 Found 964 hits with Last Name = 'rabindran' and Initial = 's'
Found 964 hits with Last Name = 'rabindran' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396542

(CHEMBL2171141)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2cccc(F)c2)c2c(N)ncnc12 Show InChI InChI=1S/C23H20FN5O/c1-28-12-18(21-22(25)26-13-27-23(21)28)15-5-6-19-16(11-15)7-8-29(19)20(30)10-14-3-2-4-17(24)9-14/h2-6,9,11-13H,7-8,10H2,1H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396539

(CHEMBL2171144)Show SMILES Cc1cccc(CC(=O)N2CCc3cc(ccc23)-c2cn(C)c3ncnc(N)c23)c1 Show InChI InChI=1S/C24H23N5O/c1-15-4-3-5-16(10-15)11-21(30)29-9-8-18-12-17(6-7-20(18)29)19-13-28(2)24-22(19)23(25)26-14-27-24/h3-7,10,12-14H,8-9,11H2,1-2H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396532
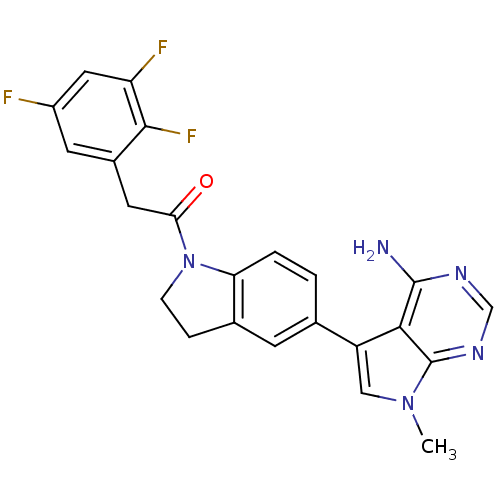
(CHEMBL2171126)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2cc(F)cc(F)c2F)c2c(N)ncnc12 Show InChI InChI=1S/C23H18F3N5O/c1-30-10-16(20-22(27)28-11-29-23(20)30)12-2-3-18-13(6-12)4-5-31(18)19(32)8-14-7-15(24)9-17(25)21(14)26/h2-3,6-7,9-11H,4-5,8H2,1H3,(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396536
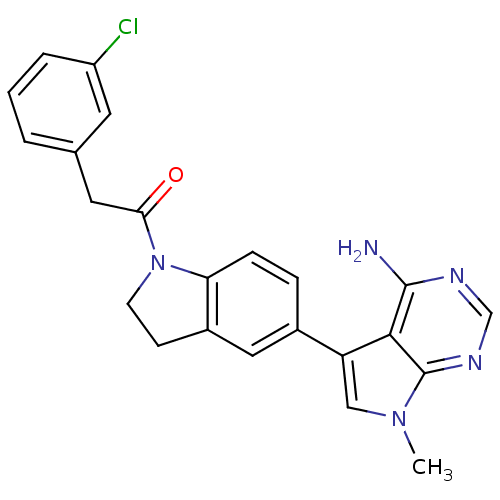
(CHEMBL2171122)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2cccc(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C23H20ClN5O/c1-28-12-18(21-22(25)26-13-27-23(21)28)15-5-6-19-16(11-15)7-8-29(19)20(30)10-14-3-2-4-17(24)9-14/h2-6,9,11-13H,7-8,10H2,1H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396540
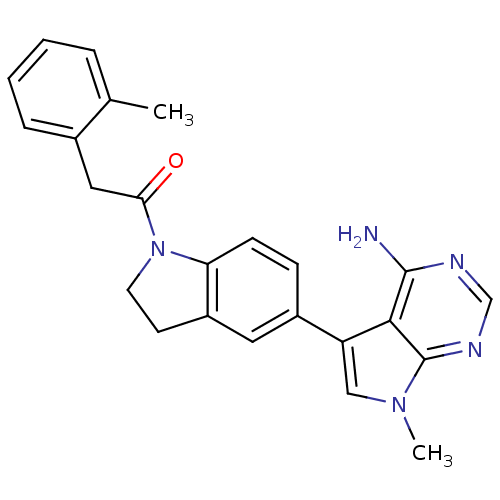
(CHEMBL2171143)Show SMILES Cc1ccccc1CC(=O)N1CCc2cc(ccc12)-c1cn(C)c2ncnc(N)c12 Show InChI InChI=1S/C24H23N5O/c1-15-5-3-4-6-16(15)12-21(30)29-10-9-18-11-17(7-8-20(18)29)19-13-28(2)24-22(19)23(25)26-14-27-24/h3-8,11,13-14H,9-10,12H2,1-2H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396533
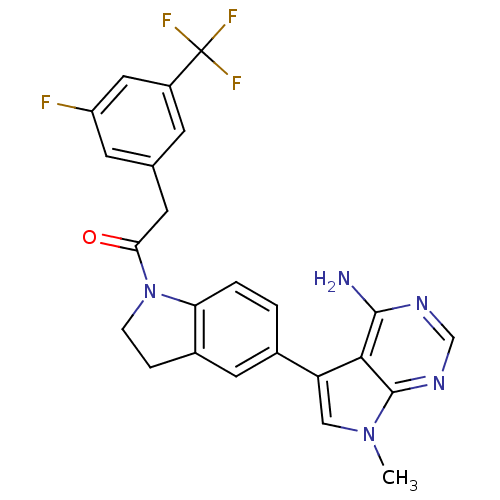
