 Found 16502 hits with Last Name = 'qu' and Initial = 'j'
Found 16502 hits with Last Name = 'qu' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204090

(CHEMBL3958859)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#8])cc(-[#16]-c2ccccc2-[#6](-[#8])=O)c1-[#8] Show InChI InChI=1S/C33H42O4S/c1-23(2)11-8-12-24(3)13-9-14-25(4)15-10-16-26(5)19-20-27-21-28(34)22-31(32(27)35)38-30-18-7-6-17-29(30)33(36)37/h6-7,11,13,15,17-19,21-22,34-35H,8-10,12,14,16,20H2,1-5H3,(H,36,37)/b24-13+,25-15+,26-19+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537072

(CHEMBL440072)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C63H88N16O16S2/c1-34(66)53(84)69-30-51(83)70-48-32-96-97-33-49(63(94)95)78-60(91)47(31-80)77-62(93)52(35(2)81)79-55(86)42(22-12-14-24-65)71-58(89)45(27-38-29-68-40-20-10-9-19-39(38)40)75-57(88)44(26-37-17-7-4-8-18-37)73-56(87)43(25-36-15-5-3-6-16-36)74-59(90)46(28-50(67)82)76-54(85)41(72-61(48)92)21-11-13-23-64/h3-10,15-20,29,34-35,41-49,52,68,80-81H,11-14,21-28,30-33,64-66H2,1-2H3,(H2,67,82)(H,69,84)(H,70,83)(H,71,89)(H,72,92)(H,73,87)(H,74,90)(H,75,88)(H,76,85)(H,77,93)(H,78,91)(H,79,86)(H,94,95)/t34-,35+,41+,42-,43+,44-,45+,46-,47-,48+,49-,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537063

(CHEMBL4590517)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(O)=O |r,t:17,19| Show InChI InChI=1S/C83H108ClN13O21S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)95-83)45(2)71-82(5,118-71)66(38-68(101)97(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)96(6)67(100)29-31-119-120-43-61(79(109)110)93-77(107)60-42-122-121-41-59(91-72(102)54(86)33-48-19-11-10-12-20-48)76(106)89-57(34-49-25-27-52(99)28-26-49)74(104)90-58(37-51-40-87-55-22-14-13-21-53(51)55)75(105)88-56(23-15-16-30-85)73(103)94-70(47(4)98)78(108)92-60/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,87,98-99,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H,88,105)(H,89,106)(H,90,104)(H,91,102)(H,92,108)(H,93,107)(H,94,103)(H,95,112)(H,109,110)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537077

(CHEMBL4550617)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCOc1ccc(C[C@@H](N)C(=O)N[C@H]2CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)cc1 |r,t:17,19| Show InChI InChI=1S/C86H114ClN13O23S4/c1-45-16-15-20-67(119-10)86(117)41-66(121-84(116)98-86)46(2)74-85(6,123-74)68(40-70(105)100(8)64-37-52(34-45)38-65(118-9)71(64)87)122-83(115)47(3)99(7)69(104)29-32-124-125-33-31-120-55-27-23-50(24-28-55)35-57(89)75(106)94-62-43-126-127-44-63(80(111)97-73(49(5)102)82(113)114)95-81(112)72(48(4)101)96-76(107)59(19-13-14-30-88)91-78(109)61(39-53-42-90-58-18-12-11-17-56(53)58)93-77(108)60(92-79(62)110)36-51-21-25-54(103)26-22-51/h11-12,15-18,20-28,37-38,42,46-49,57,59-63,66-68,72-74,90,101-103,117H,13-14,19,29-36,39-41,43-44,88-89H2,1-10H3,(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,116)(H,113,114)/b20-15+,45-16+/t46-,47+,48-,49-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537066

(CHEMBL4541310)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r,t:17,19| Show InChI InChI=1S/C87H116ClN13O22S4/c1-47-20-18-26-68(120-10)87(118)43-67(121-85(117)99-87)48(2)75-86(6,123-75)69(42-71(106)101(8)65-39-54(36-47)40-66(119-9)72(65)88)122-84(116)49(3)100(7)70(105)31-35-125-124-34-19-33-90-60(37-52-21-12-11-13-22-52)77(108)95-63-45-126-127-46-64(81(112)98-74(51(5)103)83(114)115)96-82(113)73(50(4)102)97-76(107)59(25-16-17-32-89)92-79(110)62(41-55-44-91-58-24-15-14-23-57(55)58)94-78(109)61(93-80(63)111)38-53-27-29-56(104)30-28-53/h11-15,18,20-24,26-30,39-40,44,48-51,59-64,67-69,73-75,90-91,102-104,118H,16-17,19,25,31-38,41-43,45-46,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,108)(H,96,113)(H,97,107)(H,98,112)(H,99,117)(H,114,115)/b26-18+,47-20+/t48-,49+,50-,51-,59+,60-,61+,62-,63+,64+,67+,68-,69+,73+,74+,75+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537069
(CHEMBL4584764)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC(C)(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C85H113ClN14O20S4/c1-45-20-19-26-65(117-11)85(115)41-64(118-82(114)98-85)46(2)72-84(7,120-72)66(40-68(104)100(9)62-37-51(34-45)38-63(116-10)69(62)86)119-81(113)47(3)99(8)67(103)31-33-121-124-83(5,6)71(73(89)105)97-79(111)61-44-123-122-43-60(94-74(106)55(88)35-49-21-13-12-14-22-49)78(110)92-58(36-50-27-29-53(102)30-28-50)76(108)93-59(39-52-42-90-56-24-16-15-23-54(52)56)77(109)91-57(25-17-18-32-87)75(107)96-70(48(4)101)80(112)95-61/h12-16,19-24,26-30,37-38,42,46-48,55,57-61,64-66,70-72,90,101-102,115H,17-18,25,31-36,39-41,43-44,87-88H2,1-11H3,(H2,89,105)(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,114)/b26-19+,45-20+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71-,72+,84-,85+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537076

