 Found 68 hits with Last Name = 'ray' and Initial = 'as'
Found 68 hits with Last Name = 'ray' and Initial = 'as' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259706
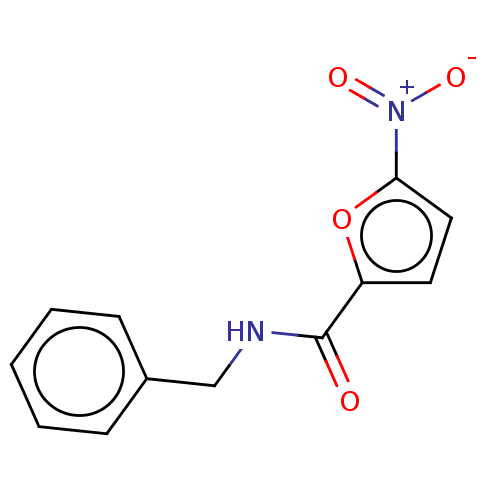
(CHEMBL2313314)Show InChI InChI=1S/C12H10N2O4/c15-12(10-6-7-11(18-10)14(16)17)13-8-9-4-2-1-3-5-9/h1-7H,8H2,(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259707

(CHEMBL4091464)Show SMILES [O-][N+](=O)c1ccc(o1)C(=O)N(Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C19H16N2O4/c22-19(17-11-12-18(25-17)21(23)24)20(13-15-7-3-1-4-8-15)14-16-9-5-2-6-10-16/h1-12H,13-14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259704
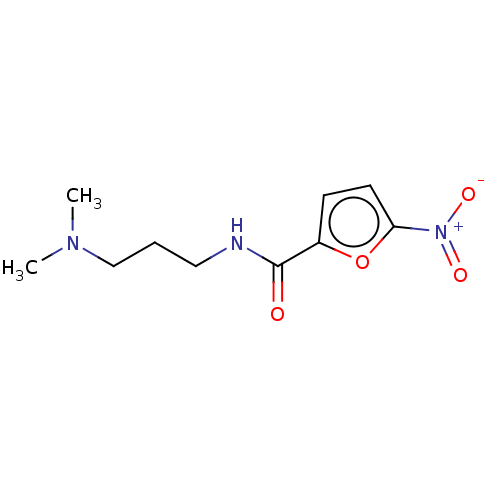
(CHEMBL4066587)Show InChI InChI=1S/C10H15N3O4/c1-12(2)7-3-6-11-10(14)8-4-5-9(17-8)13(15)16/h4-5H,3,6-7H2,1-2H3,(H,11,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259705
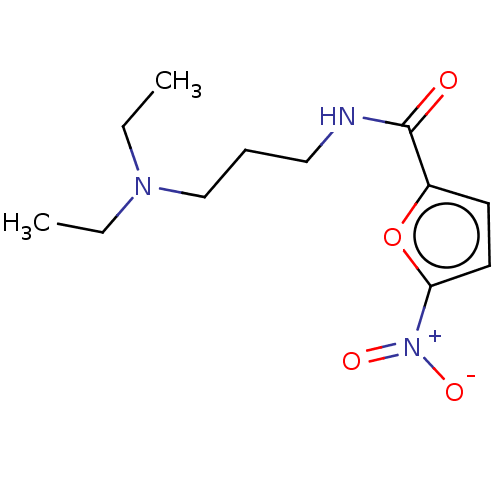
(CHEMBL4088638)Show InChI InChI=1S/C12H19N3O4/c1-3-14(4-2)9-5-8-13-12(16)10-6-7-11(19-10)15(17)18/h6-7H,3-5,8-9H2,1-2H3,(H,13,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259703

