10-9-16(23(25)26)13-18(17)29(27,28)21-19(24)22-11-3-2-4-12-22/h5-10,13,20H,2-4,11-12H2,1H3,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liège
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ29,548 from TPalpha receptor (short isoform) expressed in COS7 cells at 1 uM |
J Med Chem 49: 3701-9 (2006)
Article DOI: 10.1021/jm060108a
BindingDB Entry DOI: 10.7270/Q2X63MJT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039328

(CHEMBL3355875)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C22H20ClFN6O4/c1-33-19-12-18-15(21(27-13-26-18)28-14-4-5-17(24)16(23)10-14)11-20(19)34-9-3-2-7-29-8-6-25-22(29)30(31)32/h4-6,8,10-13H,2-3,7,9H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040438

(CHEMBL3360609)Show SMILES COc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C25H24N6O4/c1-3-18-8-7-9-19(14-18)29-24-20-15-22(34-2)23(16-21(20)27-17-28-24)35-13-6-4-5-11-30-12-10-26-25(30)31(32)33/h1,7-10,12,14-17H,4-6,11,13H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039334
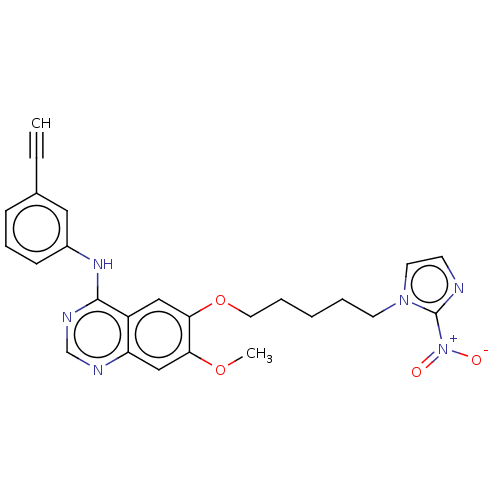
(CHEMBL3355881)Show SMILES COc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C25H24N6O4/c1-3-18-8-7-9-19(14-18)29-24-20-15-23(22(34-2)16-21(20)27-17-28-24)35-13-6-4-5-11-30-12-10-26-25(30)31(32)33/h1,7-10,12,14-17H,4-6,11,13H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040447

(CHEMBL3360617)Show SMILES COc1cc2c(Nc3cccc(Br)c3)ncnc2cc1OCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C21H19BrN6O4/c1-31-18-11-16-17(24-13-25-20(16)26-15-5-2-4-14(22)10-15)12-19(18)32-9-3-7-27-8-6-23-21(27)28(29)30/h2,4-6,8,10-13H,3,7,9H2,1H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039335
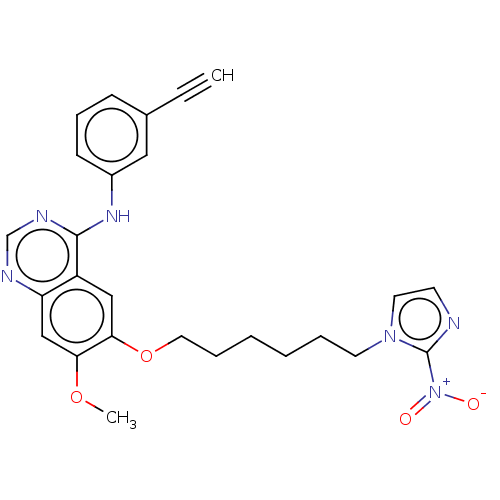
(CHEMBL3355882)Show SMILES COc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C26H26N6O4/c1-3-19-9-8-10-20(15-19)30-25-21-16-24(23(35-2)17-22(21)28-18-29-25)36-14-7-5-4-6-12-31-13-11-27-26(31)32(33)34/h1,8-11,13,15-18H,4-7,12,14H2,2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039329