(CHEMBL4564727)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C83H110ClN13O20S4/c1-45-18-17-24-66(114-9)83(112)39-65(115-81(111)95-83)46(2)72-82(5,117-72)67(38-69(102)97(7)63-35-51(32-45)36-64(113-8)70(63)84)116-80(110)47(3)96(6)68(101)29-31-118-119-42-53(41-98)88-77(107)61-43-120-121-44-62(92-73(103)56(86)33-49-19-11-10-12-20-49)78(108)90-59(34-50-25-27-54(100)28-26-50)75(105)91-60(37-52-40-87-57-22-14-13-21-55(52)57)76(106)89-58(23-15-16-30-85)74(104)94-71(48(4)99)79(109)93-61/h10-14,17-22,24-28,35-36,40,46-48,53,56,58-62,65-67,71-72,87,98-100,112H,15-16,23,29-34,37-39,41-44,85-86H2,1-9H3,(H,88,107)(H,89,106)(H,90,108)(H,91,105)(H,92,103)(H,93,109)(H,94,104)(H,95,111)/b24-17+,45-18+/t46-,47+,48-,53-,56-,58+,59+,60-,61+,62+,65+,66-,67+,71+,72+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537061
(CHEMBL4527856)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C83H109ClN14O20S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)96-83)45(2)71-82(5,118-71)66(38-68(102)98(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)97(6)67(101)29-31-119-120-41-59(72(87)103)92-78(109)61-43-122-121-42-60(93-73(104)54(86)33-48-19-11-10-12-20-48)77(108)90-57(34-49-25-27-52(100)28-26-49)75(106)91-58(37-51-40-88-55-22-14-13-21-53(51)55)76(107)89-56(23-15-16-30-85)74(105)95-70(47(4)99)79(110)94-61/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,88,99-100,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H2,87,103)(H,89,107)(H,90,108)(H,91,106)(H,92,109)(H,93,104)(H,94,110)(H,95,105)(H,96,112)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089314
(CHEMBL405182 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...)Show SMILES NCCCCC(N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CC(O)C[C@H]1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C49H69N13O12S/c50-16-6-5-11-31(51)42(68)56-32(12-7-17-54-49(52)53)46(72)60-18-8-14-36(60)48(74)61-23-30(64)21-37(61)44(70)55-22-39(65)59-41(29-19-27-9-1-2-10-28(27)20-29)45(71)57-33(25-63)43(69)58-34-26-75-38-15-4-3-13-35(38)62(47(34)73)24-40(66)67/h1-4,9-10,13,15,29-34,36-37,41,63-64H,5-8,11-12,14,16-26,50-51H2,(H,55,70)(H,56,68)(H,57,71)(H,58,69)(H,59,65)(H,66,67)(H4,52,53,54)/t30?,31?,32-,33+,34-,36+,37+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Ability to bind to human cloned B1 receptor in competition binding experiments with [3H][des-Arg10,Leu9]-Kallidin. |
J Med Chem 43: 2382-6 (2000)
BindingDB Entry DOI: 10.7270/Q2S181QZ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089316
(CHEMBL410068 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...)Show SMILES NCCCCC(N)C(=O)NC(CCCNC(N)=N)C(=O)N1CCCC1C(=O)N1CC(O)CC1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)NC(CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C49H69N13O12S/c50-16-6-5-11-31(51)42(68)56-32(12-7-17-54-49(52)53)46(72)60-18-8-14-36(60)48(74)61-23-30(64)21-37(61)44(70)55-22-39(65)59-41(29-19-27-9-1-2-10-28(27)20-29)45(71)57-33(25-63)43(69)58-34-26-75-38-15-4-3-13-35(38)62(47(34)73)24-40(66)67/h1-4,9-10,13,15,29-34,36-37,41,63-64H,5-8,11-12,14,16-26,50-51H2,(H,55,70)(H,56,68)(H,57,71)(H,58,69)(H,59,65)(H,66,67)(H4,52,53,54)/t30?,31?,32?,33?,34-,36?,37?,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human cloned B1 receptor was determined using [3H]-[des-Arg10-Leu9]-kallidin as radioligand |
J Med Chem 43: 2387-94 (2000)
BindingDB Entry DOI: 10.7270/Q2N87913 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50200340
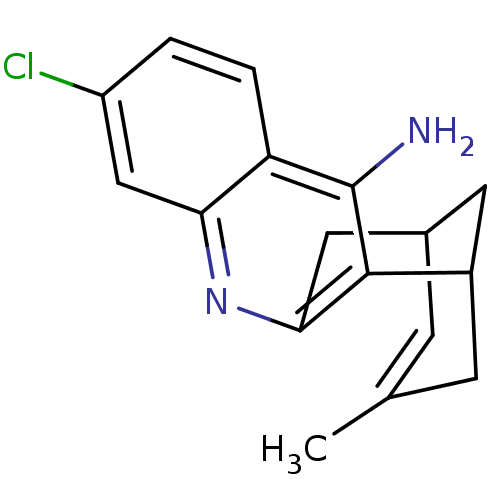
((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:1,THB:18:7:1.2.6:4,10:8:1.2.6:4| Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Binding affinity to human AChE |
J Med Chem 49: 6833-40 (2006)
Article DOI: 10.1021/jm060945c
BindingDB Entry DOI: 10.7270/Q29K4C1S |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089307