(CHEMBL4084206)Show InChI InChI=1S/C9H13N3O4/c1-11(2)6-5-10-9(13)7-3-4-8(16-7)12(14)15/h3-4H,5-6H2,1-2H3,(H,10,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259702
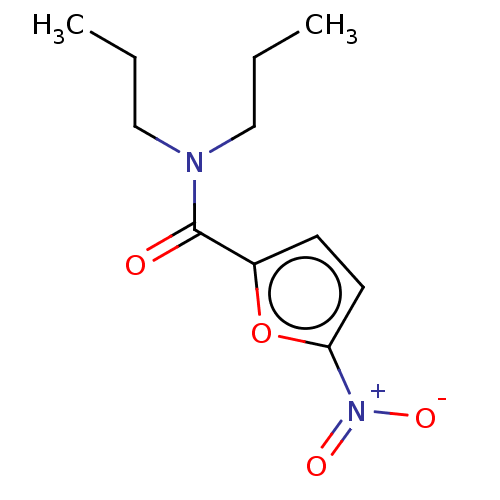
(CHEMBL4079787)Show InChI InChI=1S/C11H16N2O4/c1-3-7-12(8-4-2)11(14)9-5-6-10(17-9)13(15)16/h5-6H,3-4,7-8H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259710
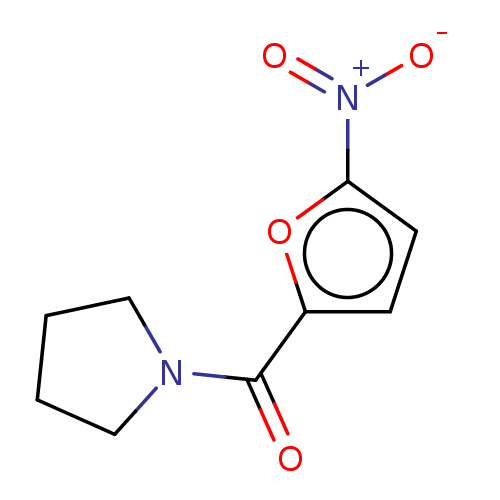
(CHEMBL4071417)Show InChI InChI=1S/C9H10N2O4/c12-9(10-5-1-2-6-10)7-3-4-8(15-7)11(13)14/h3-4H,1-2,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259708
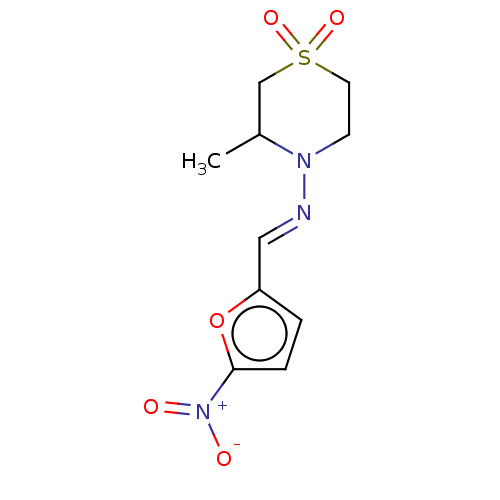
(BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...)Show SMILES CC1CS(=O)(=O)CCN1\N=C\c1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C10H13N3O5S/c1-8-7-19(16,17)5-4-12(8)11-6-9-2-3-10(18-9)13(14)15/h2-3,6,8H,4-5,7H2,1H3/b11-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259698

(CHEMBL4092991)Show InChI InChI=1S/C10H12N2O4/c13-10(11-6-2-1-3-7-11)8-4-5-9(16-8)12(14)15/h4-5H,1-3,6-7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259709
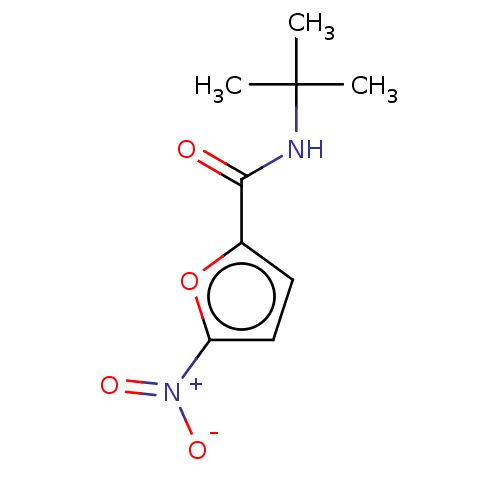
(CHEMBL4061646)Show InChI InChI=1S/C9H12N2O4/c1-9(2,3)10-8(12)6-4-5-7(15-6)11(13)14/h4-5H,1-3H3,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259700

(CHEMBL4064356)Show InChI InChI=1S/C9H12N2O4/c1-2-3-6-10-9(12)7-4-5-8(15-7)11(13)14/h4-5H,2-3,6H2,1H3,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259699

(CHEMBL4079946)Show InChI InChI=1S/C8H10N2O4/c1-2-5-9-8(11)6-3-4-7(14-6)10(12)13/h3-4H,2,5H2,1H3,(H,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259711
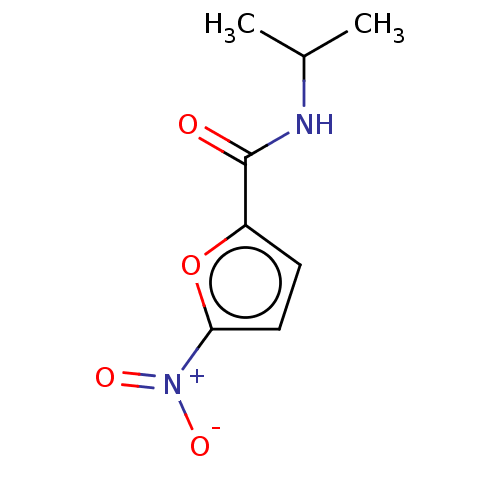
(CHEMBL4092070)Show InChI InChI=1S/C8H10N2O4/c1-5(2)9-8(11)6-3-4-7(14-6)10(12)13/h3-5H,1-2H3,(H,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50259701