(CHEMBL2171125)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2cc(F)cc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C24H19F4N5O/c1-32-11-18(21-22(29)30-12-31-23(21)32)14-2-3-19-15(9-14)4-5-33(19)20(34)8-13-6-16(24(26,27)28)10-17(25)7-13/h2-3,6-7,9-12H,4-5,8H2,1H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396534
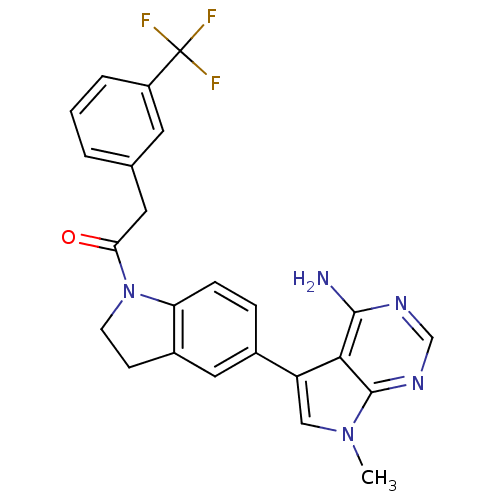
(CHEMBL2171124)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C24H20F3N5O/c1-31-12-18(21-22(28)29-13-30-23(21)31)15-5-6-19-16(11-15)7-8-32(19)20(33)10-14-3-2-4-17(9-14)24(25,26)27/h2-6,9,11-13H,7-8,10H2,1H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396544
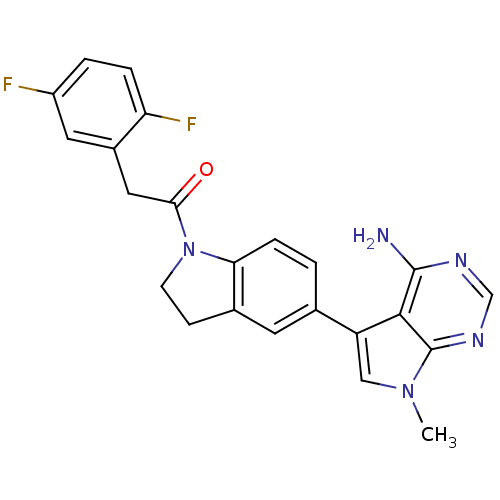
(CHEMBL2171139)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2cc(F)ccc2F)c2c(N)ncnc12 Show InChI InChI=1S/C23H19F2N5O/c1-29-11-17(21-22(26)27-12-28-23(21)29)13-2-5-19-14(8-13)6-7-30(19)20(31)10-15-9-16(24)3-4-18(15)25/h2-5,8-9,11-12H,6-7,10H2,1H3,(H2,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396535
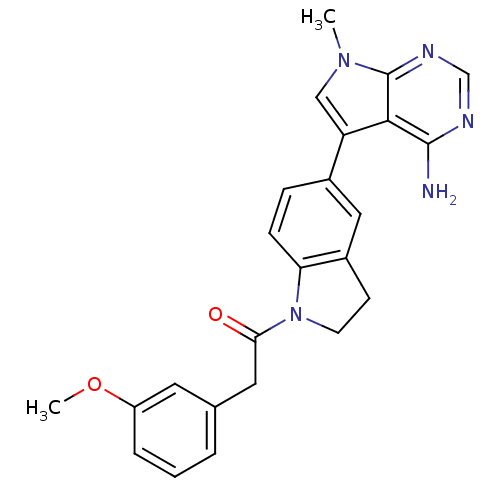
(CHEMBL2171123)Show SMILES COc1cccc(CC(=O)N2CCc3cc(ccc23)-c2cn(C)c3ncnc(N)c23)c1 Show InChI InChI=1S/C24H23N5O2/c1-28-13-19(22-23(25)26-14-27-24(22)28)16-6-7-20-17(12-16)8-9-29(20)21(30)11-15-4-3-5-18(10-15)31-2/h3-7,10,12-14H,8-9,11H2,1-2H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396548
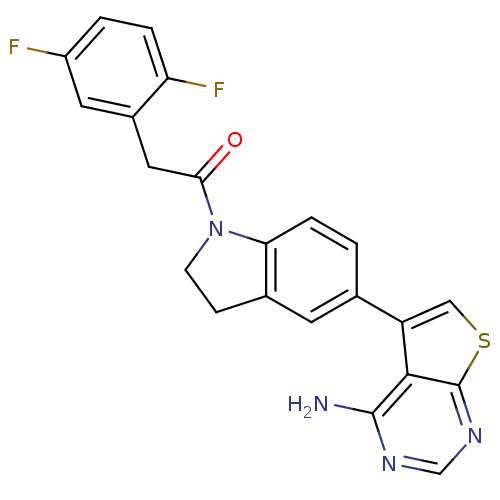
(CHEMBL2171135)Show SMILES Nc1ncnc2scc(-c3ccc4N(CCc4c3)C(=O)Cc3cc(F)ccc3F)c12 Show InChI InChI=1S/C22H16F2N4OS/c23-15-2-3-17(24)14(8-15)9-19(29)28-6-5-13-7-12(1-4-18(13)28)16-10-30-22-20(16)21(25)26-11-27-22/h1-4,7-8,10-11H,5-6,9H2,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341250
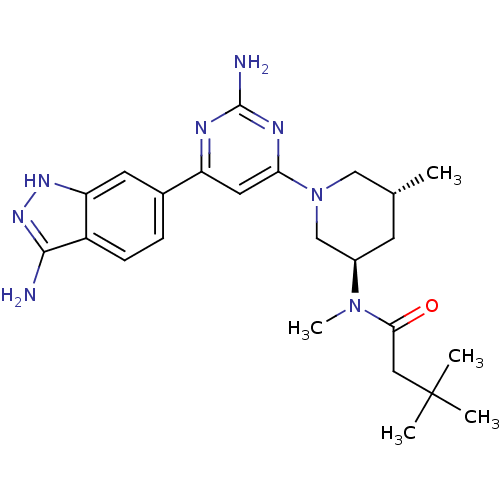
(CHEMBL1765751 | N-{(3R,5R)-1-[2-Amino-6-(3-amino-1...)Show SMILES C[C@@H]1C[C@H](CN(C1)c1cc(nc(N)n1)-c1ccc2c(N)n[nH]c2c1)N(C)C(=O)CC(C)(C)C |r| Show InChI InChI=1S/C24H34N8O/c1-14-8-16(31(5)21(33)11-24(2,3)4)13-32(12-14)20-10-18(27-23(26)28-20)15-6-7-17-19(9-15)29-30-22(17)25/h6-7,9-10,14,16H,8,11-13H2,1-5H3,(H3,25,29,30)(H2,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396543