(CHEMBL410579 | H-Lys-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D...)Show SMILES NCCCCC(N)C(=O)NC(CCCCN)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CC(O)C[C@H]1C(=O)NCC(=O)NC(C1Cc2ccccc2C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C55H81N15O13S/c56-19-7-5-13-35(58)47(76)63-36(14-6-8-20-57)48(77)64-37(15-9-21-61-55(59)60)52(81)68-22-10-17-41(68)54(83)69-27-34(72)25-42(69)50(79)62-26-44(73)67-46(33-23-31-11-1-2-12-32(31)24-33)51(80)65-38(29-71)49(78)66-39-30-84-43-18-4-3-16-40(43)70(53(39)82)28-45(74)75/h1-4,11-12,16,18,33-39,41-42,46,71-72H,5-10,13-15,17,19-30,56-58H2,(H,62,79)(H,63,76)(H,64,77)(H,65,80)(H,66,78)(H,67,73)(H,74,75)(H4,59,60,61)/t34?,35?,36?,37-,38+,39-,41+,42+,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Ability to bind to human cloned B1 receptor in competition binding experiments with [3H][des-Arg10,Leu9]-Kallidin. |
J Med Chem 43: 2382-6 (2000)
BindingDB Entry DOI: 10.7270/Q2S181QZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM22416
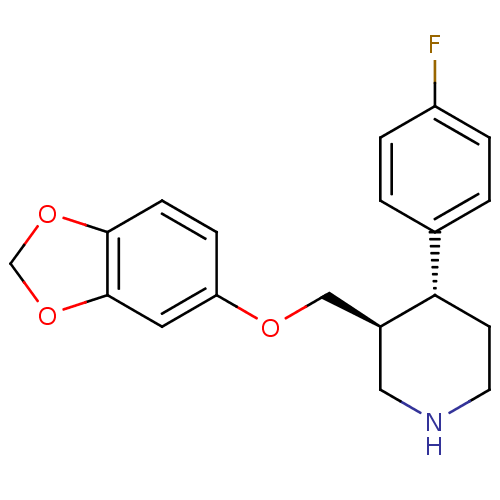
((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 349: 129-32 (1998)
Article DOI: 10.1016/s0014-2999(98)00241-6
BindingDB Entry DOI: 10.7270/Q279436B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514737
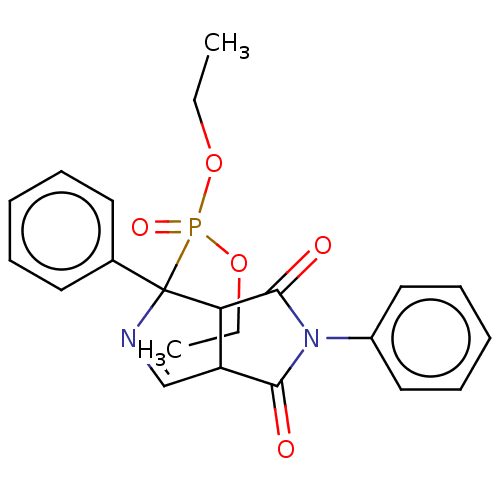
(CHEMBL4482861)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1ccccc1)c1ccccc1 |c:9| Show InChI InChI=1S/C22H23N2O5P/c1-3-28-30(27,29-4-2)22(16-11-7-5-8-12-16)19-18(15-23-22)20(25)24(21(19)26)17-13-9-6-10-14-17/h5-15,18-19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,V571I]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0520 | -59.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514738
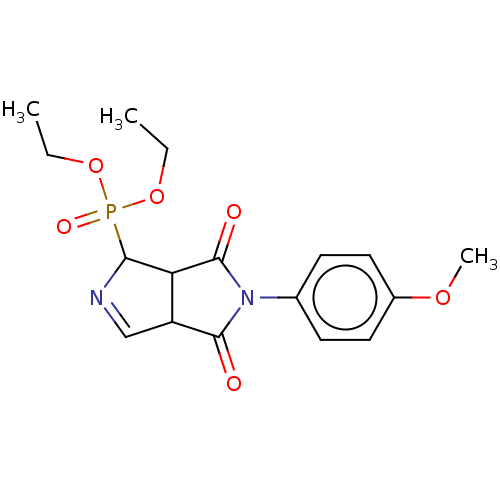
(CHEMBL4536304)Show SMILES CCOP(=O)(OCC)C1N=CC2C1C(=O)N(C2=O)c1ccc(OC)cc1 |c:9| Show InChI InChI=1S/C17H21N2O6P/c1-4-24-26(22,25-5-2)15-14-13(10-18-15)16(20)19(17(14)21)11-6-8-12(23-3)9-7-11/h6-10,13-15H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204086
(Avarol-3''-Thiosalicylate | CHEMBL238756)Show SMILES [H][C@@]12CCC=C(C)[C@@]1(C)CC[C@H](C)[C@@]2(C)Cc1cc(O)cc(Sc2ccccc2C(O)=O)c1O |r,t:4| Show InChI InChI=1S/C28H34O4S/c1-17-8-7-11-24-27(17,3)13-12-18(2)28(24,4)16-19-14-20(29)15-23(25(19)30)33-22-10-6-5-9-21(22)26(31)32/h5-6,8-10,14-15,18,24,29-30H,7,11-13,16H2,1-4H3,(H,31,32)/t18-,24+,27+,28+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130563
((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1 |r| Show InChI InChI=1S/C28H39N3O3/c1-18(2)26(30-27(34)25-13-20-8-9-24(33)12-21(20)15-29-25)17-31-11-10-28(4,19(3)16-31)22-6-5-7-23(32)14-22/h5-9,12,14,18-19,25-26,29,32-33H,10-11,13,15-17H2,1-4H3,(H,30,34)/t19-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0591 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 54: 8000-12 (2011)
Article DOI: 10.1021/jm200789r
BindingDB Entry DOI: 10.7270/Q29C6XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50403371
(Firazyr | HOE-140 | ICATIBANT)Show SMILES [#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£s Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned Bradykinin receptor B2 expressed in CHO cells by [3H]bradykinin displacement. |
J Med Chem 42: 4193-201 (1999)
BindingDB Entry DOI: 10.7270/Q2222VFV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537068
(CHEMBL4592483)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C82H108ClN13O19S4/c1-45-18-17-24-65(112-9)82(110)41-64(113-80(109)94-82)46(2)71-81(5,115-71)66(40-68(100)96(7)62-37-51(34-45)38-63(111-8)69(62)83)114-79(108)47(3)95(6)67(99)29-32-116-117-33-31-86-73(102)60-43-118-119-44-61(91-72(101)55(85)35-49-19-11-10-12-20-49)77(106)89-58(36-50-25-27-53(98)28-26-50)75(104)90-59(39-52-42-87-56-22-14-13-21-54(52)56)76(105)88-57(23-15-16-30-84)74(103)93-70(48(4)97)78(107)92-60/h10-14,17-22,24-28,37-38,42,46-48,55,57-61,64-66,70-71,87,97-98,110H,15-16,23,29-36,39-41,43-44,84-85H2,1-9H3,(H,86,102)(H,88,105)(H,89,106)(H,90,104)(H,91,101)(H,92,107)(H,93,103)(H,94,109)/b24-17+,45-18+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71+,81-,82+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474415

(CHEMBL2112882)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N[C@]1(C)O[C@@]2(O)[C@H](Cc5ccccc5)NC(=O)[C@H](CC(C)C)N2C1=O)c34 |c:12| Show InChI InChI=1S/C34H39N5O5/c1-19(2)13-27-31(41)36-28(14-20-9-6-5-7-10-20)34(43)39(27)32(42)33(3,44-34)37-30(40)22-15-24-23-11-8-12-25-29(23)21(17-35-25)16-26(24)38(4)18-22/h5-12,15,17,19,22,26-28,35,43H,13-14,16,18H2,1-4H3,(H,36,41)(H,37,40)/t22-,26-,27+,28+,33-,34+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 1A human cloned receptors in HEK293 cells using [3H]8-OH-DPAT as radioligand |
J Med Chem 46: 5117-20 (2003)
Article DOI: 10.1021/jm0341204
BindingDB Entry DOI: 10.7270/Q2Q242ZQ |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50403371

