 Found 479 hits with Last Name = 'read' and Initial = 'ja'
Found 479 hits with Last Name = 'read' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50425950
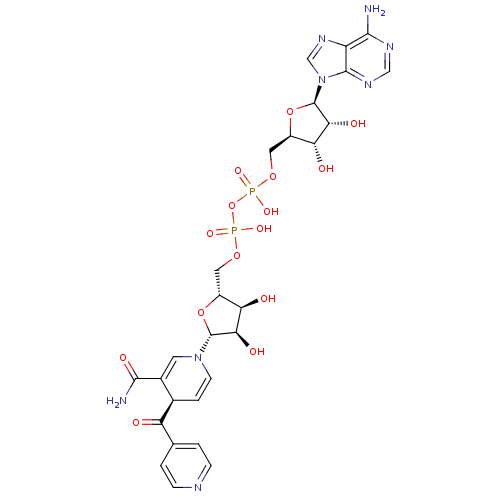
(CHEMBL2311561 | Isoniazid-NAD)Show SMILES NC(=O)C1=CN(C=C[C@H]1C(=O)c1ccncc1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r,c:6,t:3| Show InChI InChI=1S/C27H32N8O15P2/c28-23-17-25(32-10-31-23)35(11-33-17)27-22(40)20(38)16(49-27)9-47-52(44,45)50-51(42,43)46-8-15-19(37)21(39)26(48-15)34-6-3-13(14(7-34)24(29)41)18(36)12-1-4-30-5-2-12/h1-7,10-11,13,15-16,19-22,26-27,37-40H,8-9H2,(H2,29,41)(H,42,43)(H,44,45)(H2,28,31,32)/t13-,15-,16-,19-,20-,21-,22-,26-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type Mycobacterium tuberculosis inhA |
J Med Chem 56: 8533-42 (2013)
Article DOI: 10.1021/jm4012033
BindingDB Entry DOI: 10.7270/Q2SB4769 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250610