(CHEMBL2171140)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2ccccc2F)c2c(N)ncnc12 Show InChI InChI=1S/C23H20FN5O/c1-28-12-17(21-22(25)26-13-27-23(21)28)14-6-7-19-16(10-14)8-9-29(19)20(30)11-15-4-2-3-5-18(15)24/h2-7,10,12-13H,8-9,11H2,1H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341249
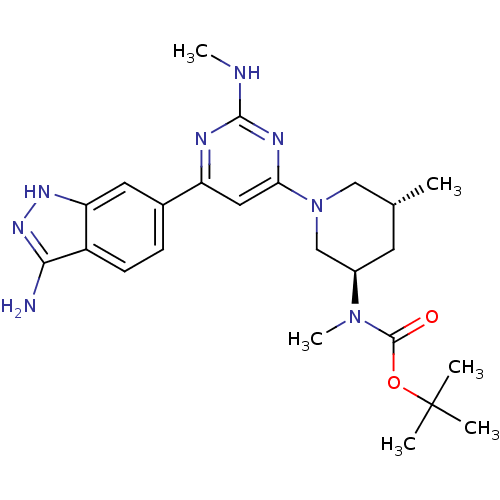
(1,1-Dimethylethyl{(3R,5R)-1-[2-Amino-6-(3-amino-1H...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](C)C[C@H](C1)N(C)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C24H34N8O2/c1-14-9-16(31(6)23(33)34-24(2,3)4)13-32(12-14)20-11-18(27-22(26-5)28-20)15-7-8-17-19(10-15)29-30-21(17)25/h7-8,10-11,14,16H,9,12-13H2,1-6H3,(H3,25,29,30)(H,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396550
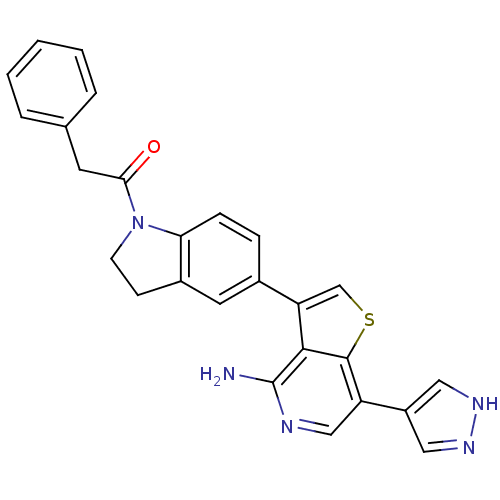
(CHEMBL2171131)Show SMILES Nc1ncc(-c2cn[nH]c2)c2scc(-c3ccc4N(CCc4c3)C(=O)Cc3ccccc3)c12 Show InChI InChI=1S/C26H21N5OS/c27-26-24-21(15-33-25(24)20(14-28-26)19-12-29-30-13-19)17-6-7-22-18(11-17)8-9-31(22)23(32)10-16-4-2-1-3-5-16/h1-7,11-15H,8-10H2,(H2,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396537

(CHEMBL2171121 | D3RKN_50)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2ccccc2Cl)c2c(N)ncnc12 Show InChI InChI=1S/C23H20ClN5O/c1-28-12-17(21-22(25)26-13-27-23(21)28)14-6-7-19-16(10-14)8-9-29(19)20(30)11-15-4-2-3-5-18(15)24/h2-7,10,12-13H,8-9,11H2,1H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396552

(CHEMBL2171129)Show SMILES Nc1ncc(-c2ccncc2)c2scc(-c3ccc4N(CCc4c3)C(=O)Cc3ccccc3)c12 Show InChI InChI=1S/C28H22N4OS/c29-28-26-23(17-34-27(26)22(16-31-28)19-8-11-30-12-9-19)20-6-7-24-21(15-20)10-13-32(24)25(33)14-18-4-2-1-3-5-18/h1-9,11-12,15-17H,10,13-14H2,(H2,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396531
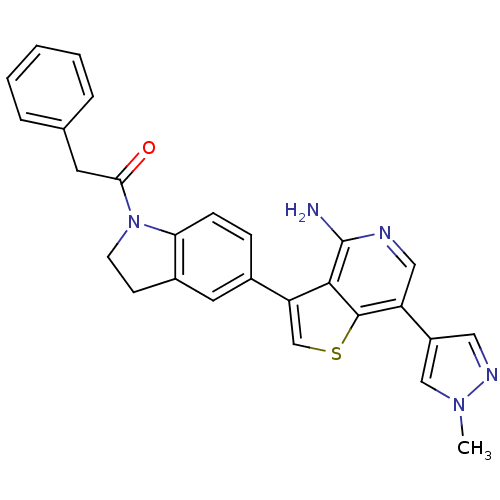
(CHEMBL2171132)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc2N(CCc2c1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C27H23N5OS/c1-31-15-20(13-30-31)21-14-29-27(28)25-22(16-34-26(21)25)18-7-8-23-19(12-18)9-10-32(23)24(33)11-17-5-3-2-4-6-17/h2-8,12-16H,9-11H2,1H3,(H2,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM6477

(4-Anilino-7,8-dialkoxybenzo[g]quinoline-3-carbonit...)Show SMILES COc1cc(Nc2c(cnc3cc4cc(OC)c(OC)cc4cc23)C#N)c(C)cc1Cl Show InChI InChI=1S/C24H20ClN3O3/c1-13-5-18(25)21(29-2)10-19(13)28-24-16(11-26)12-27-20-7-15-9-23(31-4)22(30-3)8-14(15)6-17(20)24/h5-10,12H,1-4H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 12: 423-5 (2002)
BindingDB Entry DOI: 10.7270/Q2WM1CQ7 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396541