(CHEMBL4103302)Show InChI InChI=1S/C9H12N2O4/c1-3-6(2)10-9(12)7-4-5-8(15-7)11(13)14/h4-6H,3H2,1-2H3,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method |
Eur J Med Chem 125: 1088-1097 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.055
BindingDB Entry DOI: 10.7270/Q2TM7DKH |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV 3B reverse transcriptase in MT2 cells |
Bioorg Med Chem Lett 18: 1120-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.125
BindingDB Entry DOI: 10.7270/Q24Q7XRS |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350205
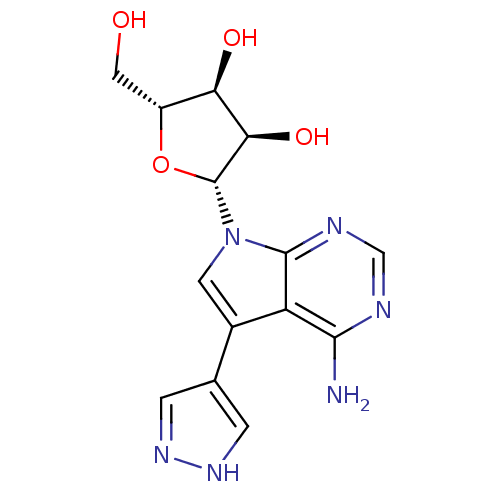
(CHEMBL1814774)Show SMILES Nc1ncnc2n(cc(-c3cn[nH]c3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-12-9-7(6-1-18-19-2-6)3-20(13(9)17-5-16-12)14-11(23)10(22)8(4-21)24-14/h1-3,5,8,10-11,14,21-23H,4H2,(H,18,19)(H2,15,16,17)/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479807
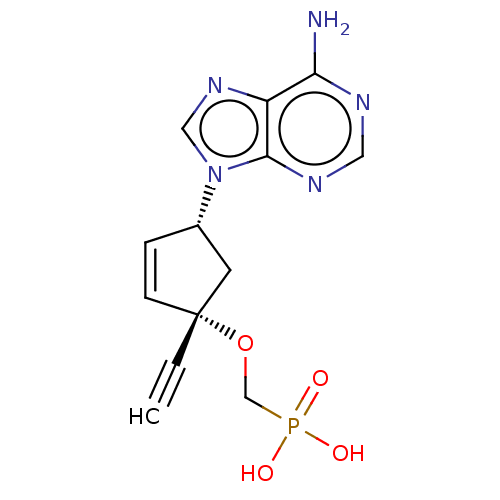
(CHEMBL525256)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@](OCP(O)(O)=O)(C=C1)C#C |r,c:21| Show InChI InChI=1S/C13H14N5O4P/c1-2-13(22-8-23(19,20)21)4-3-9(5-13)18-7-17-10-11(14)15-6-16-12(10)18/h1,3-4,6-7,9H,5,8H2,(H2,14,15,16)(H2,19,20,21)/t9-,13+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50366816

(ABACAVIR | Epzicom | Ziagen)Show SMILES Nc1nc(NC2CC2)c2ncn([C@@H]3C[C@H](CO)C=C3)c2n1 |r,c:18| Show InChI InChI=1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350207

(CHEMBL1814776)Show SMILES Nc1ncnc2n(cc(C#C)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H14N4O4/c1-2-6-3-17(12-8(6)11(14)15-5-16-12)13-10(20)9(19)7(4-18)21-13/h1,3,5,7,9-10,13,18-20H,4H2,(H2,14,15,16)/t7-,9-,10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50450813

(Atripla | CHEBI:63625 | GS-1275 | PMP-A | Tenofovi...)Show InChI InChI=1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV 3B reverse transcriptase in MT2 cells |
Bioorg Med Chem Lett 18: 1120-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.125
BindingDB Entry DOI: 10.7270/Q24Q7XRS |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50450813

(Atripla | CHEBI:63625 | GS-1275 | PMP-A | Tenofovi...)Show InChI InChI=1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478099
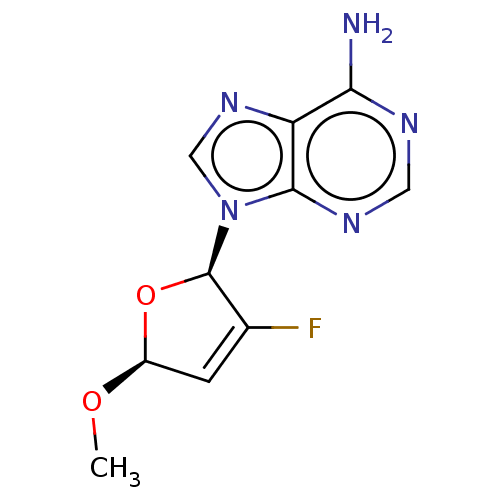
(CHEMBL271681)Show SMILES CO[C@H]1O[C@H](C(F)=C1)n1cnc2c(N)ncnc12 |c:6| Show InChI InChI=1S/C10H10FN5O2/c1-17-6-2-5(11)10(18-6)16-4-15-7-8(12)13-3-14-9(7)16/h2-4,6,10H,1H3,(H2,12,13,14)/t6-,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV 3B reverse transcriptase in MT2 cells |
Bioorg Med Chem Lett 18: 1120-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.125
BindingDB Entry DOI: 10.7270/Q24Q7XRS |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478098
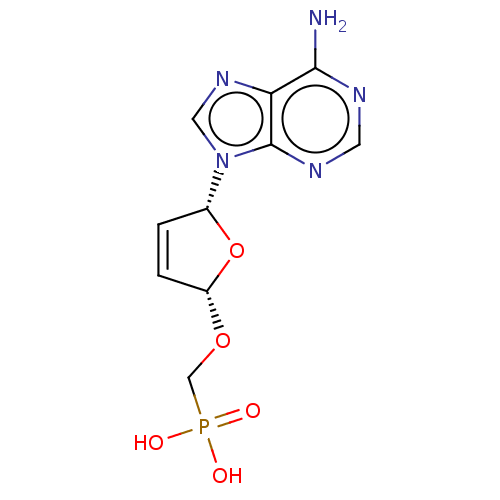
(CHEMBL420843)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](OCP(O)(O)=O)C=C1 |r,c:21| Show InChI InChI=1S/C10H12N5O5P/c11-9-8-10(13-3-12-9)15(4-14-8)6-1-2-7(20-6)19-5-21(16,17)18/h1-4,6-7H,5H2,(H2,11,12,13)(H2,16,17,18)/t6-,7+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478098