(Firazyr | HOE-140 | ICATIBANT)Show SMILES [#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£s Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity against human cloned Bradykinin receptor B2 expressed in CHO cells using [3H]-bradykinin as radioligand |
J Med Chem 42: 4185-92 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WTF |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50049949
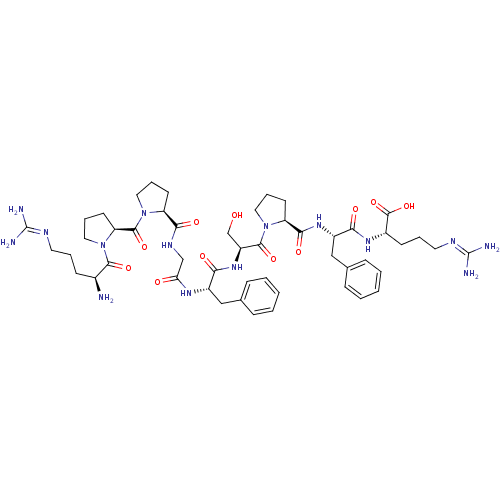
((BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH | (b...)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C50H73N15O11/c51-32(16-7-21-56-49(52)53)45(72)65-25-11-20-39(65)47(74)64-24-9-18-37(64)43(70)58-28-40(67)59-34(26-30-12-3-1-4-13-30)41(68)62-36(29-66)46(73)63-23-10-19-38(63)44(71)61-35(27-31-14-5-2-6-15-31)42(69)60-33(48(75)76)17-8-22-57-50(54)55/h1-6,12-15,32-39,66H,7-11,16-29,51H2,(H,58,70)(H,59,67)(H,60,69)(H,61,71)(H,62,68)(H,75,76)(H4,52,53,56)(H4,54,55,57)/t32-,33-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£s Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned Bradykinin receptor B2 expressed in CHO cells by [3H]bradykinin displacement. |
J Med Chem 42: 4193-201 (1999)
BindingDB Entry DOI: 10.7270/Q2222VFV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514722
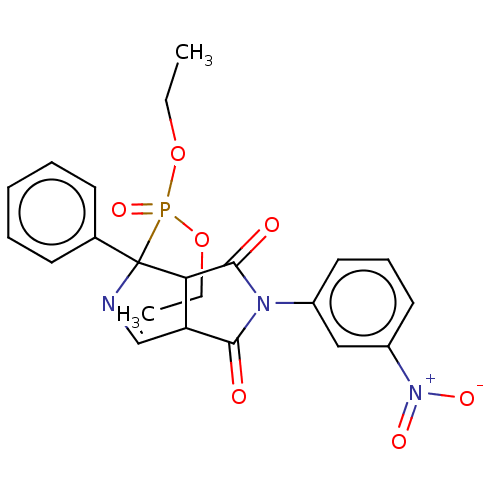
(CHEMBL4438801)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1cccc(c1)[N+]([O-])=O)c1ccccc1 |c:9| Show InChI InChI=1S/C22H22N3O7P/c1-3-31-33(30,32-4-2)22(15-9-6-5-7-10-15)19-18(14-23-22)20(26)24(21(19)27)16-11-8-12-17(13-16)25(28)29/h5-14,18-19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50081659
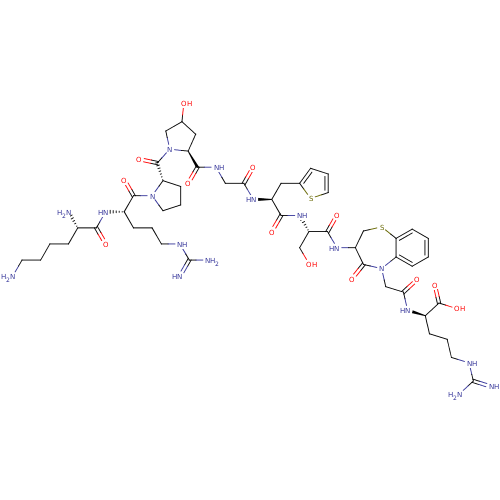
((3-(Ser-Thi-Gly-Hyp-Pro-Arg-Lys-H)-4-oxo-3,4-dihyd...)Show SMILES NCCCC[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CC(O)C[C@H]1C(=O)NCC(=O)N[C@@H](Cc1cccs1)C(=O)N[C@@H](CO)C(=O)NC1CSc2ccccc2N(CC(=O)N[C@H](CCCNC(N)=N)C(O)=O)C1=O Show InChI InChI=1S/C51H77N17O13S2/c52-16-4-3-10-30(53)42(73)63-31(11-5-17-58-50(54)55)46(77)66-19-7-14-37(66)48(79)67-24-28(70)21-38(67)45(76)60-23-40(71)62-33(22-29-9-8-20-82-29)43(74)64-34(26-69)44(75)65-35-27-83-39-15-2-1-13-36(39)68(47(35)78)25-41(72)61-32(49(80)81)12-6-18-59-51(56)57/h1-2,8-9,13,15,20,28,30-35,37-38,69-70H,3-7,10-12,14,16-19,21-27,52-53H2,(H,60,76)(H,61,72)(H,62,71)(H,63,73)(H,64,74)(H,65,75)(H,80,81)(H4,54,55,58)(H4,56,57,59)/t28?,30-,31-,32+,33-,34-,35?,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£s Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity towards human cloned Bradykinin receptor B2 expressed in CHO cells by [3H]bradykinin displacement. |
J Med Chem 42: 4193-201 (1999)
BindingDB Entry DOI: 10.7270/Q2222VFV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537070

(CHEMBL4581874)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCC(C)(C)SSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O20S4/c1-46-20-19-26-67(118-11)86(116)41-66(119-83(115)99-86)47(2)73-85(7,121-73)68(40-70(105)101(9)64-37-52(34-46)38-65(117-10)71(64)87)120-82(114)48(3)100(8)69(104)31-32-84(5,6)125-124-43-61(74(90)106)95-80(112)63-45-123-122-44-62(96-75(107)56(89)35-50-21-13-12-14-22-50)79(111)93-59(36-51-27-29-54(103)30-28-51)77(109)94-60(39-53-42-91-57-24-16-15-23-55(53)57)78(110)92-58(25-17-18-33-88)76(108)98-72(49(4)102)81(113)97-63/h12-16,19-24,26-30,37-38,42,47-49,56,58-63,66-68,72-73,91,102-103,116H,17-18,25,31-36,39-41,43-45,88-89H2,1-11H3,(H2,90,106)(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,107)(H,97,113)(H,98,108)(H,99,115)/b26-19+,46-20+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67-,68+,72+,73+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50403371