(CHEMBL2171142)Show SMILES Cn1cc(-c2ccc3N(CCc3c2)C(=O)Cc2ccc(F)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C23H20FN5O/c1-28-12-18(21-22(25)26-13-27-23(21)28)15-4-7-19-16(11-15)8-9-29(19)20(30)10-14-2-5-17(24)6-3-14/h2-7,11-13H,8-10H2,1H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341241
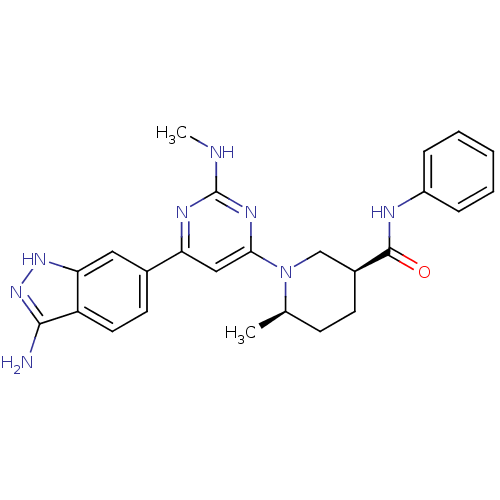
((3S,6R)-1-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](CC[C@H]1C)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C25H28N8O/c1-15-8-9-17(24(34)28-18-6-4-3-5-7-18)14-33(15)22-13-20(29-25(27-2)30-22)16-10-11-19-21(12-16)31-32-23(19)26/h3-7,10-13,15,17H,8-9,14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t15-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396551

(CHEMBL2171130)Show SMILES Nc1ncc(C2CCNCC2)c2scc(-c3ccc4N(CCc4c3)C(=O)Cc3ccccc3)c12 Show InChI InChI=1S/C28H28N4OS/c29-28-26-23(17-34-27(26)22(16-31-28)19-8-11-30-12-9-19)20-6-7-24-21(15-20)10-13-32(24)25(33)14-18-4-2-1-3-5-18/h1-7,15-17,19,30H,8-14H2,(H2,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50267899

((4Z)-6-(3-Furyl)-4-{[(3-hydroxy-4-methoxybenzyl)am...)Show SMILES COc1ccc(CNC=C2C(=O)NC(=O)c3ccc(cc23)-c2ccoc2)cc1O |w:8.7| Show InChI InChI=1S/C22H18N2O5/c1-28-20-5-2-13(8-19(20)25)10-23-11-18-17-9-14(15-6-7-29-12-15)3-4-16(17)21(26)24-22(18)27/h2-9,11-12,23,25H,10H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of cytoplasmic domain of epidermal growth factor receptor |
J Med Chem 46: 49-63 (2002)
Article DOI: 10.1021/jm020241c
BindingDB Entry DOI: 10.7270/Q2PV6M3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50267898
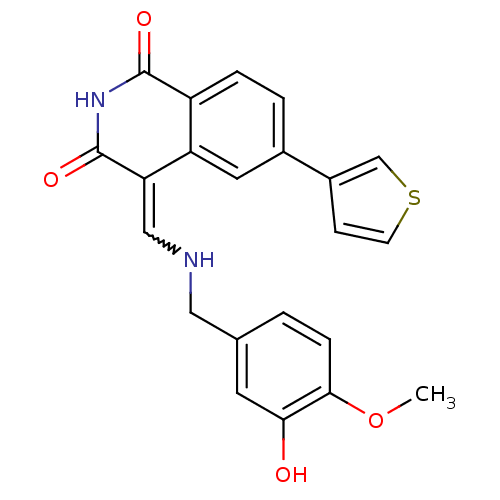
((4Z)-4-{[(3-Hydroxy-4-methoxybenzyl)amino]methylen...)Show SMILES COc1ccc(CNC=C2C(=O)NC(=O)c3ccc(cc23)-c2ccsc2)cc1O |w:8.7| Show InChI InChI=1S/C22H18N2O4S/c1-28-20-5-2-13(8-19(20)25)10-23-11-18-17-9-14(15-6-7-29-12-15)3-4-16(17)21(26)24-22(18)27/h2-9,11-12,23,25H,10H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396549
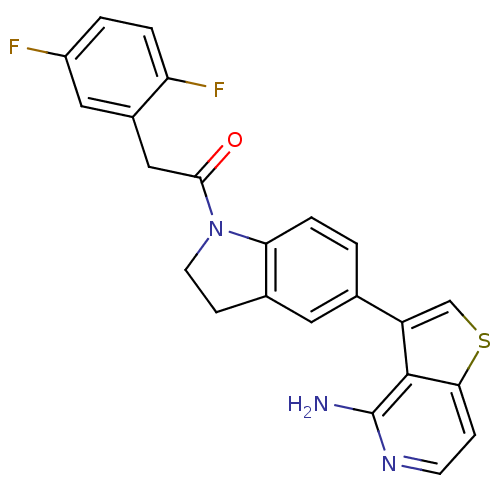
(CHEMBL2171134)Show SMILES Nc1nccc2scc(-c3ccc4N(CCc4c3)C(=O)Cc3cc(F)ccc3F)c12 Show InChI InChI=1S/C23H17F2N3OS/c24-16-2-3-18(25)15(10-16)11-21(29)28-8-6-14-9-13(1-4-19(14)28)17-12-30-20-5-7-27-23(26)22(17)20/h1-5,7,9-10,12H,6,8,11H2,(H2,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341243
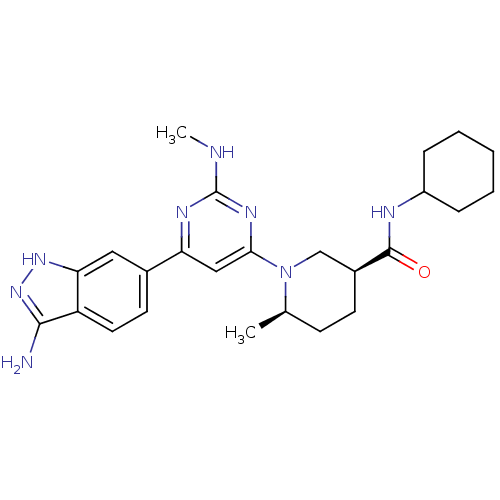
((3S,6R)-1-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](CC[C@H]1C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C25H34N8O/c1-15-8-9-17(24(34)28-18-6-4-3-5-7-18)14-33(15)22-13-20(29-25(27-2)30-22)16-10-11-19-21(12-16)31-32-23(19)26/h10-13,15,17-18H,3-9,14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t15-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341239