(CHEMBL3355876)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C23H22ClFN6O4/c1-34-20-13-19-16(22(28-14-27-19)29-15-5-6-18(25)17(24)11-15)12-21(20)35-10-4-2-3-8-30-9-7-26-23(30)31(32)33/h5-7,9,11-14H,2-4,8,10H2,1H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040433

(CHEMBL3360604)Show SMILES COc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1OCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C23H22ClFN6O4/c1-34-20-12-16-19(27-14-28-22(16)29-15-5-6-18(25)17(24)11-15)13-21(20)35-10-4-2-3-8-30-9-7-26-23(30)31(32)33/h5-7,9,11-14H,2-4,8,10H2,1H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039459
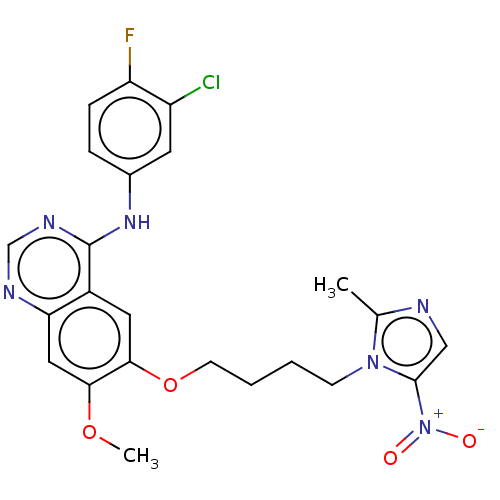
(CHEMBL3355889)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCCn1c(C)ncc1[N+]([O-])=O Show InChI InChI=1S/C23H22ClFN6O4/c1-14-26-12-22(31(32)33)30(14)7-3-4-8-35-21-10-16-19(11-20(21)34-2)27-13-28-23(16)29-15-5-6-18(25)17(24)9-15/h5-6,9-13H,3-4,7-8H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040441
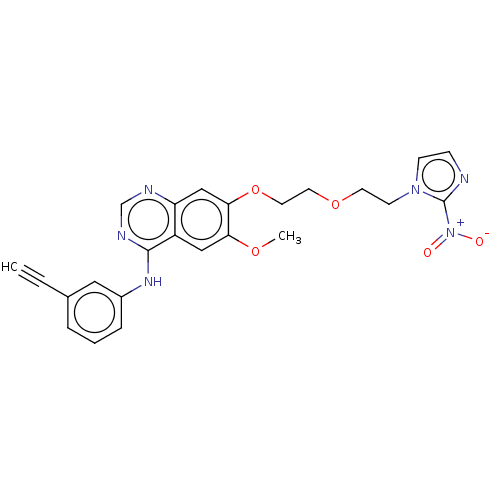
(CHEMBL3360611)Show SMILES COc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCOCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C24H22N6O5/c1-3-17-5-4-6-18(13-17)28-23-19-14-21(33-2)22(15-20(19)26-16-27-23)35-12-11-34-10-9-29-8-7-25-24(29)30(31)32/h1,4-8,13-16H,9-12H2,2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM248583

(US9434711, 496)Show SMILES Cc1c(sc2ccccc12)N(Cc1ccc(OC(F)(F)F)cc1)S(=O)(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H18F3NO5S2/c1-15-20-4-2-3-5-21(20)34-22(15)28(14-16-6-10-18(11-7-16)33-24(25,26)27)35(31,32)19-12-8-17(9-13-19)23(29)30/h2-13H,14H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.554 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica, N.V.