(CHEMBL420843)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](OCP(O)(O)=O)C=C1 |r,c:21| Show InChI InChI=1S/C10H12N5O5P/c11-9-8-10(13-3-12-9)15(4-14-8)6-1-2-7(20-6)19-5-21(16,17)18/h1-4,6-7H,5H2,(H2,11,12,13)(H2,16,17,18)/t6-,7+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV 3B reverse transcriptase in MT2 cells |
Bioorg Med Chem Lett 18: 1120-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.125
BindingDB Entry DOI: 10.7270/Q24Q7XRS |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479808
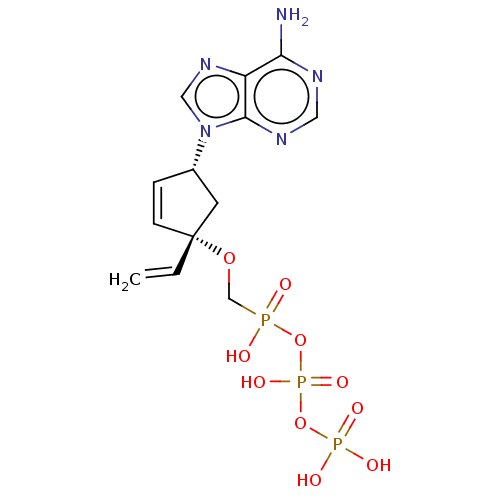
(CHEMBL512522)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@](OCP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C1 |r,c:31| Show InChI InChI=1S/C13H18N5O10P3/c1-2-13(26-8-29(19,20)27-31(24,25)28-30(21,22)23)4-3-9(5-13)18-7-17-10-11(14)15-6-16-12(10)18/h2-4,6-7,9H,1,5,8H2,(H,19,20)(H,24,25)(H2,14,15,16)(H2,21,22,23)/t9-,13+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50164648
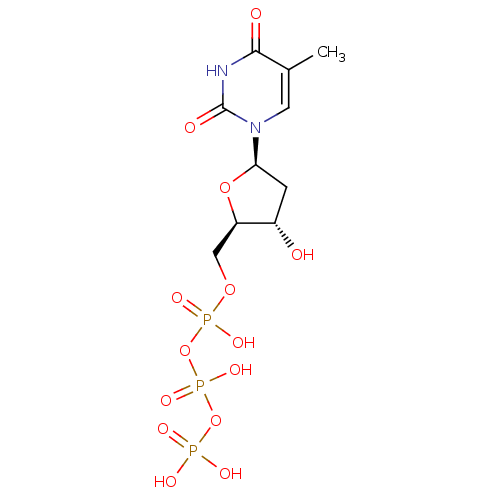
(2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O14P3/c1-5-3-12(10(15)11-9(5)14)8-2-6(13)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8,13H,2,4H2,1H3,(H,19,20)(H,21,22)(H,11,14,15)(H2,16,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase-beta |
Bioorg Med Chem Lett 27: 1840-1847 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.037
BindingDB Entry DOI: 10.7270/Q2M047Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50335554
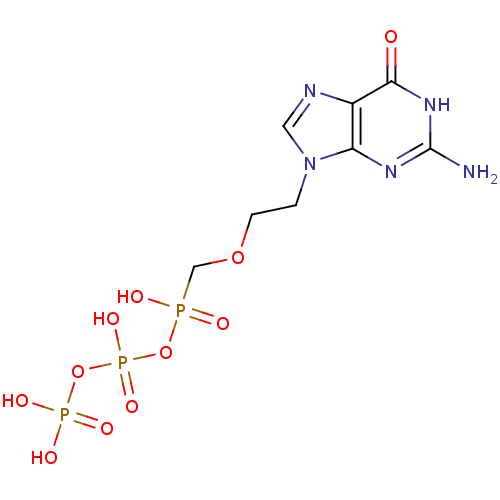
(({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H14N5O11P3/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-22-4-25(15,16)23-27(20,21)24-26(17,18)19/h3H,1-2,4H2,(H,15,16)(H,20,21)(H2,17,18,19)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478100
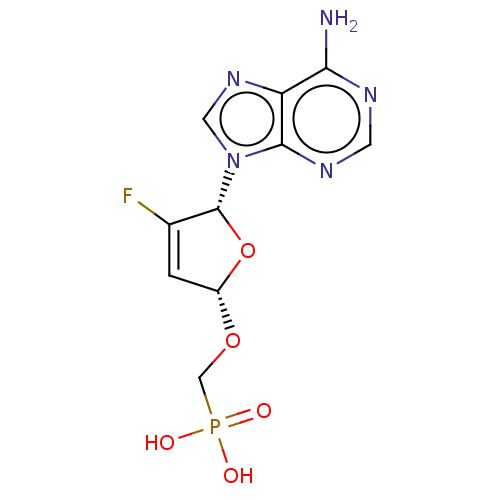
(CHEMBL252036 | GS-9148)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](OCP(O)(O)=O)C=C1F |r,c:21| Show InChI InChI=1S/C10H11FN5O5P/c11-5-1-6(20-4-22(17,18)19)21-10(5)16-3-15-7-8(12)13-2-14-9(7)16/h1-3,6,10H,4H2,(H2,12,13,14)(H2,17,18,19)/t6-,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Science, Inc
Curated by ChEMBL
| Assay Description
Inhibition on HIV1 reverse transcriptase p66/p51 |
Bioorg Med Chem 17: 1739-46 (2009)
Article DOI: 10.1016/j.bmc.2008.12.028
BindingDB Entry DOI: 10.7270/Q21Z4774 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482144
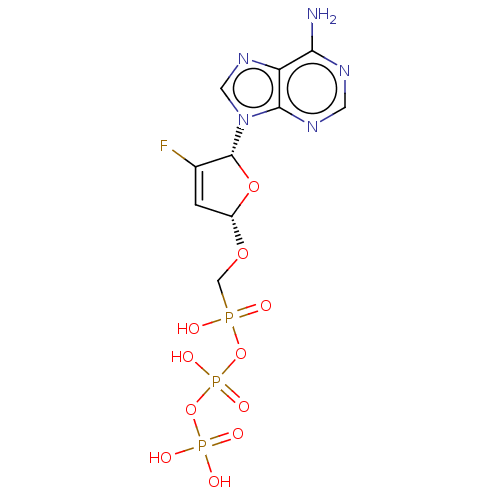
(CHEMBL1098407)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](OCP(O)(=O)OP(O)(=O)OP(O)(O)=O)C=C1F |r,c:29| Show InChI InChI=1S/C10H13FN5O11P3/c11-5-1-6(24-4-28(17,18)26-30(22,23)27-29(19,20)21)25-10(5)16-3-15-7-8(12)13-2-14-9(7)16/h1-3,6,10H,4H2,(H,17,18)(H,22,23)(H2,12,13,14)(H2,19,20,21)/t6-,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
Bioorg Med Chem 18: 3606-17 (2010)
Article DOI: 10.1016/j.bmc.2010.03.041
BindingDB Entry DOI: 10.7270/Q2M04892 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478100