((3S,6R)-1-[2-Amino-6-(3-amino-1H-indazol-6-yl)-4-p...)Show SMILES C[C@@H]1CC[C@@H](CN1c1cc(nc(N)n1)-c1ccc2c(N)[nH]nc2c1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C24H26N8O/c1-14-7-8-16(23(33)27-17-5-3-2-4-6-17)13-32(14)21-12-19(28-24(26)29-21)15-9-10-18-20(11-15)30-31-22(18)25/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,27,33)(H3,25,30,31)(H2,26,28,29)/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT phosphorylation at Thr308 residue in human PC3 cells by ELISA |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396528

(CHEMBL2171128)Show SMILES Nc1ncc(-c2cccnc2)c2scc(-c3ccc4N(CCc4c3)C(=O)Cc3ccccc3)c12 Show InChI InChI=1S/C28H22N4OS/c29-28-26-23(17-34-27(26)22(16-31-28)21-7-4-11-30-15-21)19-8-9-24-20(14-19)10-12-32(24)25(33)13-18-5-2-1-3-6-18/h1-9,11,14-17H,10,12-13H2,(H2,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341245

((2S,5R)-4-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CC[C@@H]1CO[C@@H](CN1c1cc(nc(NC)n1)-c1ccc2c(N)n[nH]c2c1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C25H28N8O2/c1-3-17-14-35-21(24(34)28-16-7-5-4-6-8-16)13-33(17)22-12-19(29-25(27-2)30-22)15-9-10-18-20(11-15)31-32-23(18)26/h4-12,17,21H,3,13-14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341246
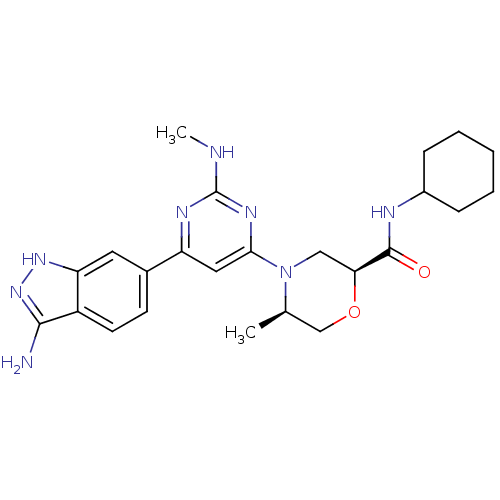
((2S,5R)-4-[6-(3-amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](OC[C@H]1C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C24H32N8O2/c1-14-13-34-20(23(33)27-16-6-4-3-5-7-16)12-32(14)21-11-18(28-24(26-2)29-21)15-8-9-17-19(10-15)30-31-22(17)25/h8-11,14,16,20H,3-7,12-13H2,1-2H3,(H,27,33)(H3,25,30,31)(H,26,28,29)/t14-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50267867
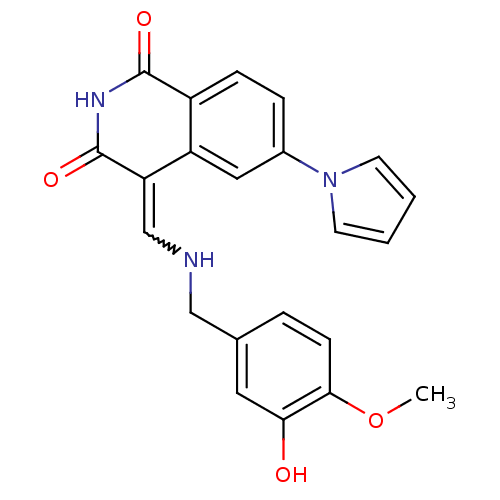
((4Z)-4-{[(3-hydroxy-4-methoxybenzyl)amino]methylen...)Show SMILES COc1ccc(CNC=C2C(=O)NC(=O)c3ccc(cc23)-n2cccc2)cc1O |w:8.7| Show InChI InChI=1S/C22H19N3O4/c1-29-20-7-4-14(10-19(20)26)12-23-13-18-17-11-15(25-8-2-3-9-25)5-6-16(17)21(27)24-22(18)28/h2-11,13,23,26H,12H2,1H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM4531

(4-Phenylamino-3-quinolinecarbonitrile deriv. 1i | ...)Show SMILES COc1cc(Nc2c(cnc3cc(OC)c(OC)cc23)C#N)c(C)cc1Cl Show InChI InChI=1S/C20H18ClN3O3/c1-11-5-14(21)17(25-2)7-15(11)24-20-12(9-22)10-23-16-8-19(27-4)18(26-3)6-13(16)20/h5-8,10H,1-4H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 12: 423-5 (2002)
BindingDB Entry DOI: 10.7270/Q2WM1CQ7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50267540
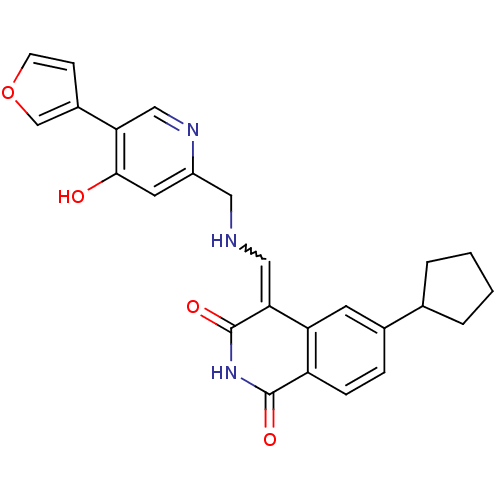
(6-Cyclopentyl-4-{[(5-furan-3-yl-4-hydroxy-pyridin-...)Show SMILES Oc1cc(CNC=C2C(=O)NC(=O)c3ccc(cc23)C2CCCC2)ncc1-c1ccoc1 |w:6.5| Show InChI InChI=1S/C25H23N3O4/c29-23-10-18(27-13-21(23)17-7-8-32-14-17)11-26-12-22-20-9-16(15-3-1-2-4-15)5-6-19(20)24(30)28-25(22)31/h5-10,12-15,26H,1-4,11H2,(H,27,29)(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341244