US Patent
| Assay Description
For patch clamp experiments, HEK293 cells are stably transfected with canine TRPM8 and cultured in DMEM supplemented with 10% fetal bovine serum, 100... |
US Patent US9434711 (2016)
BindingDB Entry DOI: 10.7270/Q2H41QB6 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50188608
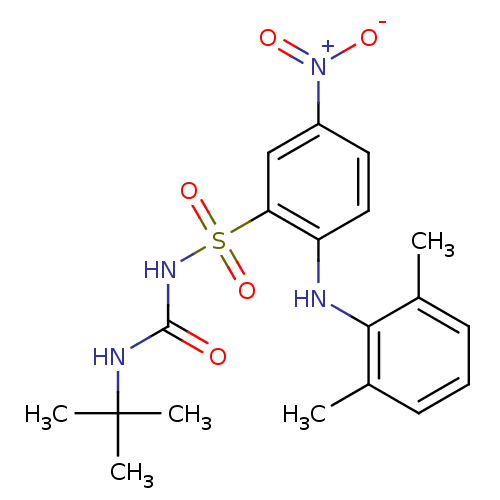
(CHEMBL209306 | N-tert-butyl-N'-[2-(2,6-dimethylphe...)Show SMILES Cc1cccc(C)c1Nc1ccc(cc1S(=O)(=O)NC(=O)NC(C)(C)C)[N+]([O-])=O Show InChI InChI=1S/C19H24N4O5S/c1-12-7-6-8-13(2)17(12)20-15-10-9-14(23(25)26)11-16(15)29(27,28)22-18(24)21-19(3,4)5/h6-11,20H,1-5H3,(H2,21,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liège
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ29,548 from TPbeta receptor (long isoform) expressed in COS7 cells at 1 uM |
J Med Chem 49: 3701-9 (2006)
Article DOI: 10.1021/jm060108a
BindingDB Entry DOI: 10.7270/Q2X63MJT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039333

(CHEMBL3355880)Show SMILES COc1cc2ncnc(Nc3cccc(c3)C#C)c2cc1OCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C24H22N6O4/c1-3-17-7-6-8-18(13-17)28-23-19-14-22(21(33-2)15-20(19)26-16-27-23)34-12-5-4-10-29-11-9-25-24(29)30(31)32/h1,6-9,11,13-16H,4-5,10,12H2,2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040440
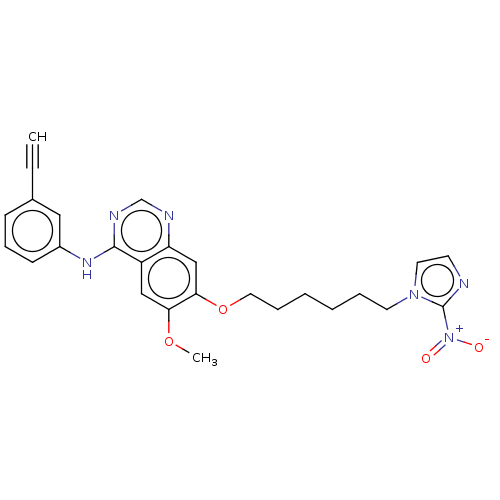
(CHEMBL3360610)Show SMILES COc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCCCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C26H26N6O4/c1-3-19-9-8-10-20(15-19)30-25-21-16-23(35-2)24(17-22(21)28-18-29-25)36-14-7-5-4-6-12-31-13-11-27-26(31)32(33)34/h1,8-11,13,15-18H,4-7,12,14H2,2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50188613
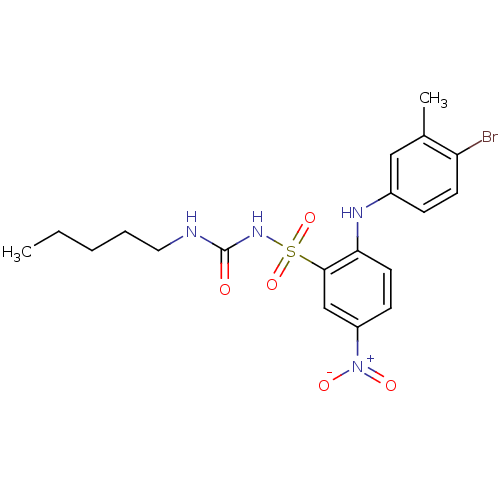
(CHEMBL209134 | N-n-pentyl-N'-[2-(3-methyl-4-bromop...)Show SMILES CCCCCNC(=O)NS(=O)(=O)c1cc(ccc1Nc1ccc(Br)c(C)c1)[N+]([O-])=O Show InChI InChI=1S/C19H23BrN4O5S/c1-3-4-5-10-21-19(25)23-30(28,29)18-12-15(24(26)27)7-9-17(18)22-14-6-8-16(20)13(2)11-14/h6-9,11-12,22H,3-5,10H2,1-2H3,(H2,21,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liège