(CHEMBL4076072)Show SMILES OP(O)(=O)C(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H22Cl3FNO6PS/c25-20-14-22(27)21(26)13-18(20)12-17-2-1-3-19(23(17)28)15-4-6-16(7-5-15)24(36(30,31)32)37(33,34)29-8-10-35-11-9-29/h1-7,13-14,24H,8-12H2,(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250608
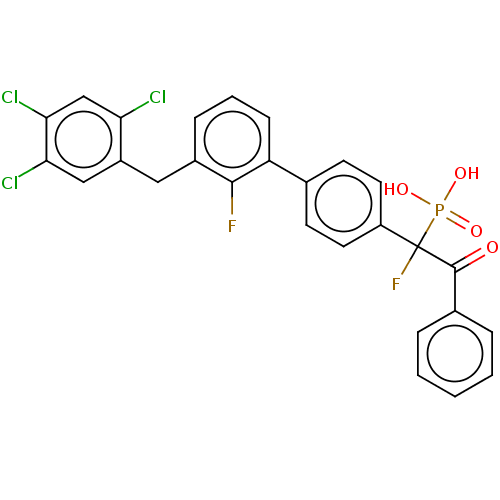
(CHEMBL4092589)Show SMILES OP(O)(=O)C(F)(C(=O)c1ccccc1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C27H18Cl3F2O4P/c28-22-15-24(30)23(29)14-19(22)13-18-7-4-8-21(25(18)31)16-9-11-20(12-10-16)27(32,37(34,35)36)26(33)17-5-2-1-3-6-17/h1-12,14-15H,13H2,(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250601
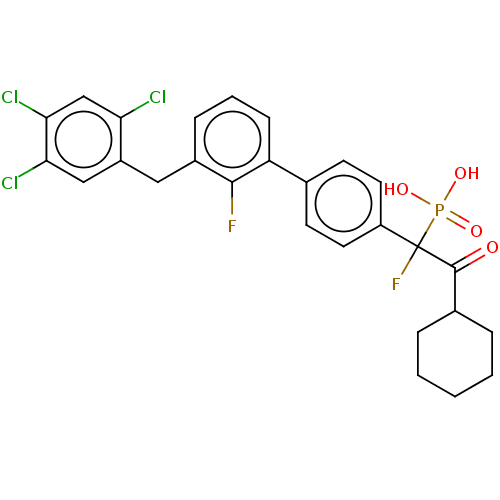
(CHEMBL4070970)Show SMILES OP(O)(=O)C(F)(C(=O)C1CCCCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C27H24Cl3F2O4P/c28-22-15-24(30)23(29)14-19(22)13-18-7-4-8-21(25(18)31)16-9-11-20(12-10-16)27(32,37(34,35)36)26(33)17-5-2-1-3-6-17/h4,7-12,14-15,17H,1-3,5-6,13H2,(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250600
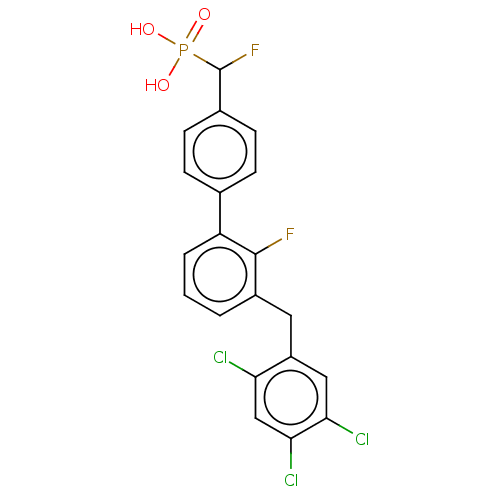
(CHEMBL4103050)Show SMILES OP(O)(=O)C(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C20H14Cl3F2O3P/c21-16-10-18(23)17(22)9-14(16)8-13-2-1-3-15(19(13)24)11-4-6-12(7-5-11)20(25)29(26,27)28/h1-7,9-10,20H,8H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50250606
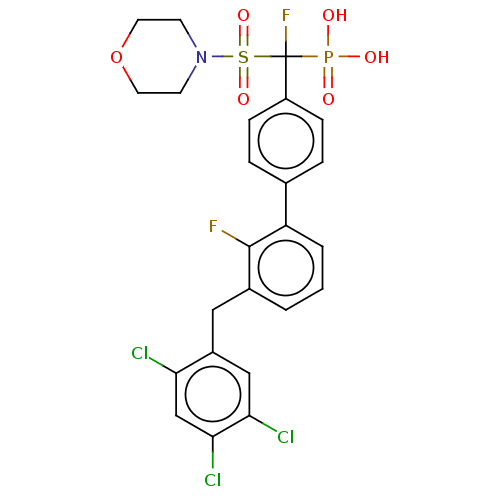
(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of LAR (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectrophot... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250603
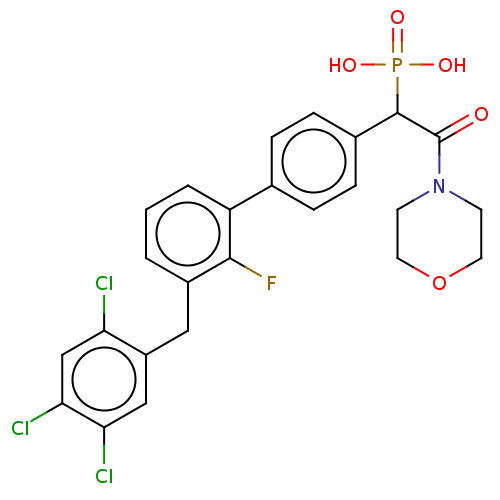
(CHEMBL4102693)Show SMILES OP(O)(=O)C(C(=O)N1CCOCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C25H22Cl3FNO5P/c26-20-14-22(28)21(27)13-18(20)12-17-2-1-3-19(23(17)29)15-4-6-16(7-5-15)24(36(32,33)34)25(31)30-8-10-35-11-9-30/h1-7,13-14,24H,8-12H2,(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250604
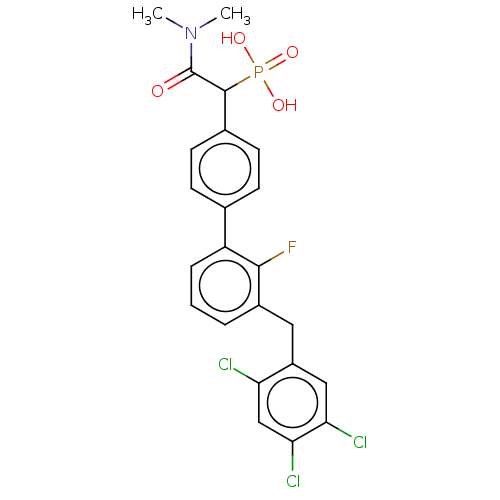
(CHEMBL4084768)Show SMILES CN(C)C(=O)C(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O Show InChI InChI=1S/C23H20Cl3FNO4P/c1-28(2)23(29)22(33(30,31)32)14-8-6-13(7-9-14)17-5-3-4-15(21(17)27)10-16-11-19(25)20(26)12-18(16)24/h3-9,11-12,22H,10H2,1-2H3,(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250607
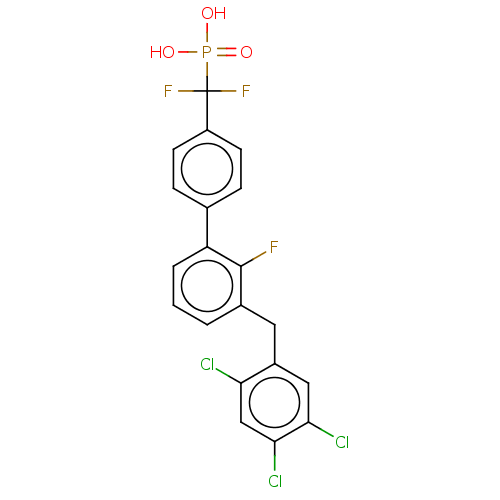
(CHEMBL4069436)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C20H13Cl3F3O3P/c21-16-10-18(23)17(22)9-13(16)8-12-2-1-3-15(19(12)24)11-4-6-14(7-5-11)20(25,26)30(27,28)29/h1-7,9-10H,8H2,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250599
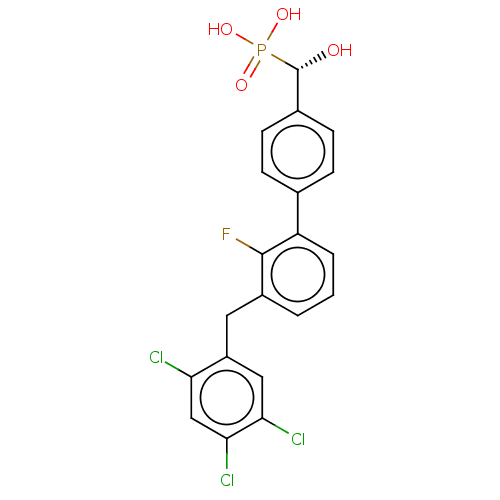
(CHEMBL4088863)Show SMILES O[C@H](c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O |r| Show InChI InChI=1S/C20H15Cl3FO4P/c21-16-10-18(23)17(22)9-14(16)8-13-2-1-3-15(19(13)24)11-4-6-12(7-5-11)20(25)29(26,27)28/h1-7,9-10,20,25H,8H2,(H2,26,27,28)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250609