((2S,5R)-4-[2-Amino-6-(3-amino-1H-indazol-6-yl)-4-p...)Show SMILES C[C@@H]1CO[C@@H](CN1c1cc(nc(N)n1)-c1ccc2c(N)n[nH]c2c1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C23H24N8O2/c1-13-12-33-19(22(32)26-15-5-3-2-4-6-15)11-31(13)20-10-17(27-23(25)28-20)14-7-8-16-18(9-14)29-30-21(16)24/h2-10,13,19H,11-12H2,1H3,(H,26,32)(H3,24,29,30)(H2,25,27,28)/t13-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50267482
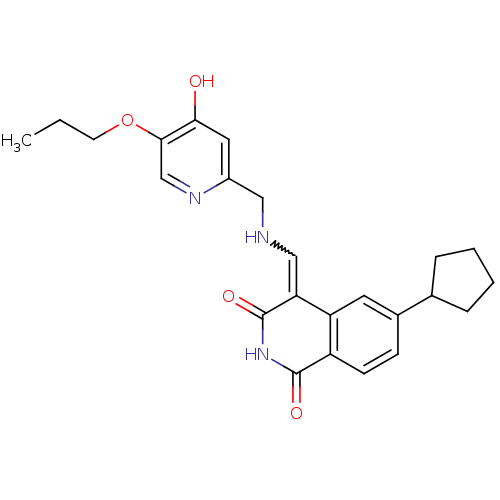
(6-Cyclopentyl-4-{[(4-hydroxy-5-propoxy-pyridin-2-y...)Show SMILES CCCOc1cnc(CNC=C2C(=O)NC(=O)c3ccc(cc23)C2CCCC2)cc1O |w:10.9| Show InChI InChI=1S/C24H27N3O4/c1-2-9-31-22-14-26-17(11-21(22)28)12-25-13-20-19-10-16(15-5-3-4-6-15)7-8-18(19)23(29)27-24(20)30/h7-8,10-11,13-15,25H,2-6,9,12H2,1H3,(H,26,28)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396545

(CHEMBL2171138)Show SMILES Cn1nc(-c2ccc3N(CCc3c2)C(=O)Cc2cc(F)ccc2F)c2c(N)ncnc12 Show InChI InChI=1S/C22H18F2N6O/c1-29-22-19(21(25)26-11-27-22)20(28-29)13-2-5-17-12(8-13)6-7-30(17)18(31)10-14-9-15(23)3-4-16(14)24/h2-5,8-9,11H,6-7,10H2,1H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341247

((2S,5R)-4-[6-(3-Amino-1H-indazol-6-yl)-2-(methylam...)Show SMILES CC[C@@H]1CO[C@@H](CN1c1cc(nc(NC)n1)-c1ccc2c(N)n[nH]c2c1)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C25H34N8O2/c1-3-17-14-35-21(24(34)28-16-7-5-4-6-8-16)13-33(17)22-12-19(29-25(27-2)30-22)15-9-10-18-20(11-15)31-32-23(18)26/h9-12,16-17,21H,3-8,13-14H2,1-2H3,(H,28,34)(H3,26,31,32)(H,27,29,30)/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341248
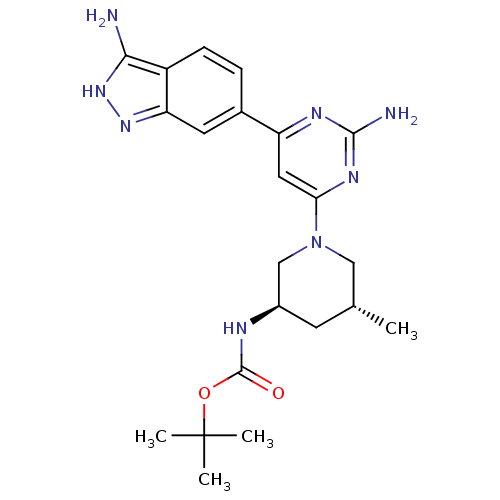
(1,1-Dimethylethyl{(3R,5R)-1-[2-amino-6-(3-amino-1H...)Show SMILES C[C@@H]1C[C@H](CN(C1)c1cc(nc(N)n1)-c1ccc2c(N)[nH]nc2c1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C22H30N8O2/c1-12-7-14(25-21(31)32-22(2,3)4)11-30(10-12)18-9-16(26-20(24)27-18)13-5-6-15-17(8-13)28-29-19(15)23/h5-6,8-9,12,14H,7,10-11H2,1-4H3,(H,25,31)(H3,23,28,29)(H2,24,26,27)/t12-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT phosphorylation at Thr308 residue in human PC3 cells by ELISA |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161879
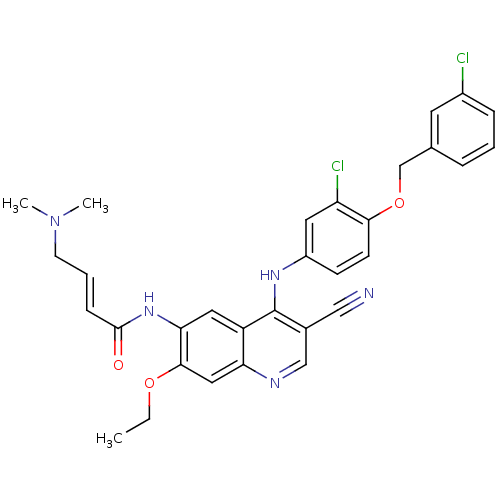
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(3...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4cccc(Cl)c4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C31H29Cl2N5O3/c1-4-40-29-16-26-24(15-27(29)37-30(39)9-6-12-38(2)3)31(21(17-34)18-35-26)36-23-10-11-28(25(33)14-23)41-19-20-7-5-8-22(32)13-20/h5-11,13-16,18H,4,12,19H2,1-3H3,(H,35,36)(H,37,39)/b9-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor (EGFR) autophosphorylation |
J Med Chem 48: 1107-31 (2005)
Article DOI: 10.1021/jm040159c
BindingDB Entry DOI: 10.7270/Q2BP03KG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50003643
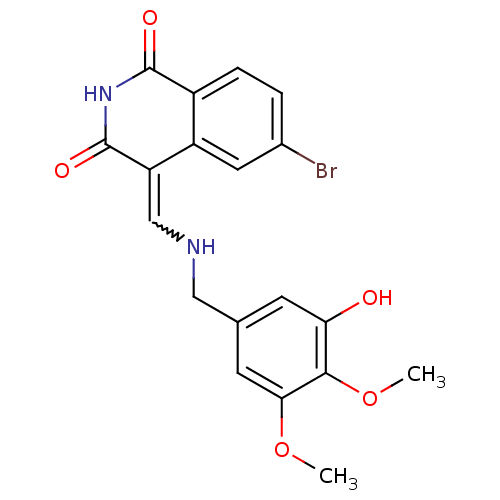
(CHEMBL484477)Show SMILES COc1cc(CN\C=C2/C(=O)NC(=O)c3ccc(Br)cc23)cc(O)c1OC Show InChI InChI=1S/C19H17BrN2O5/c1-26-16-6-10(5-15(23)17(16)27-2)8-21-9-14-13-7-11(20)3-4-12(13)18(24)22-19(14)25/h3-7,9,21,23H,8H2,1-2H3,(H,22,24,25)/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396547

