

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254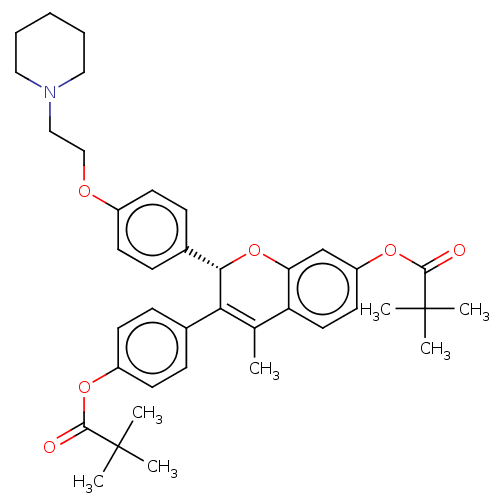 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471256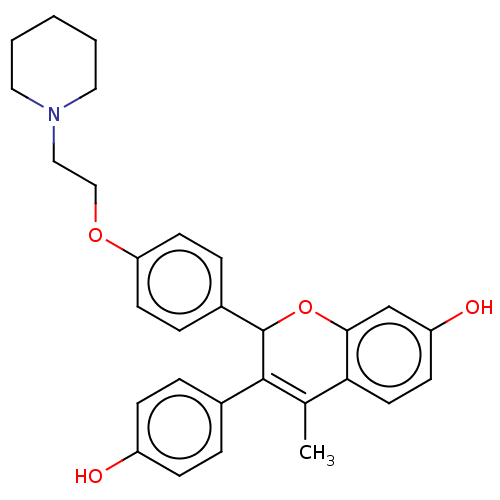 (CHEMBL291808) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471255 (Acolbifene | EM-652 | SCH-57068) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated ZR-75-1-cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20625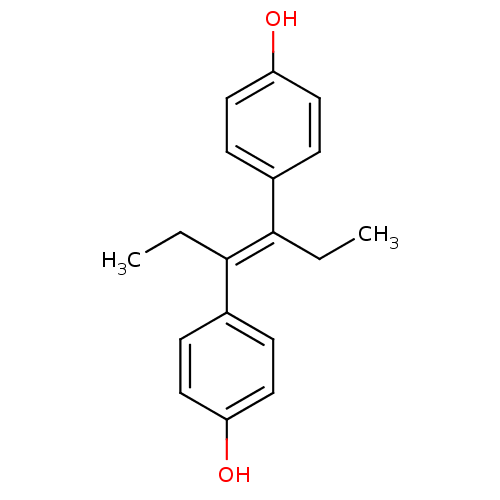 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20625 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50276802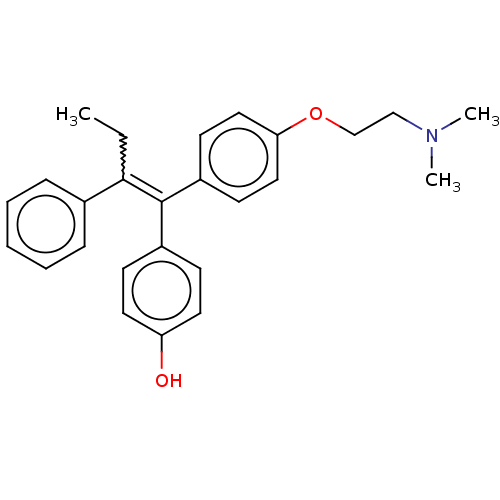 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 0.249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18778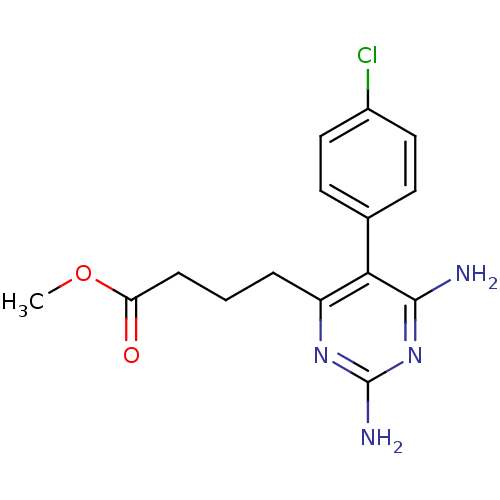 (CHEMBL22405 | P16 | methyl 4-[2,6-diamino-5-(4-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18781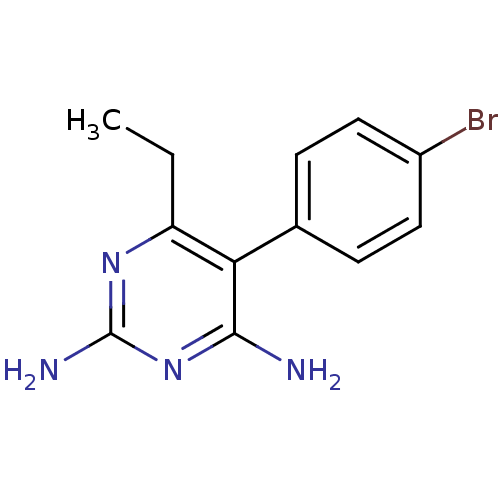 (5-(4-bromophenyl)-6-ethylpyrimidine-2,4-diamine | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18790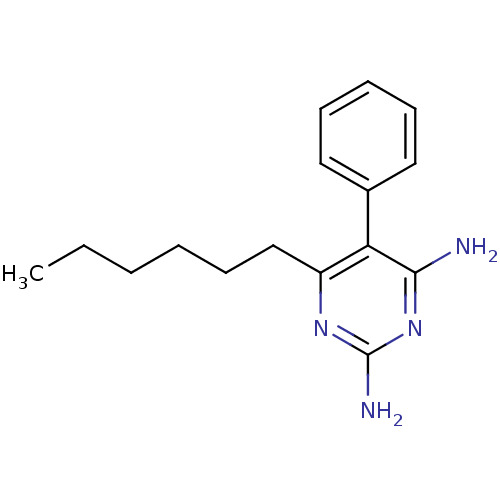 (6-hexyl-5-phenylpyrimidine-2,4-diamine | CHEMBL416...