(CHEMBL4105477)Show SMILES OP(O)(=O)C(C(=O)N1CCCCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C26H24Cl3FNO4P/c27-21-15-23(29)22(28)14-19(21)13-18-5-4-6-20(24(18)30)16-7-9-17(10-8-16)25(36(33,34)35)26(32)31-11-2-1-3-12-31/h4-10,14-15,25H,1-3,11-13H2,(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250602

(CHEMBL4087599)Show SMILES OP(O)(=O)C(F)(C(=O)N1CCCCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C26H23Cl3F2NO4P/c27-21-15-23(29)22(28)14-18(21)13-17-5-4-6-20(24(17)30)16-7-9-19(10-8-16)26(31,37(34,35)36)25(33)32-11-2-1-3-12-32/h4-10,14-15H,1-3,11-13H2,(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250597
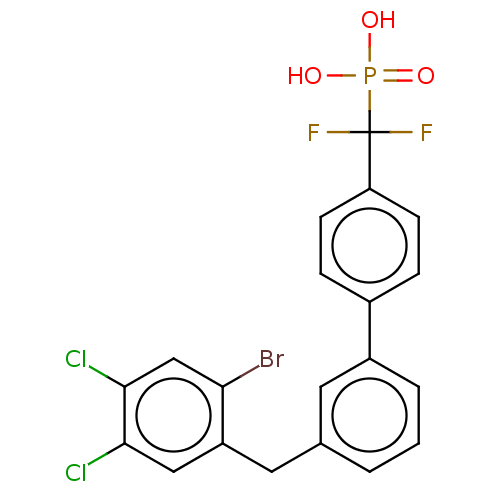
(CHEMBL4096664)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Br)c1 Show InChI InChI=1S/C20H14BrCl2F2O3P/c21-17-11-19(23)18(22)10-15(17)9-12-2-1-3-14(8-12)13-4-6-16(7-5-13)20(24,25)29(26,27)28/h1-8,10-11H,9H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250596