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ29,548 from TPbeta receptor (long isoform) expressed in COS7 cells at 1 uM |
J Med Chem 49: 3701-9 (2006)
Article DOI: 10.1021/jm060108a
BindingDB Entry DOI: 10.7270/Q2X63MJT |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM248576
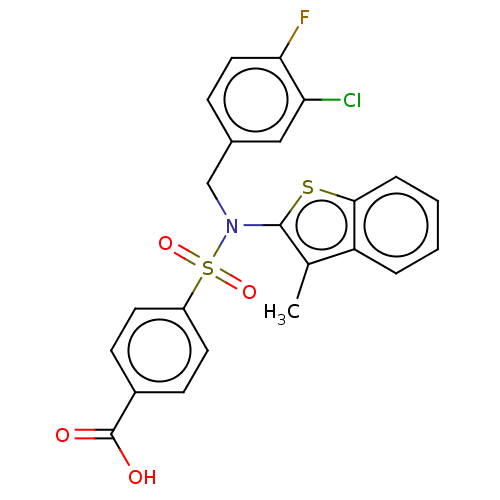
(US9434711, 489)Show SMILES Cc1c(sc2ccccc12)N(Cc1ccc(F)c(Cl)c1)S(=O)(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H17ClFNO4S2/c1-14-18-4-2-3-5-21(18)31-22(14)26(13-15-6-11-20(25)19(24)12-15)32(29,30)17-9-7-16(8-10-17)23(27)28/h2-12H,13H2,1H3,(H,27,28) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V.
US Patent
| Assay Description
The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... |
US Patent US9434711 (2016)
BindingDB Entry DOI: 10.7270/Q2H41QB6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM248370
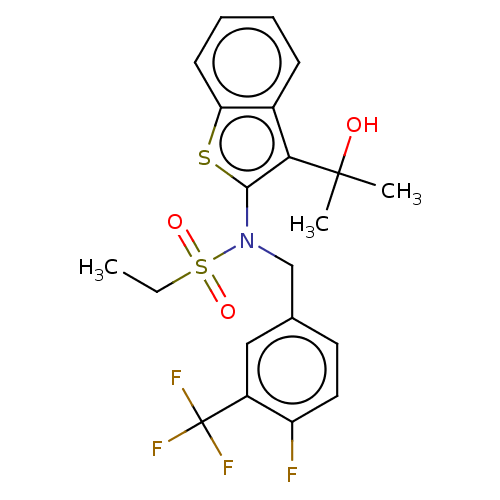
(US9434711, 227)Show SMILES CCS(=O)(=O)N(Cc1ccc(F)c(c1)C(F)(F)F)c1sc2ccccc2c1C(C)(C)O Show InChI InChI=1S/C21H21F4NO3S2/c1-4-31(28,29)26(12-13-9-10-16(22)15(11-13)21(23,24)25)19-18(20(2,3)27)14-7-5-6-8-17(14)30-19/h5-11,27H,4,12H2,1-3H3 | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V.
US Patent
| Assay Description
The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... |
US Patent US9434711 (2016)
BindingDB Entry DOI: 10.7270/Q2H41QB6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential M8 protein
(Canis lupus familiaris (Dog)) | BDBM50335170

(3-[5-(2-Fluoro-6-trifluoromethylphenyl)-1H-benzimi...)Show SMILES Fc1cccc(c1-c1ccc2nc([nH]c2c1)C1=NOC2(C1)CCCCC2)C(F)(F)F |t:19| Show InChI InChI=1S/C22H19F4N3O/c23-15-6-4-5-14(22(24,25)26)19(15)13-7-8-16-17(11-13)28-20(27-16)18-12-21(30-29-18)9-2-1-3-10-21/h4-8,11H,1-3,9-10,12H2,(H,27,28) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at canine TRPM8 expressed in HEK293 cells assessed as inhibition of intracellular calcium accumulation by FLIPR assay |