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110753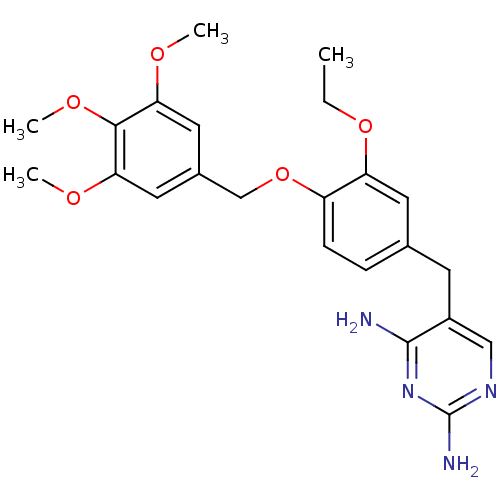 (5-(3-ethoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110753 (5-(3-ethoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004767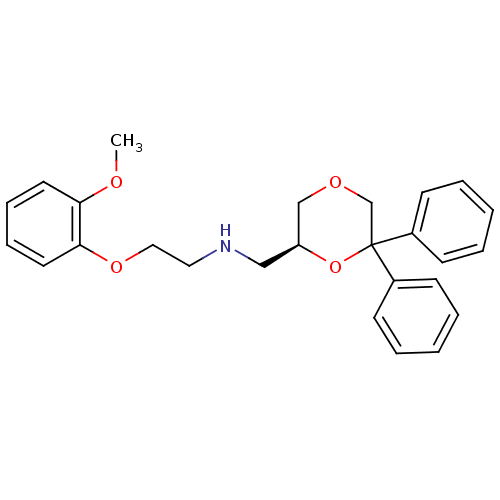 (CHEMBL2312536) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HeLa cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50276802 (4-OHT | Afimoxifene | TamoGel) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50026917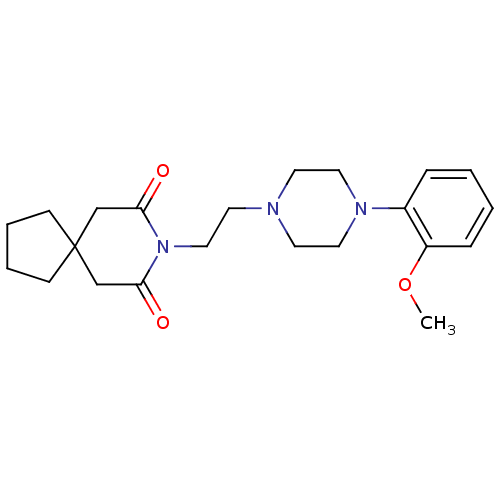 (8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HeLa cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50026917 (8-(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-8-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in human HeLa cells | J Med Chem 51: 6359-70 (2008) Article DOI: 10.1021/jm800461k BindingDB Entry DOI: 10.7270/Q2S183R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110769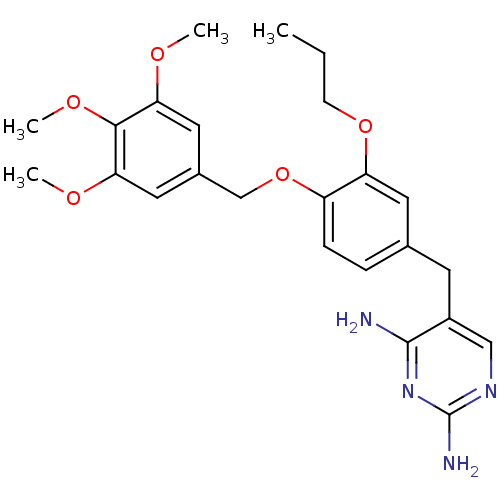 (5-(3-propoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18779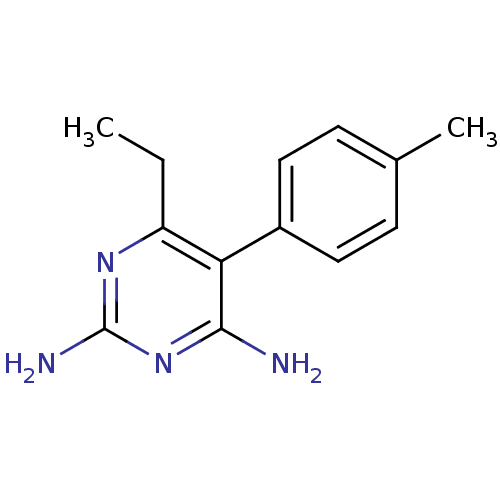 (6-ethyl-5-(4-methylphenyl)pyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110577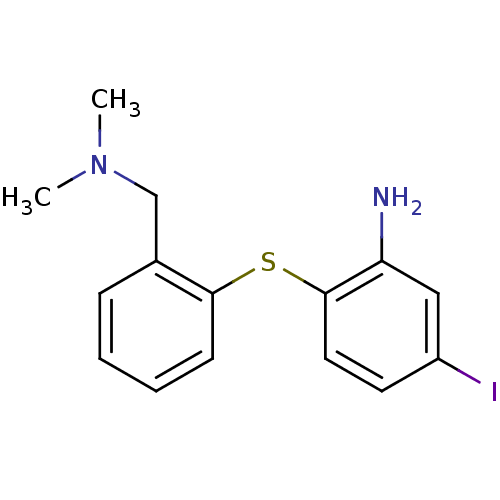 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane | J Med Chem 45: 1253-8 (2002) BindingDB Entry DOI: 10.7270/Q2TH8M15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18787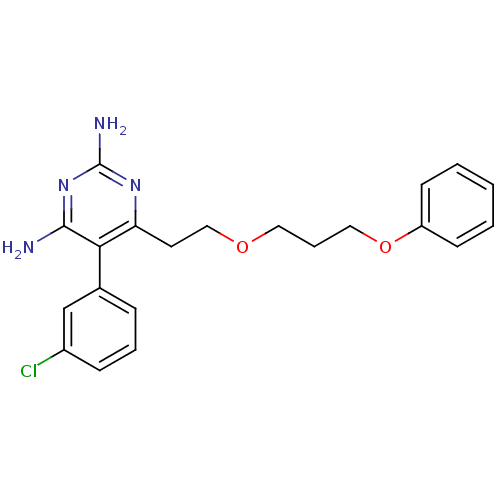 (5-(3-chlorophenyl)-6-[2-(3-phenoxypropoxy)ethyl]py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110769 (5-(3-propoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110755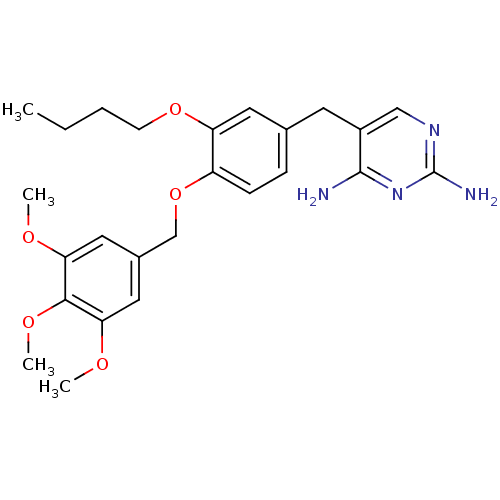 (5-(3-butoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004768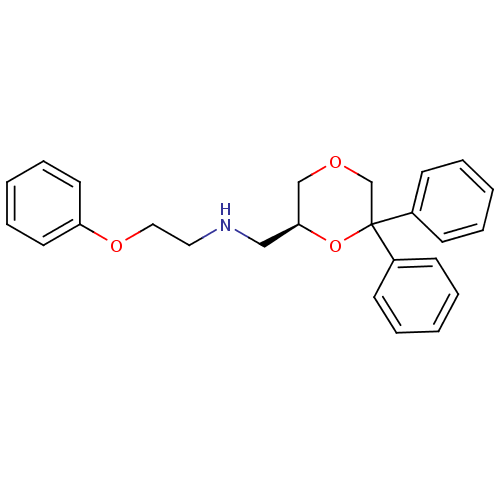 (CHEMBL2312538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HeLa cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110755 (5-(3-butoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against wild-type dihydrofolate reductase (S108N DHFR) | J Med Chem 47: 345-54 (2004) Article DOI: 10.1021/jm0303352 BindingDB Entry DOI: 10.7270/Q237784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50392637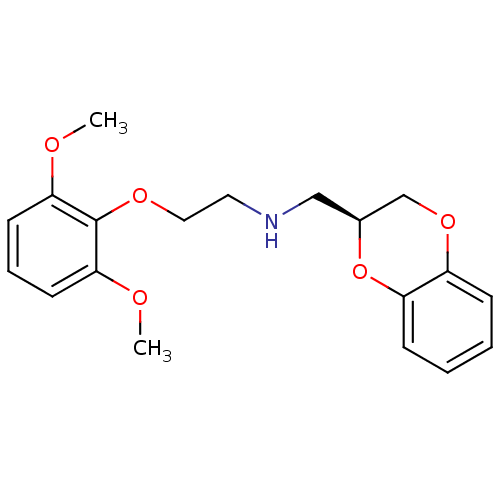 (CHEMBL215421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant alpha1A adrenergic receptor expressed in CHO cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM69602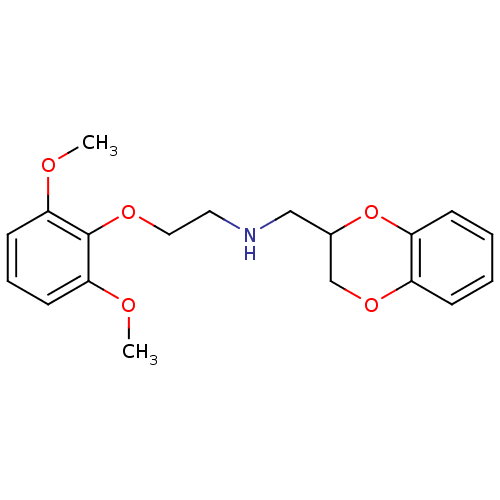 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human cloned alpha1A adrenoceptor expressed in CHO cells | J Med Chem 51: 6359-70 (2008) Article DOI: 10.1021/jm800461k BindingDB Entry DOI: 10.7270/Q2S183R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant alpha1A adrenergic receptor expressed in CHO cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18791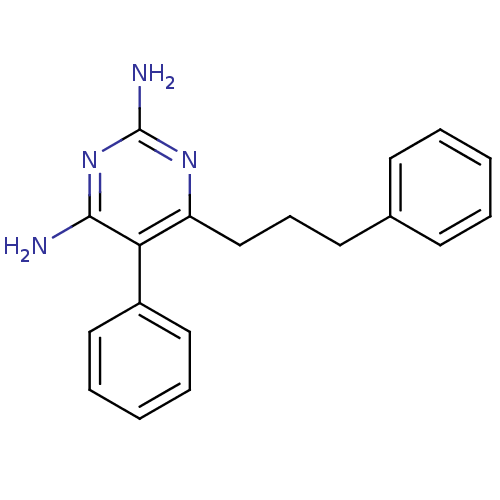 (5-phenyl-6-(3-phenylpropyl)pyrimidine-2,4-diamine ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110767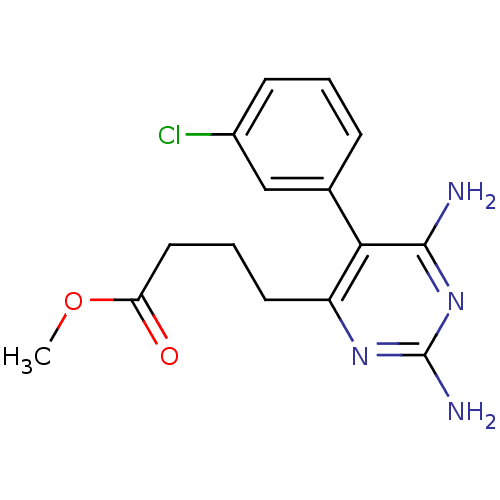 (4-[2,6-Diamino-5-(3-chloro-phenyl)-pyrimidin-4-yl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50514929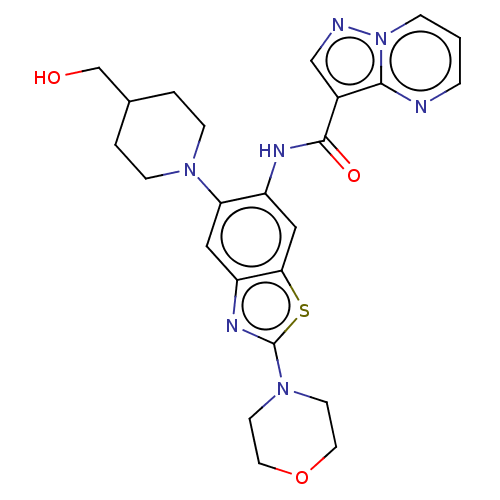 (CHEMBL4474636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... | J Med Chem 62: 6223-6240 (2019) Article DOI: 10.1021/acs.jmedchem.9b00439 BindingDB Entry DOI: 10.7270/Q2CC1419 