(Firazyr | HOE-140 | ICATIBANT)Show SMILES [#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£s Montpellier I et II
Curated by ChEMBL
| Assay Description
Binding affinity against rat bradykinin B2 receptors expressed in CHO cells using [3H]bradykinin as radioligand |
J Med Chem 42: 4185-92 (1999)
BindingDB Entry DOI: 10.7270/Q25Q4WTF |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50089308
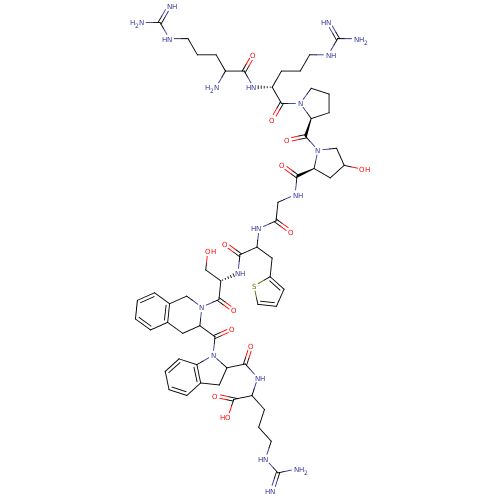
(CHEMBL412349 | H-DArg-Arg-Pro-Hyp-Gly-Thi-Ser-DTic...)Show SMILES NC(CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CC(O)C[C@H]1C(=O)NCC(=O)NC(Cc1cccs1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2CC1C(=O)N1C(Cc2ccccc12)C(=O)NC(CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C59H83N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-4,9-13,17,23,35,37-41,43-46,79-80H,5-8,14-16,18-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t35?,37?,38-,39?,40?,41+,43+,44+,45?,46?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Ability to bind to human cloned B2 receptor in competition binding experiments with [3H]- bradykinin |
J Med Chem 43: 2382-6 (2000)
BindingDB Entry DOI: 10.7270/Q2S181QZ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537074

(CHEMBL4556000)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCC(=O)NCCCC[C@@H]1N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C84H111ClN12O19S2/c1-48-21-20-28-67(113-10)84(111)46-66(114-82(110)94-84)49(2)74-83(5,116-74)68(45-71(102)96(7)64-42-54(39-48)43-65(112-9)72(64)85)115-81(109)50(3)95(6)70(101)34-38-118-117-37-33-69(100)87-36-19-17-27-63-78(106)91-60(40-53-29-31-56(99)32-30-53)76(104)90-61(44-55-47-88-58-25-15-14-24-57(55)58)77(105)89-59(26-16-18-35-86)75(103)93-73(51(4)98)79(107)92-62(80(108)97(63)8)41-52-22-12-11-13-23-52/h11-15,20-25,28-32,42-43,47,49-51,59-63,66-68,73-74,88,98-99,111H,16-19,26-27,33-41,44-46,86H2,1-10H3,(H,87,100)(H,89,105)(H,90,104)(H,91,106)(H,92,107)(H,93,103)(H,94,110)/b28-20+,48-21+/t49-,50+,51-,59+,60+,61-,62+,63+,66+,67-,68+,73+,74+,83-,84+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50049949

((BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH | (b...)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C50H73N15O11/c51-32(16-7-21-56-49(52)53)45(72)65-25-11-20-39(65)47(74)64-24-9-18-37(64)43(70)58-28-40(67)59-34(26-30-12-3-1-4-13-30)41(68)62-36(29-66)46(73)63-23-10-19-38(63)44(71)61-35(27-31-14-5-2-6-15-31)42(69)60-33(48(75)76)17-8-22-57-50(54)55/h1-6,12-15,32-39,66H,7-11,16-29,51H2,(H,58,70)(H,59,67)(H,60,69)(H,61,71)(H,62,68)(H,75,76)(H4,52,53,56)(H4,54,55,57)/t32-,33-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II
Curated by ChEMBL
| Assay Description
Ability to bind to human cloned B2 receptor in competition binding experiments with [3H]- bradykinin |
J Med Chem 43: 2382-6 (2000)
BindingDB Entry DOI: 10.7270/Q2S181QZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50335985
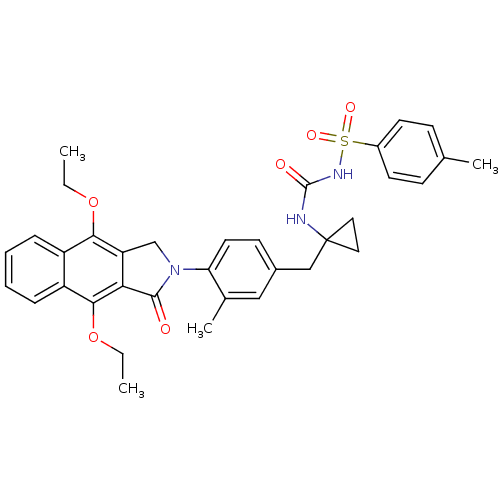
(CHEMBL1669013 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC2(CC2)NC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1C Show InChI InChI=1S/C35H37N3O6S/c1-5-43-31-26-9-7-8-10-27(26)32(44-6-2)30-28(31)21-38(33(30)39)29-16-13-24(19-23(29)4)20-35(17-18-35)36-34(40)37-45(41,42)25-14-11-22(3)12-15-25/h7-16,19H,5-6,17-18,20-21H2,1-4H3,(H2,36,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1041-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.014
BindingDB Entry DOI: 10.7270/Q2VH5P4H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587,L565M]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.114 | -57.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50372064
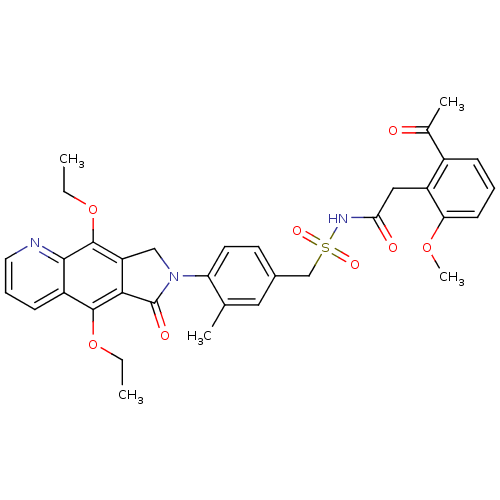
(CHEMBL256873)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2cccnc12)c1ccc(CS(=O)(=O)NC(=O)Cc2c(OC)cccc2C(C)=O)cc1C Show InChI InChI=1S/C34H35N3O8S/c1-6-44-32-24-11-9-15-35-31(24)33(45-7-2)26-18-37(34(40)30(26)32)27-14-13-22(16-20(27)3)19-46(41,42)36-29(39)17-25-23(21(4)38)10-8-12-28(25)43-5/h8-16H,6-7,17-19H2,1-5H3,(H,36,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 2048-54 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.103
BindingDB Entry DOI: 10.7270/Q2J96770 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537067
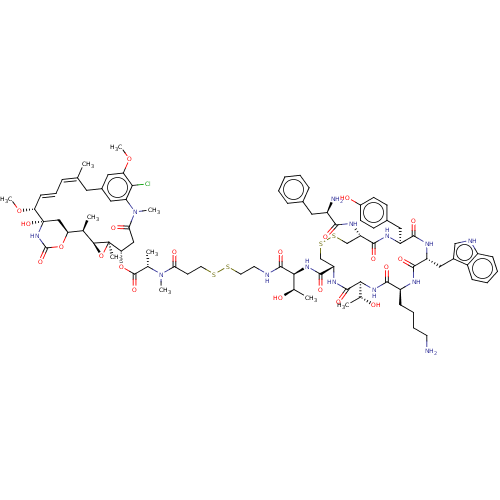
(CHEMBL4532058)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCNC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C86H115ClN14O21S4/c1-46-19-18-25-67(119-10)86(117)42-66(120-84(116)99-86)47(2)74-85(6,122-74)68(41-70(106)101(8)64-38-53(35-46)39-65(118-9)71(64)87)121-83(115)48(3)100(7)69(105)30-33-123-124-34-32-90-81(113)72(49(4)102)97-80(112)63-45-126-125-44-62(95-75(107)57(89)36-51-20-12-11-13-21-51)79(111)93-60(37-52-26-28-55(104)29-27-52)77(109)94-61(40-54-43-91-58-23-15-14-22-56(54)58)78(110)92-59(24-16-17-31-88)76(108)98-73(50(5)103)82(114)96-63/h11-15,18-23,25-29,38-39,43,47-50,57,59-63,66-68,72-74,91,102-104,117H,16-17,24,30-37,40-42,44-45,88-89H2,1-10H3,(H,90,113)(H,92,110)(H,93,111)(H,94,109)(H,95,107)(H,96,114)(H,97,112)(H,98,108)(H,99,116)/b25-18+,46-19+/t47-,48+,49-,50-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537062