(CHEMBL252036 | GS-9148)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](OCP(O)(O)=O)C=C1F |r,c:21| Show InChI InChI=1S/C10H11FN5O5P/c11-5-1-6(20-4-22(17,18)19)21-10(5)16-3-15-7-8(12)13-2-14-9(7)16/h1-3,6,10H,4H2,(H2,12,13,14)(H2,17,18,19)/t6-,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV 3B reverse transcriptase in MT2 cells |
Bioorg Med Chem Lett 18: 1120-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.125
BindingDB Entry DOI: 10.7270/Q24Q7XRS |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350195
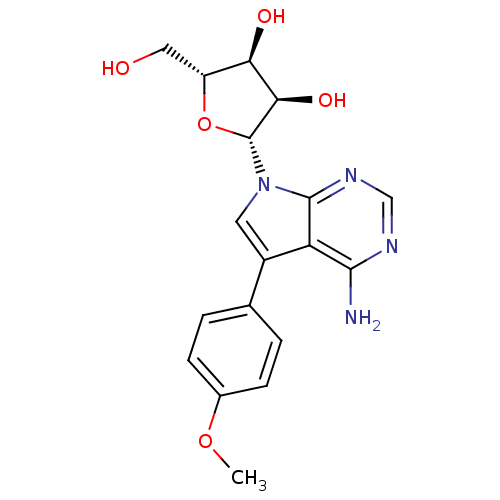
(CHEMBL1814763)Show SMILES COc1ccc(cc1)-c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C18H20N4O5/c1-26-10-4-2-9(3-5-10)11-6-22(17-13(11)16(19)20-8-21-17)18-15(25)14(24)12(7-23)27-18/h2-6,8,12,14-15,18,23-25H,7H2,1H3,(H2,19,20,21)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350199
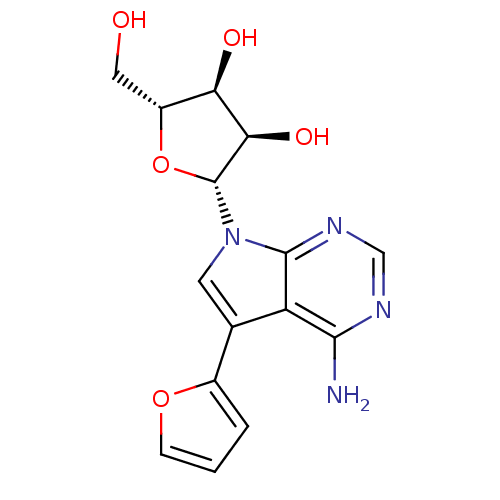
(CHEMBL1814767)Show SMILES Nc1ncnc2n(cc(-c3ccco3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O5/c16-13-10-7(8-2-1-3-23-8)4-19(14(10)18-6-17-13)15-12(22)11(21)9(5-20)24-15/h1-4,6,9,11-12,15,20-22H,5H2,(H2,16,17,18)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50335554