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50514930 (CHEMBL4464832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... | J Med Chem 62: 6223-6240 (2019) Article DOI: 10.1021/acs.jmedchem.9b00439 BindingDB Entry DOI: 10.7270/Q2CC1419 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50392637 (CHEMBL215421) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant alpha1D adrenergic receptor expressed in CHO cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant alpha1D adrenergic receptor expressed in CHO cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human cloned alpha1D adrenoceptor expressed in CHO cells | J Med Chem 51: 6359-70 (2008) Article DOI: 10.1021/jm800461k BindingDB Entry DOI: 10.7270/Q2S183R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471258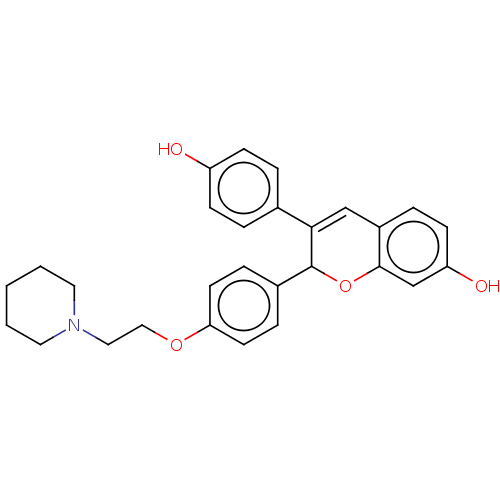 (CHEMBL67783) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004766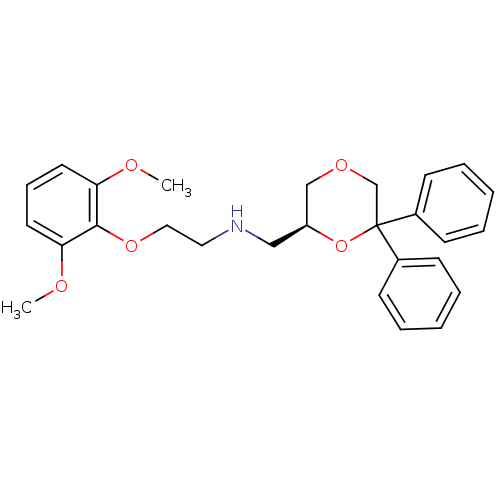 (CHEMBL2312226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HeLa cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50412784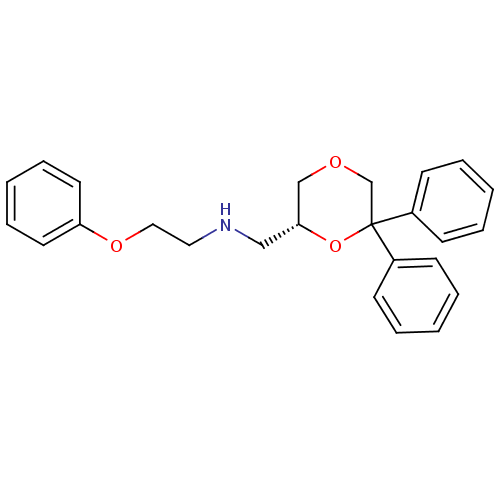 (CHEMBL493285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in human HeLa cells | J Med Chem 51: 6359-70 (2008) Article DOI: 10.1021/jm800461k BindingDB Entry DOI: 10.7270/Q2S183R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50004769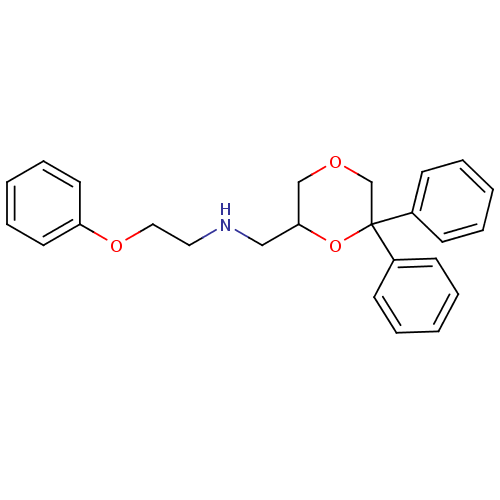 (CHEMBL2312537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HeLa cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50412783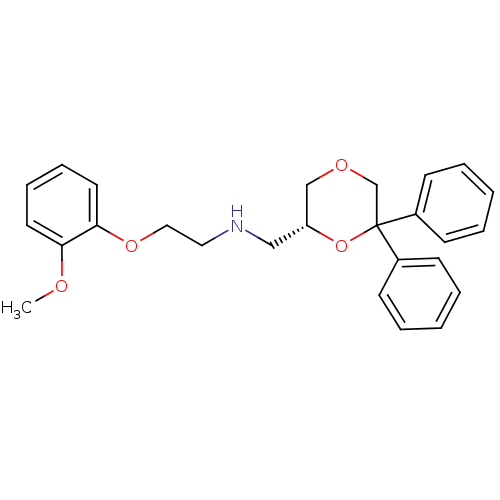 (CHEMBL493486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in HeLa cell membranes after 30 mins | J Med Chem 56: 584-8 (2013) Article DOI: 10.1021/jm301525w BindingDB Entry DOI: 10.7270/Q2474CDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50110760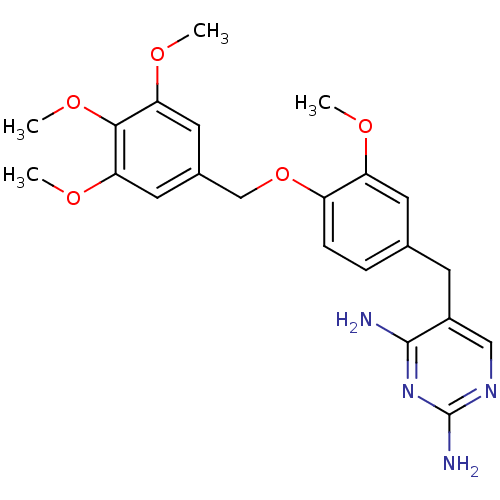 (5-(3-methoxy-4-(3,4,5-trimethoxybenzyloxy)benzyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Genetic Engineering and Biotechnology Curated by ChEMBL | Assay Description Inhibition of the wild-type dihydrofolate reductase (DHFR) | J Med Chem 45: 1244-52 (2002) BindingDB Entry DOI: 10.7270/Q2Z89BQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4732 total ) | Next | Last >> |