(CHEMBL4549303)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O |r,t:17,19| Show InChI InChI=1S/C87H118ClN13O20S4/c1-49-23-21-31-70(118-10)87(116)44-69(119-85(115)99-87)50(2)76-86(6,121-76)71(43-73(106)101(8)67-40-56(37-49)41-68(117-9)74(67)88)120-84(114)51(3)100(7)72(105)32-36-123-122-35-22-34-90-61(38-54-24-13-11-14-25-54)78(108)96-65-47-124-125-48-66(82(112)95-64(46-102)52(4)103)97-83(113)75(53(5)104)98-77(107)60(30-19-20-33-89)92-80(110)63(42-57-45-91-59-29-18-17-28-58(57)59)94-79(109)62(93-81(65)111)39-55-26-15-12-16-27-55/h11-18,21,23-29,31,40-41,45,50-53,60-66,69-71,75-76,90-91,102-104,116H,19-20,22,30,32-39,42-44,46-48,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,112)(H,96,108)(H,97,113)(H,98,107)(H,99,115)/b31-21+,49-23+/t50-,51+,52-,53-,60+,61-,62+,63-,64-,65+,66+,69+,70-,71+,75+,76+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50560224
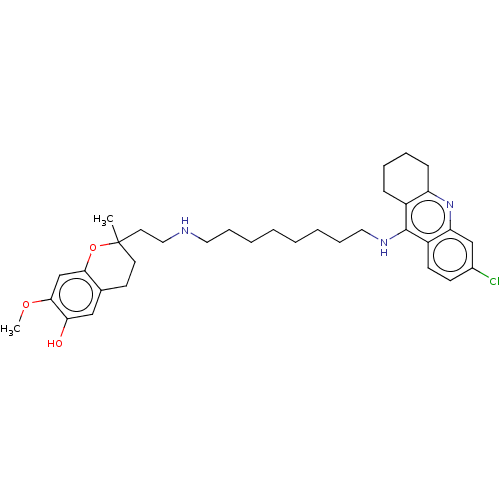
(CHEMBL4751100)Show SMILES COc1cc2OC(C)(CCNCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)CCc2cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of recombinant human AChE expressed in HEK293 cells assessed as dissociation constant for enzyme-inhibitor complex using varying lev... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00528
BindingDB Entry DOI: 10.7270/Q23N2733 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537075