(({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H14N5O11P3/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-22-4-25(15,16)23-27(20,21)24-26(17,18)19/h3H,1-2,4H2,(H,15,16)(H,20,21)(H2,17,18,19)(H3,9,11,12,14) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350209
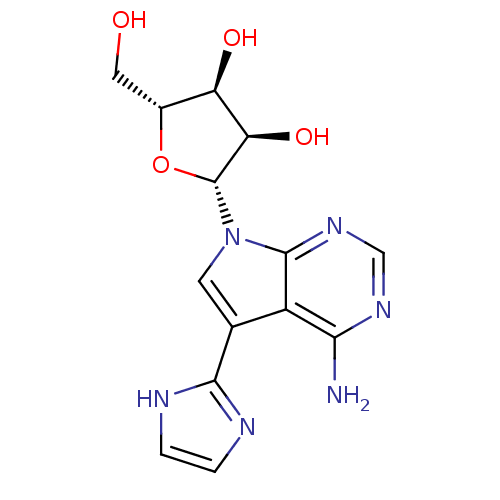
(CHEMBL1814780)Show SMILES Nc1ncnc2n(cc(-c3ncc[nH]3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-11-8-6(12-16-1-2-17-12)3-20(13(8)19-5-18-11)14-10(23)9(22)7(4-21)24-14/h1-3,5,7,9-10,14,21-23H,4H2,(H,16,17)(H2,15,18,19)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350194
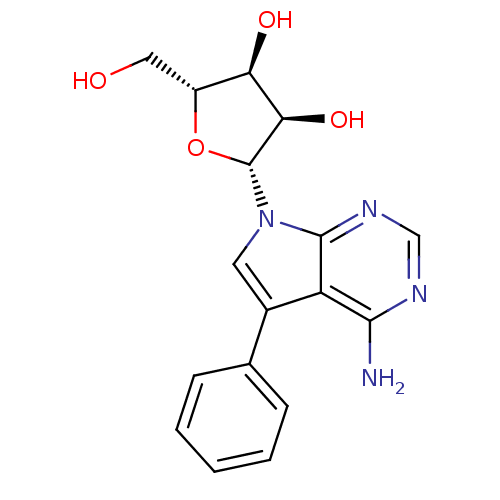
(CHEMBL1814762)Show SMILES Nc1ncnc2n(cc(-c3ccccc3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H18N4O4/c18-15-12-10(9-4-2-1-3-5-9)6-21(16(12)20-8-19-15)17-14(24)13(23)11(7-22)25-17/h1-6,8,11,13-14,17,22-24H,7H2,(H2,18,19,20)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350200
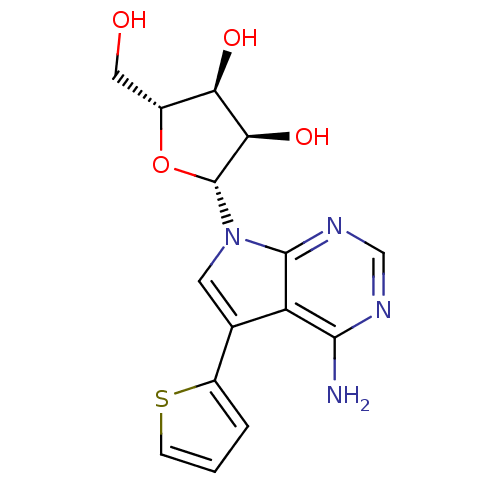
(CHEMBL1814768)Show SMILES Nc1ncnc2n(cc(-c3cccs3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O4S/c16-13-10-7(9-2-1-3-24-9)4-19(14(10)18-6-17-13)15-12(22)11(21)8(5-20)23-15/h1-4,6,8,11-12,15,20-22H,5H2,(H2,16,17,18)/t8-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350202
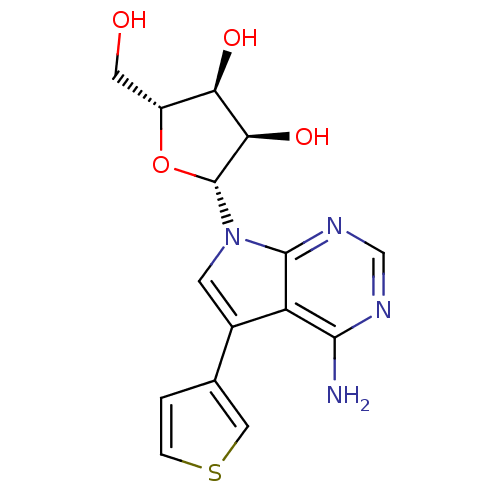
(CHEMBL1814770)Show SMILES Nc1ncnc2n(cc(-c3ccsc3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O4S/c16-13-10-8(7-1-2-24-5-7)3-19(14(10)18-6-17-13)15-12(22)11(21)9(4-20)23-15/h1-3,5-6,9,11-12,15,20-22H,4H2,(H2,16,17,18)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350198