(CHEMBL4073186)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1 Show InChI InChI=1S/C20H14Cl3F2O3P/c21-17-11-19(23)18(22)10-15(17)9-12-2-1-3-14(8-12)13-4-6-16(7-5-13)20(24,25)29(26,27)28/h1-8,10-11H,9H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectrop... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250595
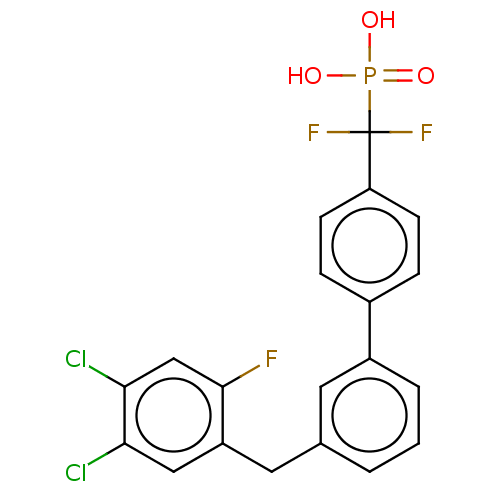
(CHEMBL4100037)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2F)c1 Show InChI InChI=1S/C20H14Cl2F3O3P/c21-17-10-15(19(23)11-18(17)22)9-12-2-1-3-14(8-12)13-4-6-16(7-5-13)20(24,25)29(26,27)28/h1-8,10-11H,9H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectroph... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of LMW-PTP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectro... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250605

(CHEMBL4075995)Show SMILES CNC(=O)C(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O Show InChI InChI=1S/C22H18Cl3FNO4P/c1-27-22(28)21(32(29,30)31)13-7-5-12(6-8-13)16-4-2-3-14(20(16)26)9-15-10-18(24)19(25)11-17(15)23/h2-8,10-11,21H,9H2,1H3,(H,27,28)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 10
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of MKP5 (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441304