(CHEMBL4548228)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CC(=O)NC[C@H](NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](N)Cc3ccccc3)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@H](Cc3c[nH]c4ccccc34)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N2)C(N)=O)C1=O |r,t:17,19| Show InChI InChI=1S/C89H115ClN16O23S3/c1-46-18-17-24-68(126-9)89(124)40-66(127-87(123)103-89)47(2)76-88(5,129-76)69(39-72(111)105(7)64-35-52(32-46)36-65(125-8)74(64)90)128-86(122)48(3)104(6)71(110)29-31-130-67-38-73(112)106(85(67)121)43-70(109)95-42-61(77(93)113)99-83(119)63-45-132-131-44-62(100-78(114)56(92)33-50-19-11-10-12-20-50)82(118)97-59(34-51-25-27-54(108)28-26-51)80(116)98-60(37-53-41-94-57-22-14-13-21-55(53)57)81(117)96-58(23-15-16-30-91)79(115)102-75(49(4)107)84(120)101-63/h10-14,17-22,24-28,35-36,41,47-49,56,58-63,66-69,75-76,94,107-108,124H,15-16,23,29-34,37-40,42-45,91-92H2,1-9H3,(H2,93,113)(H,95,109)(H,96,117)(H,97,118)(H,98,116)(H,99,119)(H,100,114)(H,101,120)(H,102,115)(H,103,123)/b24-17+,46-18+/t47-,48+,49-,56-,58+,59+,60-,61+,62+,63+,66+,67?,68-,69+,75+,76+,88-,89+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537064
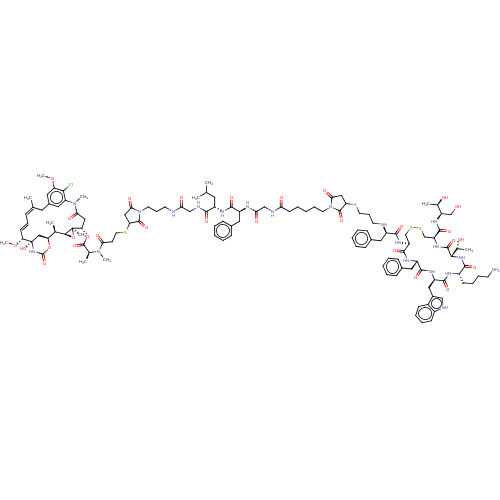
(CHEMBL4563111)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CCCNC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CCCCCN2C(=O)CC(SCCCN[C@H](Cc3ccccc3)C(=O)N[C@H]3CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@H](CO)[C@@H](C)O)C2=O)C1=O |r,t:17,19| Show InChI InChI=1S/C123H167ClN20O29S4/c1-70(2)52-85(109(155)130-65-100(149)127-46-30-49-144-105(154)61-96(119(144)165)175-51-44-102(151)141(9)73(5)120(166)172-98-62-103(152)142(10)92-57-79(58-93(169-11)106(92)124)53-71(3)32-29-42-97(170-12)123(168)63-94(171-121(167)140-123)72(4)108-122(98,8)173-108)133-112(158)86(55-77-35-19-14-20-36-77)131-101(150)66-129-99(148)43-23-16-28-48-143-104(153)60-95(118(143)164)174-50-31-47-126-84(54-76-33-17-13-18-34-76)111(157)137-90-68-176-177-69-91(116(162)136-89(67-145)74(6)146)138-117(163)107(75(7)147)139-110(156)83(41-26-27-45-125)132-114(160)88(59-80-64-128-82-40-25-24-39-81(80)82)135-113(159)87(134-115(90)161)56-78-37-21-15-22-38-78/h13-15,17-22,24-25,29,32-40,42,57-58,64,70,72-75,83-91,94-98,107-108,126,128,145-147,168H,16,23,26-28,30-31,41,43-56,59-63,65-69,125H2,1-12H3,(H,127,149)(H,129,148)(H,130,155)(H,131,150)(H,132,160)(H,133,158)(H,134,161)(H,135,159)(H,136,162)(H,137,157)(H,138,163)(H,139,156)(H,140,167)/b42-29+,71-32+/t72-,73+,74-,75-,83+,84-,85+,86+,87+,88-,89-,90+,91+,94+,95?,96?,97-,98+,107+,108+,122-,123+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045775
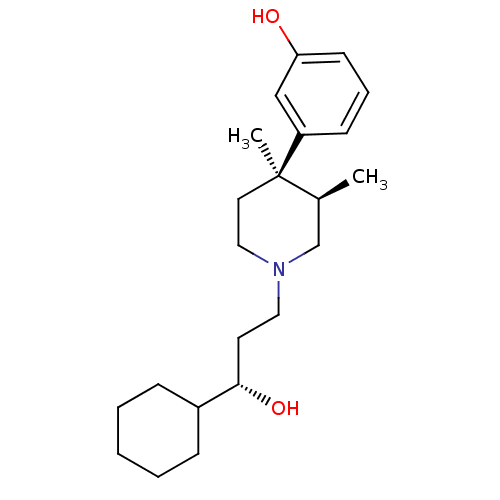
((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...)Show SMILES C[C@H]1CN(CC[C@H](O)C2CCCCC2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H35NO2/c1-17-16-23(13-11-21(25)18-7-4-3-5-8-18)14-12-22(17,2)19-9-6-10-20(24)15-19/h6,9-10,15,17-18,21,24-25H,3-5,7-8,11-14,16H2,1-2H3/t17-,21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 6841-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.025
BindingDB Entry DOI: 10.7270/Q2125TGQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551
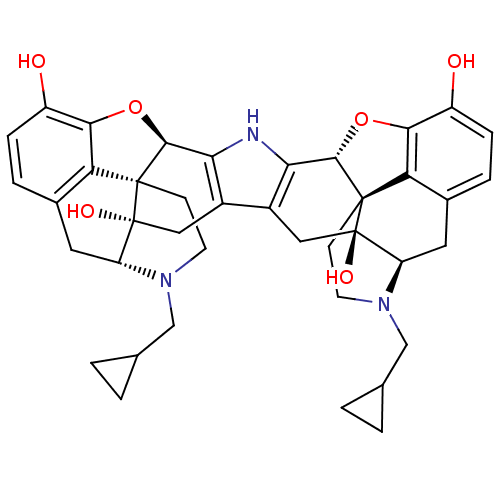
(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 54: 8000-12 (2011)
Article DOI: 10.1021/jm200789r
BindingDB Entry DOI: 10.7270/Q29C6XVV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026614
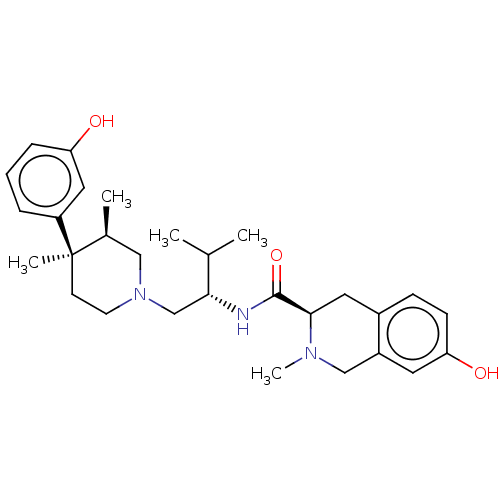
(CHEMBL575508)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccc(O)cc2CN1C |r| Show InChI InChI=1S/C29H41N3O3/c1-19(2)26(30-28(35)27-14-21-9-10-25(34)13-22(21)17-31(27)5)18-32-12-11-29(4,20(3)16-32)23-7-6-8-24(33)15-23/h6-10,13,15,19-20,26-27,33-34H,11-12,14,16-18H2,1-5H3,(H,30,35)/t20-,26+,27+,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 54: 8000-12 (2011)
Article DOI: 10.1021/jm200789r
BindingDB Entry DOI: 10.7270/Q29C6XVV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50335986
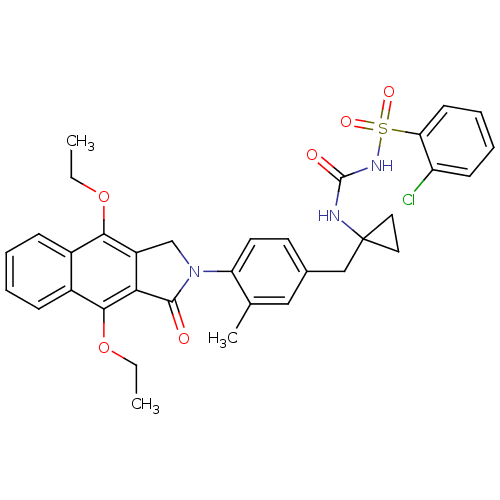
(2-chloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]is...)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC2(CC2)NC(=O)NS(=O)(=O)c2ccccc2Cl)cc1C Show InChI InChI=1S/C34H34ClN3O6S/c1-4-43-30-23-10-6-7-11-24(23)31(44-5-2)29-25(30)20-38(32(29)39)27-15-14-22(18-21(27)3)19-34(16-17-34)36-33(40)37-45(41,42)28-13-9-8-12-26(28)35/h6-15,18H,4-5,16-17,19-20H2,1-3H3,(H2,36,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1041-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.014
BindingDB Entry DOI: 10.7270/Q2VH5P4H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50335980
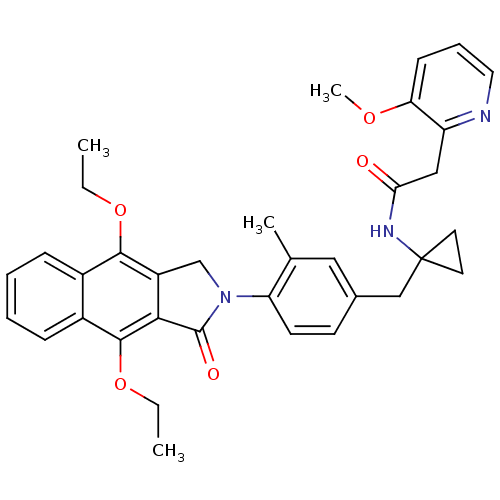
(CHEMBL1669017 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC2(CC2)NC(=O)Cc2ncccc2OC)cc1C Show InChI InChI=1S/C35H37N3O5/c1-5-42-32-24-10-7-8-11-25(24)33(43-6-2)31-26(32)21-38(34(31)40)28-14-13-23(18-22(28)3)20-35(15-16-35)37-30(39)19-27-29(41-4)12-9-17-36-27/h7-14,17-18H,5-6,15-16,19-21H2,1-4H3,(H,37,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1041-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.014
BindingDB Entry DOI: 10.7270/Q2VH5P4H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50335989
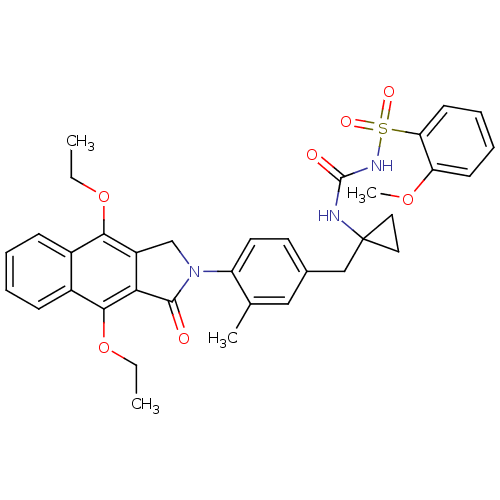
(CHEMBL1669009 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC2(CC2)NC(=O)NS(=O)(=O)c2ccccc2OC)cc1C Show InChI InChI=1S/C35H37N3O7S/c1-5-44-31-24-11-7-8-12-25(24)32(45-6-2)30-26(31)21-38(33(30)39)27-16-15-23(19-22(27)3)20-35(17-18-35)36-34(40)37-46(41,42)29-14-10-9-13-28(29)43-4/h7-16,19H,5-6,17-18,20-21H2,1-4H3,(H2,36,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1041-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.014
BindingDB Entry DOI: 10.7270/Q2VH5P4H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919
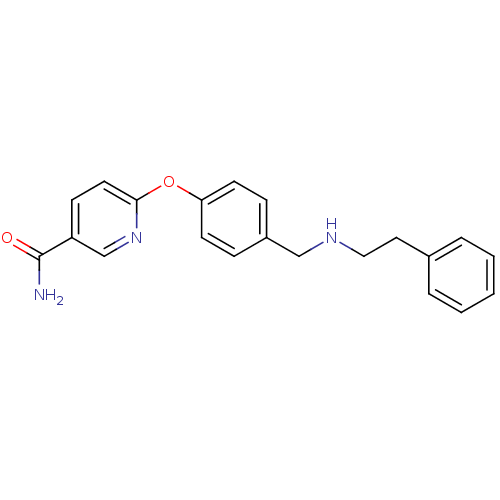
(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 6841-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.025
BindingDB Entry DOI: 10.7270/Q2125TGQ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537071