J Med Chem 54: 233-47 (2011)
Article DOI: 10.1021/jm101075v
BindingDB Entry DOI: 10.7270/Q20C4WRJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50040436
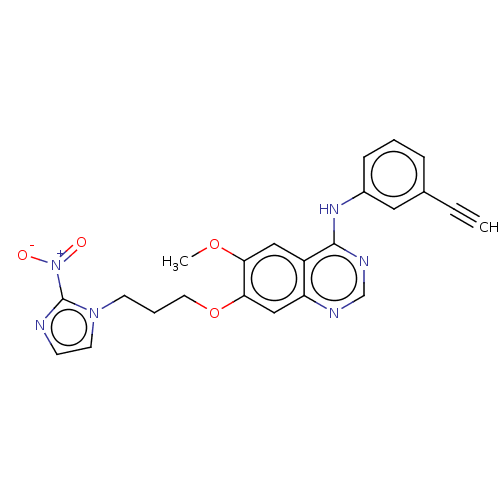
(CHEMBL3360607)Show SMILES COc1cc2c(Nc3cccc(c3)C#C)ncnc2cc1OCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C23H20N6O4/c1-3-16-6-4-7-17(12-16)27-22-18-13-20(32-2)21(14-19(18)25-15-26-22)33-11-5-9-28-10-8-24-23(28)29(30)31/h1,4,6-8,10,12-15H,5,9,11H2,2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Bioorg Med Chem 22: 6796-805 (2015)
Article DOI: 10.1016/j.bmc.2014.10.038
BindingDB Entry DOI: 10.7270/Q22J6DHQ |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50188615
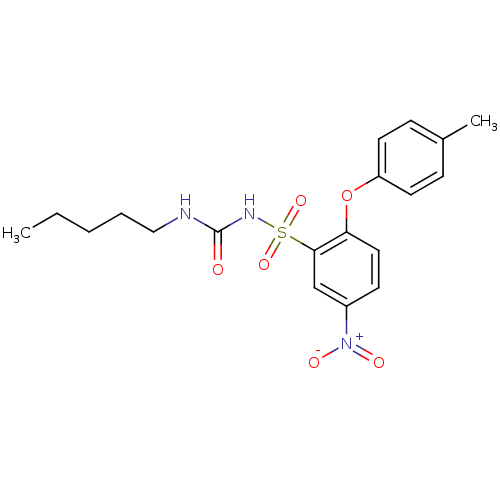
(CHEMBL274415 | N-n-pentyl-N'-[2-(4-methylphenoxy)-...)Show SMILES CCCCCNC(=O)NS(=O)(=O)c1cc(ccc1Oc1ccc(C)cc1)[N+]([O-])=O Show InChI InChI=1S/C19H23N3O6S/c1-3-4-5-12-20-19(23)21-29(26,27)18-13-15(22(24)25)8-11-17(18)28-16-9-6-14(2)7-10-16/h6-11,13H,3-5,12H2,1-2H3,(H2,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liège
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ29,548 from TPbeta receptor (long isoform) expressed in COS7 cells at 1 uM |
J Med Chem 49: 3701-9 (2006)
Article DOI: 10.1021/jm060108a
BindingDB Entry DOI: 10.7270/Q2X63MJT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50039331
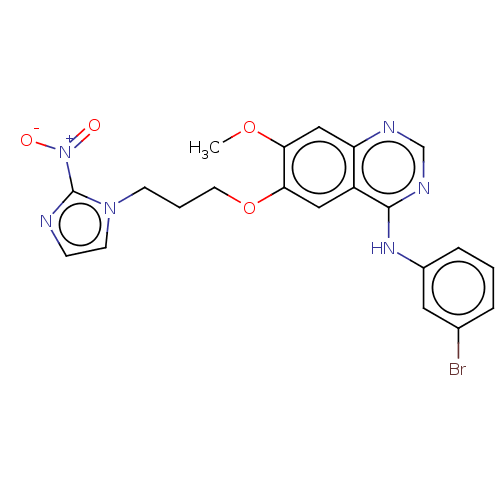
(CHEMBL3355878)Show SMILES COc1cc2ncnc(Nc3cccc(Br)c3)c2cc1OCCCn1ccnc1[N+]([O-])=O Show InChI InChI=1S/C21H19BrN6O4/c1-31-18-12-17-16(20(25-13-24-17)26-15-5-2-4-14(22)10-15)11-19(18)32-9-3-7-27-8-6-23-21(27)28(29)30/h2,4-6,8,10-13H,3,7,9H2,1H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) by Z'-Lyte kinase assay |
Eur J Med Chem 89: 826-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.11.010
BindingDB Entry DOI: 10.7270/Q24F1SC5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data