(CHEMBL1814766)Show SMILES Nc1ncnc2n(cc(-c3ccc4ccccc4c3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20N4O4/c22-19-16-14(13-6-5-11-3-1-2-4-12(11)7-13)8-25(20(16)24-10-23-19)21-18(28)17(27)15(9-26)29-21/h1-8,10,15,17-18,21,26-28H,9H2,(H2,22,23,24)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350204
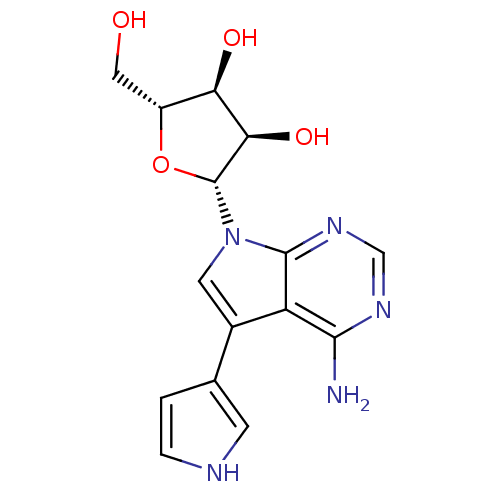
(CHEMBL1814773)Show SMILES Nc1ncnc2n(cc(-c3cc[nH]c3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H17N5O4/c16-13-10-8(7-1-2-17-3-7)4-20(14(10)19-6-18-13)15-12(23)11(22)9(5-21)24-15/h1-4,6,9,11-12,15,17,21-23H,5H2,(H2,16,18,19)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350201
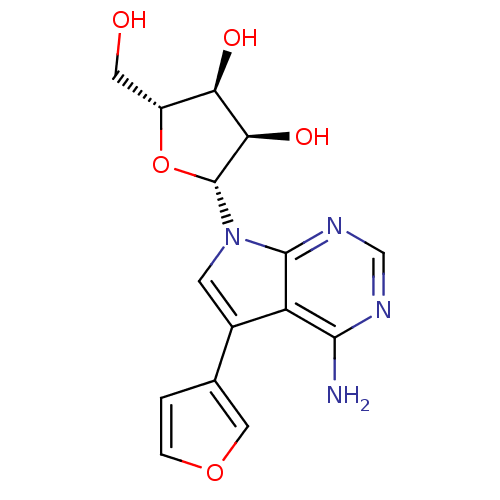
(CHEMBL1814769)Show SMILES Nc1ncnc2n(cc(-c3ccoc3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O5/c16-13-10-8(7-1-2-23-5-7)3-19(14(10)18-6-17-13)15-12(22)11(21)9(4-20)24-15/h1-3,5-6,9,11-12,15,20-22H,4H2,(H2,16,17,18)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc
Curated by ChEMBL
| Assay Description
Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cells |
Antimicrob Agents Chemother 51: 3498-504 (2007)
Article DOI: 10.1128/AAC.00671-07
BindingDB Entry DOI: 10.7270/Q24Q7VX6 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350208