(CHEMBL4581646)Show SMILES [H][C@]1(CCCN1C(=O)C[C@@H](OC)[C@H]([C@@H](C)CC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O)C(C)C)[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1 |r| Show InChI InChI=1S/C90H132N16O19S4/c1-14-52(6)76(71(123-12)45-72(109)106-39-25-33-70(106)78(124-13)53(7)80(112)95-54(8)77(110)58-28-19-16-20-29-58)104(10)89(121)73(50(2)3)102-88(120)75(51(4)5)105(11)90(122)125-40-41-126-127-47-67(79(93)111)99-86(118)69-49-129-128-48-68(100-81(113)62(92)42-56-26-17-15-18-27-56)85(117)97-65(43-57-34-36-60(108)37-35-57)83(115)98-66(44-59-46-94-63-31-22-21-30-61(59)63)84(116)96-64(32-23-24-38-91)82(114)103-74(55(9)107)87(119)101-69/h15-22,26-31,34-37,46,50-55,62,64-71,73-78,94,107-108,110H,14,23-25,32-33,38-45,47-49,91-92H2,1-13H3,(H2,93,111)(H,95,112)(H,96,116)(H,97,117)(H,98,115)(H,99,118)(H,100,113)(H,101,119)(H,102,120)(H,103,114)/t52-,53+,54+,55+,62+,64-,65-,66+,67-,68-,69-,70-,71+,73-,74-,75-,76-,77+,78+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50335981
(3-(2-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]isoindol...)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC2(CC2)NC(=O)Cc2cc(ccc2OC)C(O)=O)cc1C Show InChI InChI=1S/C37H38N2O7/c1-5-45-33-26-9-7-8-10-27(26)34(46-6-2)32-28(33)21-39(35(32)41)29-13-11-23(17-22(29)3)20-37(15-16-37)38-31(40)19-25-18-24(36(42)43)12-14-30(25)44-4/h7-14,17-18H,5-6,15-16,19-21H2,1-4H3,(H,38,40)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay |
Bioorg Med Chem Lett 21: 1041-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.014
BindingDB Entry DOI: 10.7270/Q2VH5P4H |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537065
(CHEMBL4537192)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,t:17,19| Show InChI InChI=1S/C95H122ClN15O19S4/c1-53(2)82-91(122)107-73(89(120)103-68(84(98)115)45-60-49-100-65-27-16-14-25-63(60)65)52-134-133-51-72(90(121)104-70(42-58-31-33-62(112)34-32-58)87(118)105-71(46-61-50-101-66-28-17-15-26-64(61)66)88(119)102-67(85(116)108-82)29-18-19-36-97)106-86(117)69(41-57-23-12-11-13-24-57)99-37-21-38-131-132-39-35-79(113)110(7)56(5)92(123)129-78-47-80(114)111(8)74-43-59(44-75(126-9)81(74)96)40-54(3)22-20-30-77(127-10)95(125)48-76(128-93(124)109-95)55(4)83-94(78,6)130-83/h11-17,20,22-28,30-34,43-44,49-50,53,55-56,67-73,76-78,82-83,99-101,112,125H,18-19,21,29,35-42,45-48,51-52,97H2,1-10H3,(H2,98,115)(H,102,119)(H,103,120)(H,104,121)(H,105,118)(H,106,117)(H,107,122)(H,108,116)(H,109,124)/b30-20+,54-22+/t55-,56+,67+,68+,69-,70+,71-,72+,73+,76+,77-,78+,82+,83+,94-,95+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data























