(CHEMBL2431686)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C20H15Cl2F2O3P/c21-18-9-4-14(12-19(18)22)10-13-2-1-3-16(11-13)15-5-7-17(8-6-15)20(23,24)28(25,26)27/h1-9,11-12H,10H2,(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250594
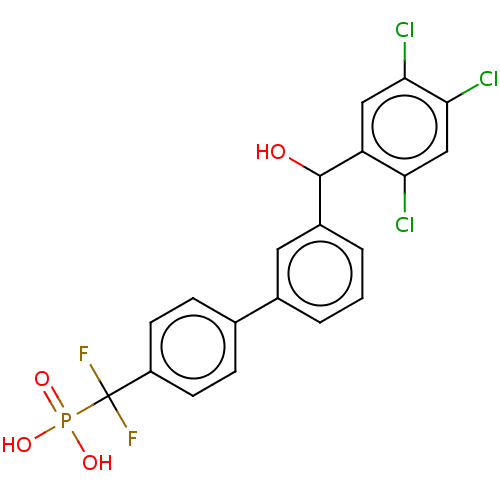
(CHEMBL4092285)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1cc(Cl)c(Cl)cc1Cl Show InChI InChI=1S/C20H14Cl3F2O4P/c21-16-10-18(23)17(22)9-15(16)19(26)13-3-1-2-12(8-13)11-4-6-14(7-5-11)20(24,25)30(27,28)29/h1-10,19,26H,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250593
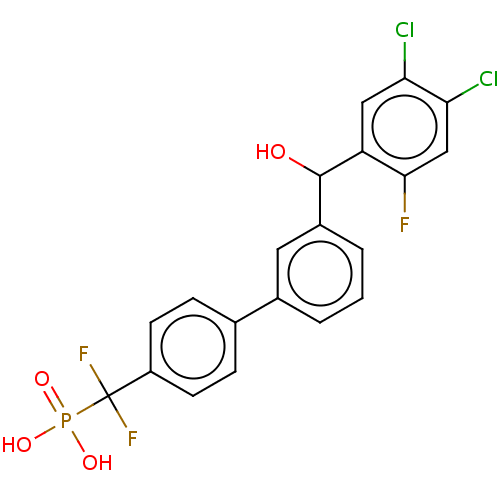
(CHEMBL4091376)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1cc(Cl)c(Cl)cc1F Show InChI InChI=1S/C20H14Cl2F3O4P/c21-16-9-15(18(23)10-17(16)22)19(26)13-3-1-2-12(8-13)11-4-6-14(7-5-11)20(24,25)30(27,28)29/h1-10,19,26H,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250598
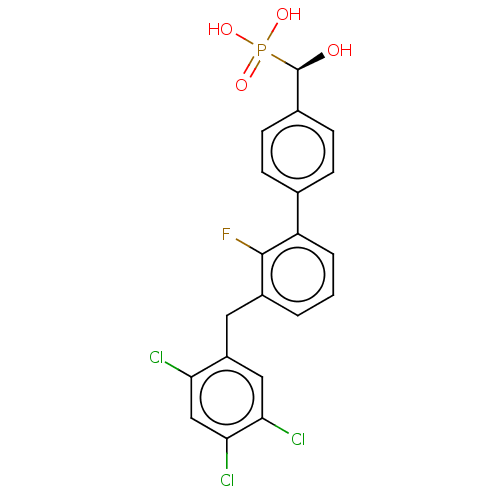
(CHEMBL4066498)Show SMILES O[C@@H](c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O |r| Show InChI InChI=1S/C20H15Cl3FO4P/c21-16-10-18(23)17(22)9-14(16)8-13-2-1-3-15(19(13)24)11-4-6-12(7-5-11)20(25)29(26,27)28/h1-7,9-10,20,25H,8H2,(H2,26,27,28)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441303
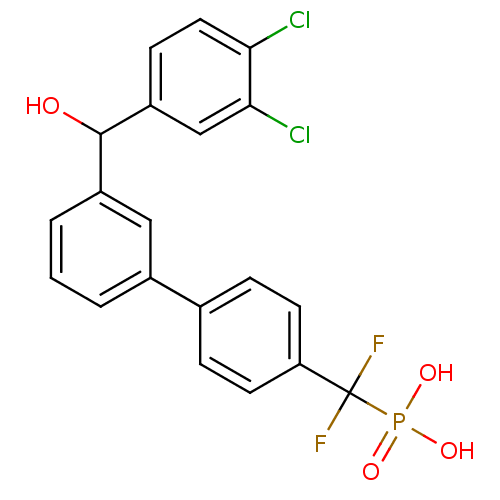
(CHEMBL2431687)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H15Cl2F2O4P/c21-17-9-6-15(11-18(17)22)19(25)14-3-1-2-13(10-14)12-4-7-16(8-5-12)20(23,24)29(26,27)28/h1-11,19,25H,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50499821
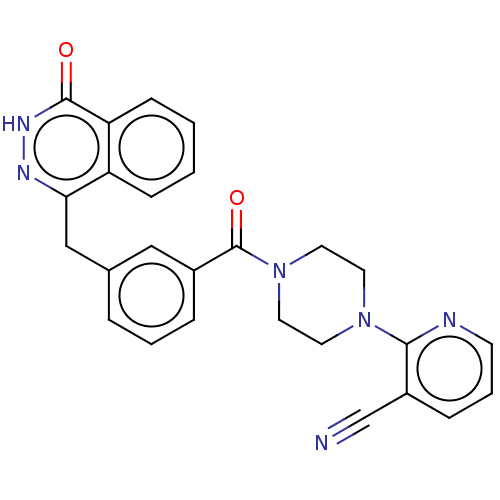
(CHEMBL3740104)Show SMILES O=C(N1CCN(CC1)c1ncccc1C#N)c1cccc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C26H22N6O2/c27-17-20-7-4-10-28-24(20)31-11-13-32(14-12-31)26(34)19-6-3-5-18(15-19)16-23-21-8-1-2-9-22(21)25(33)30-29-23/h1-10,15H,11-14,16H2,(H,30,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length human PARP1 expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidin-... |
Bioorg Med Chem Lett 25: 5743-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.079
BindingDB Entry DOI: 10.7270/Q2WH2T0J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of full length human PARP1 expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidin-... |
Bioorg Med Chem Lett 25: 5743-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.079
BindingDB Entry DOI: 10.7270/Q2WH2T0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50499821