(CHEMBL1814779)Show SMILES Nc1ncnc2n(cc(-c3c[nH]cn3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-12-9-6(7-1-16-4-17-7)2-20(13(9)19-5-18-12)14-11(23)10(22)8(3-21)24-14/h1-2,4-5,8,10-11,14,21-23H,3H2,(H,16,17)(H2,15,18,19)/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350196
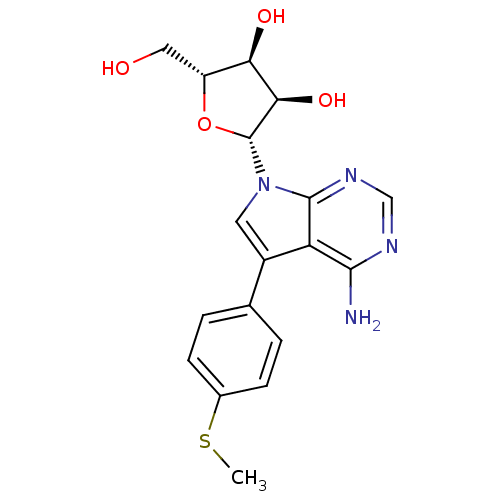
(CHEMBL1814764)Show SMILES CSc1ccc(cc1)-c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C18H20N4O4S/c1-27-10-4-2-9(3-5-10)11-6-22(17-13(11)16(19)20-8-21-17)18-15(25)14(24)12(7-23)26-18/h2-6,8,12,14-15,18,23-25H,7H2,1H3,(H2,19,20,21)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350206
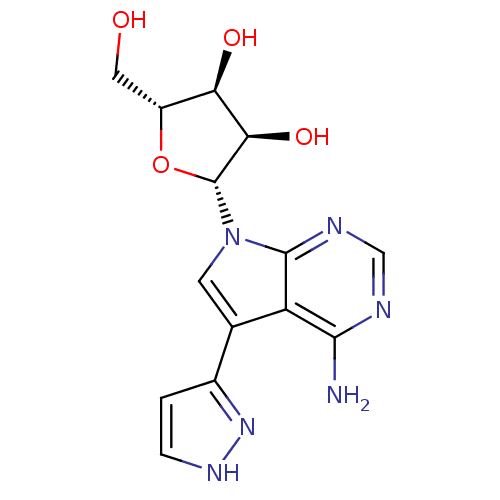
(CHEMBL1814775)Show SMILES Nc1ncnc2n(cc(-c3cc[nH]n3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-12-9-6(7-1-2-18-19-7)3-20(13(9)17-5-16-12)14-11(23)10(22)8(4-21)24-14/h1-3,5,8,10-11,14,21-23H,4H2,(H,18,19)(H2,15,16,17)/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350203
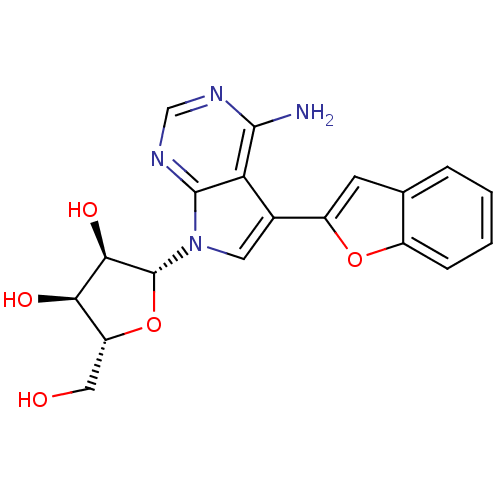
(CHEMBL1814771)Show SMILES Nc1ncnc2n(cc(-c3cc4ccccc4o3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H18N4O5/c20-17-14-10(12-5-9-3-1-2-4-11(9)27-12)6-23(18(14)22-8-21-17)19-16(26)15(25)13(7-24)28-19/h1-6,8,13,15-16,19,24-26H,7H2,(H2,20,21,22)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350197
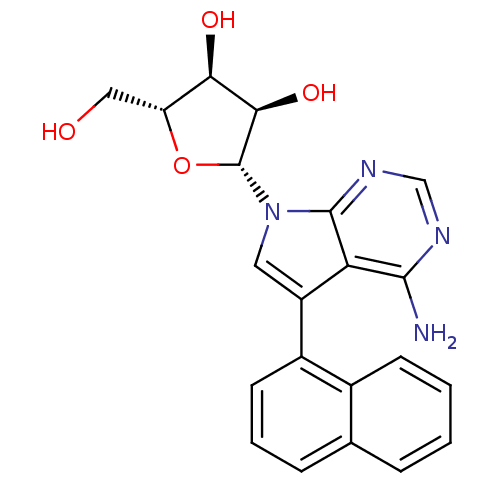
(CHEMBL1814765)Show SMILES Nc1ncnc2n(cc(-c3cccc4ccccc34)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20N4O4/c22-19-16-14(13-7-3-5-11-4-1-2-6-12(11)13)8-25(20(16)24-10-23-19)21-18(28)17(27)15(9-26)29-21/h1-8,10,15,17-18,21,26-28H,9H2,(H2,22,23,24)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc
Curated by ChEMBL
| Assay Description
Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cells |
Antimicrob Agents Chemother 51: 3498-504 (2007)
Article DOI: 10.1128/AAC.00671-07
BindingDB Entry DOI: 10.7270/Q24Q7VX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50335553
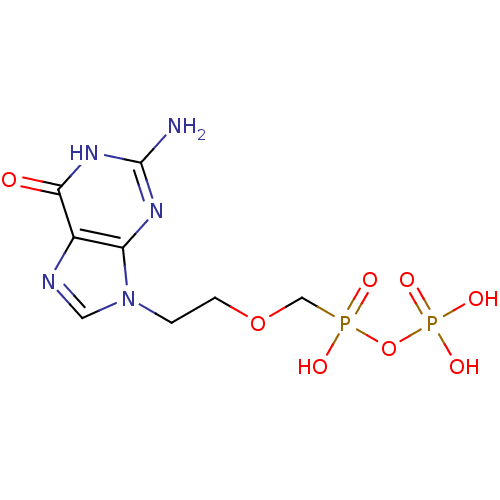
(CHEMBL1652466 | [({[2-(2-amino-6-oxo-6,9-dihydro-3...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H13N5O8P2/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-20-4-22(15,16)21-23(17,18)19/h3H,1-2,4H2,(H,15,16)(H2,17,18,19)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50366785

(NELFINAVIR)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26+,27-,29+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc
Curated by ChEMBL
| Assay Description
Inhibition of human MDR1-dependent accumulation of calcein-AM expressed in MDCK2 cells |
Antimicrob Agents Chemother 51: 3498-504 (2007)
Article DOI: 10.1128/AAC.00671-07
BindingDB Entry DOI: 10.7270/Q24Q7VX6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data