(CHEMBL2171136)Show SMILES Nc1ncnc2occ(-c3ccc4N(CCc4c3)C(=O)Cc3cc(F)ccc3F)c12 Show InChI InChI=1S/C22H16F2N4O2/c23-15-2-3-17(24)14(8-15)9-19(29)28-6-5-13-7-12(1-4-18(13)28)16-10-30-22-20(16)21(25)26-11-27-22/h1-4,7-8,10-11H,5-6,9H2,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341242

((3S,6R)-1-[2-Amino-6-(3-amino-1H-indazol-6-yl)-4-p...)Show SMILES C[C@@H]1CC[C@@H](CN1c1cc(nc(N)n1)-c1ccc2c(N)n[nH]c2c1)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C24H32N8O/c1-14-7-8-16(23(33)27-17-5-3-2-4-6-17)13-32(14)21-12-19(28-24(26)29-21)15-9-10-18-20(11-15)30-31-22(18)25/h9-12,14,16-17H,2-8,13H2,1H3,(H,27,33)(H3,25,30,31)(H2,26,28,29)/t14-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50161879

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(3...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4cccc(Cl)c4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C31H29Cl2N5O3/c1-4-40-29-16-26-24(15-27(29)37-30(39)9-6-12-38(2)3)31(21(17-34)18-35-26)36-23-10-11-28(25(33)14-23)41-19-20-7-5-8-22(32)13-20/h5-11,13-16,18H,4,12,19H2,1-3H3,(H,35,36)(H,37,39)/b9-6+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor-2 (HER-2) autophosphorylation |
J Med Chem 48: 1107-31 (2005)
Article DOI: 10.1021/jm040159c
BindingDB Entry DOI: 10.7270/Q2BP03KG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50267399
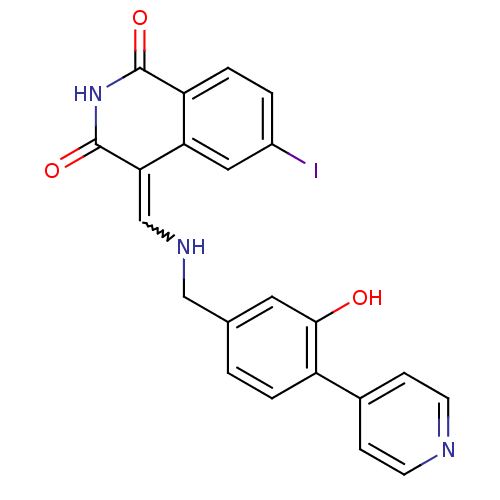
(4-[(3-Hydroxy-4-pyridin-4-yl-benzylamino)-methylen...)Show SMILES Oc1cc(CNC=C2C(=O)NC(=O)c3ccc(I)cc23)ccc1-c1ccncc1 |w:6.5| Show InChI InChI=1S/C22H16IN3O3/c23-15-2-4-17-18(10-15)19(22(29)26-21(17)28)12-25-11-13-1-3-16(20(27)9-13)14-5-7-24-8-6-14/h1-10,12,25,27H,11H2,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylation |
J Med Chem 52: 2289-310 (2009)
Article DOI: 10.1021/jm801026e
BindingDB Entry DOI: 10.7270/Q27H1JG7 |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50396546
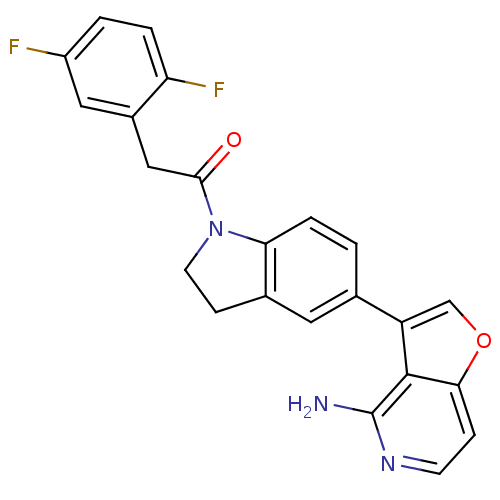
(CHEMBL2171137)Show SMILES Nc1nccc2occ(-c3ccc4N(CCc4c3)C(=O)Cc3cc(F)ccc3F)c12 Show InChI InChI=1S/C23H17F2N3O2/c24-16-2-3-18(25)15(10-16)11-21(29)28-8-6-14-9-13(1-4-19(14)28)17-12-30-20-5-7-27-23(26)22(17)20/h1-5,7,9-10,12H,6,8,11H2,(H2,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation |
J Med Chem 55: 7193-207 (2012)
Article DOI: 10.1021/jm300713s
BindingDB Entry DOI: 10.7270/Q2222VWP |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50095226
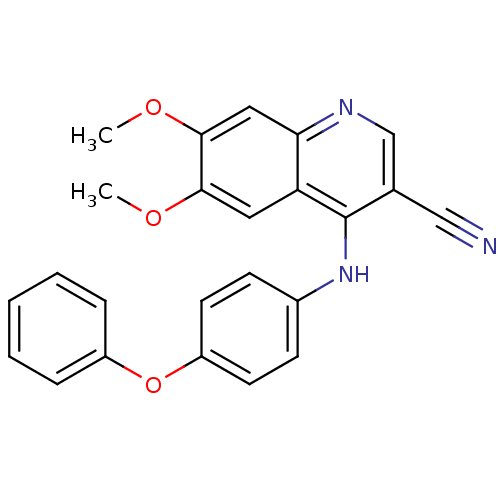
(6,7-Dimethoxy-4-(4-phenoxy-phenylamino)-quinoline-...)Show SMILES COc1cc2ncc(C#N)c(Nc3ccc(Oc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C24H19N3O3/c1-28-22-12-20-21(13-23(22)29-2)26-15-16(14-25)24(20)27-17-8-10-19(11-9-17)30-18-6-4-3-5-7-18/h3-13,15H,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of Dual specificity mitogen-activated protein kinase kinase (MEK-1) |
Bioorg Med Chem Lett 12: 423-5 (2002)
BindingDB Entry DOI: 10.7270/Q2WM1CQ7 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50341249