(CHEMBL3740104)Show SMILES O=C(N1CCN(CC1)c1ncccc1C#N)c1cccc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C26H22N6O2/c27-17-20-7-4-10-28-24(20)31-11-13-32(14-12-31)26(34)19-6-3-5-18(15-19)16-23-21-8-1-2-9-22(21)25(33)30-29-23/h1-10,15H,11-14,16H2,(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PARP2 (2 to 583 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by strep... |
Bioorg Med Chem Lett 25: 5743-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.079
BindingDB Entry DOI: 10.7270/Q2WH2T0J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50446982
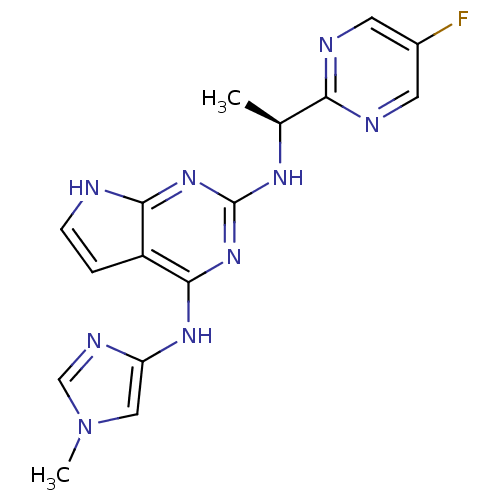
(CHEMBL3116050)Show SMILES C[C@H](Nc1nc(Nc2cn(C)cn2)c2cc[nH]c2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C16H16FN9/c1-9(13-19-5-10(17)6-20-13)22-16-24-14-11(3-4-18-14)15(25-16)23-12-7-26(2)8-21-12/h3-9H,1-2H3,(H3,18,22,23,24,25)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha'
(Homo sapiens (Human)) | BDBM50446982

(CHEMBL3116050)Show SMILES C[C@H](Nc1nc(Nc2cn(C)cn2)c2cc[nH]c2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C16H16FN9/c1-9(13-19-5-10(17)6-20-13)22-16-24-14-11(3-4-18-14)15(25-16)23-12-7-26(2)8-21-12/h3-9H,1-2H3,(H3,18,22,23,24,25)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human CK2alpha2 |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575300
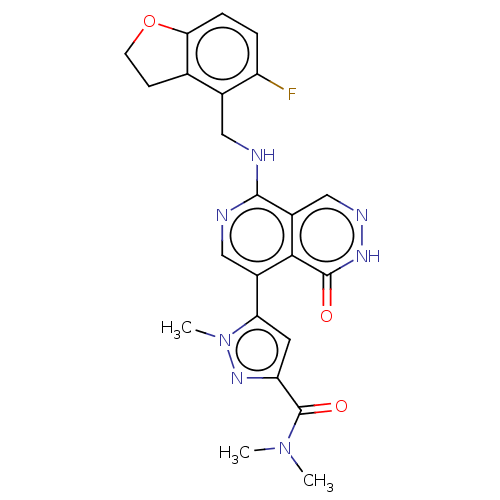
(CHEMBL4851414)Show SMILES CN(C)C(=O)c1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50288511