(1,1-Dimethylethyl{(3R,5R)-1-[2-Amino-6-(3-amino-1H...)Show SMILES CNc1nc(cc(n1)-c1ccc2c(N)n[nH]c2c1)N1C[C@H](C)C[C@H](C1)N(C)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C24H34N8O2/c1-14-9-16(31(6)23(33)34-24(2,3)4)13-32(12-14)20-11-18(27-22(26-5)28-20)15-7-8-17-19(10-15)29-30-21(17)25/h7-8,10-11,14,16H,9,12-13H2,1-6H3,(H3,25,29,30)(H,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT phosphorylation at Thr308 residue in human PC3 cells by ELISA |
J Med Chem 54: 1871-95 (2011)
Article DOI: 10.1021/jm101527u
BindingDB Entry DOI: 10.7270/Q2HQ406Q |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161948
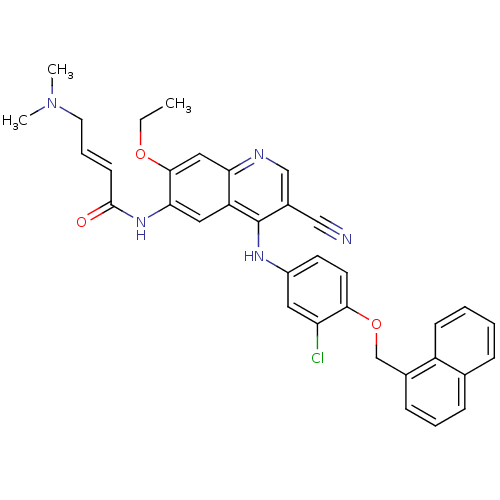
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(n...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4cccc5ccccc45)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C35H32ClN5O3/c1-4-43-33-19-30-28(18-31(33)40-34(42)13-8-16-41(2)3)35(25(20-37)21-38-30)39-26-14-15-32(29(36)17-26)44-22-24-11-7-10-23-9-5-6-12-27(23)24/h5-15,17-19,21H,4,16,22H2,1-3H3,(H,38,39)(H,40,42)/b13-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor (EGFR) autophosphorylation |
J Med Chem 48: 1107-31 (2005)
Article DOI: 10.1021/jm040159c
BindingDB Entry DOI: 10.7270/Q2BP03KG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50161903
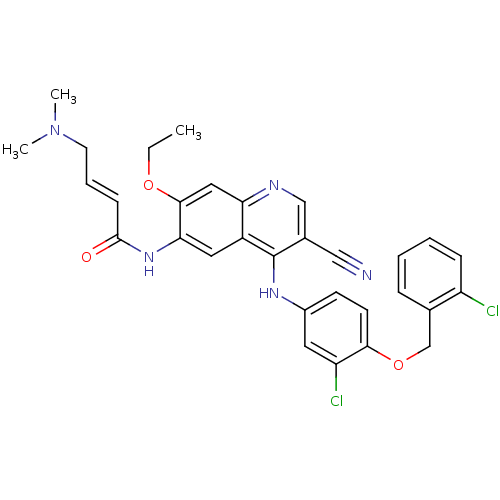
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(2...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccc4Cl)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C31H29Cl2N5O3/c1-4-40-29-16-26-23(15-27(29)37-30(39)10-7-13-38(2)3)31(21(17-34)18-35-26)36-22-11-12-28(25(33)14-22)41-19-20-8-5-6-9-24(20)32/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,35,36)(H,37,39)/b10-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor-2 (HER-2) autophosphorylation |
J Med Chem 48: 1107-31 (2005)
Article DOI: 10.1021/jm040159c
BindingDB Entry DOI: 10.7270/Q2BP03KG |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50109282
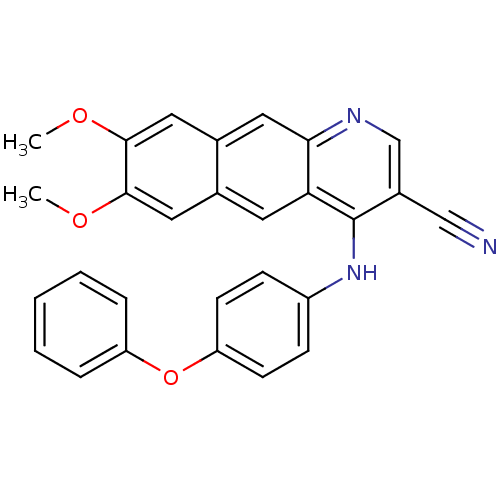
(7,8-Dimethoxy-4-(4-phenoxy-phenylamino)-benzo[g]qu...)Show SMILES COc1cc2cc3ncc(C#N)c(Nc4ccc(Oc5ccccc5)cc4)c3cc2cc1OC Show InChI InChI=1S/C28H21N3O3/c1-32-26-14-18-12-24-25(13-19(18)15-27(26)33-2)30-17-20(16-29)28(24)31-21-8-10-23(11-9-21)34-22-6-4-3-5-7-22/h3-15,17H,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of Dual specificity mitogen-activated protein kinase kinase (MEK-1) |
Bioorg Med Chem Lett 12: 423-5 (2002)
BindingDB Entry DOI: 10.7270/Q2WM1CQ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data