(CHEMBL4171692)Show SMILES CN1CCN(CC1)c1cc(Nc2ncc(C)c(Nc3cccc(CO)c3)n2)cc(c1)S(C)(=O)=O Show InChI InChI=1S/C24H30N6O3S/c1-17-15-25-24(28-23(17)26-19-6-4-5-18(11-19)16-31)27-20-12-21(14-22(13-20)34(3,32)33)30-9-7-29(2)8-10-30/h4-6,11-15,31H,7-10,16H2,1-3H3,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50288439
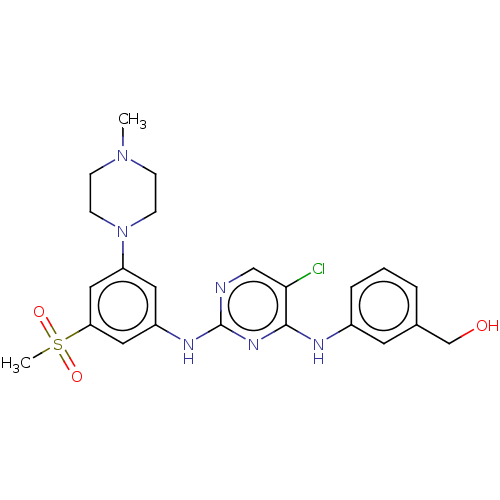
(CHEMBL4163817)Show SMILES CN1CCN(CC1)c1cc(Nc2ncc(Cl)c(Nc3cccc(CO)c3)n2)cc(c1)S(C)(=O)=O Show InChI InChI=1S/C23H27ClN6O3S/c1-29-6-8-30(9-7-29)19-11-18(12-20(13-19)34(2,32)33)27-23-25-14-21(24)22(28-23)26-17-5-3-4-16(10-17)15-31/h3-5,10-14,31H,6-9,15H2,1-2H3,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50288443

(CHEMBL4166989)Show SMILES [H][C@]12CN(c3cc(Nc4ncc(C)c(Nc5cccc(CO)c5)n4)cc(c3)S(C)(=O)=O)[C@]([H])(CN1C)C2 |r| Show InChI InChI=1S/C25H30N6O3S/c1-16-12-26-25(29-24(16)27-18-6-4-5-17(7-18)15-32)28-19-8-20(11-23(9-19)35(3,33)34)31-14-21-10-22(31)13-30(21)2/h4-9,11-12,21-22,32H,10,13-15H2,1-3H3,(H2,26,27,28,29)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PARP2 (2 to 583 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by strep... |
Bioorg Med Chem Lett 25: 5743-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.079
BindingDB Entry DOI: 10.7270/Q2WH2T0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50446990
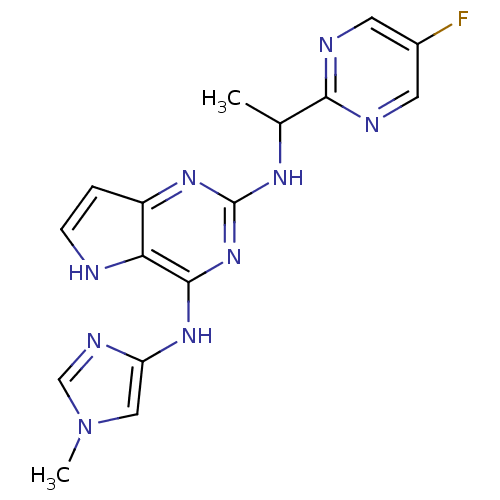
(CHEMBL3116055)Show SMILES CC(Nc1nc(Nc2cn(C)cn2)c2[nH]ccc2n1)c1ncc(F)cn1 Show InChI InChI=1S/C16H16FN9/c1-9(14-19-5-10(17)6-20-14)22-16-23-11-3-4-18-13(11)15(25-16)24-12-7-26(2)8-21-12/h3-9,18H,1-2H3,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50446982

(CHEMBL3116050)Show SMILES C[C@H](Nc1nc(Nc2cn(C)cn2)c2cc[nH]c2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C16H16FN9/c1-9(13-19-5-10(17)6-20-13)22-16-24-14-11(3-4-18-14)15(25-16)23-12-7-26(2)8-21-12/h3-9H,1-2H3,(H3,18,22,23,24,25)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Jak1 (unknown origin) |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50446982

(CHEMBL3116050)Show SMILES C[C@H](Nc1nc(Nc2cn(C)cn2)c2cc[nH]c2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C16H16FN9/c1-9(13-19-5-10(17)6-20-13)22-16-24-14-11(3-4-18-14)15(25-16)23-12-7-26(2)8-21-12/h3-9H,1-2H3,(H3,18,22,23,24,25)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50446985

(CHEMBL3116063)Show SMILES CC(Nc1nc(Nc2cn(C)cn2)c2ccc(Cl)nc2n1)c1ncc(F)cn1 Show InChI InChI=1S/C17H15ClFN9/c1-9(14-20-5-10(19)6-21-14)23-17-26-15-11(3-4-12(18)24-15)16(27-17)25-13-7-28(2)8-22-13/h3-9H,1-2H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50442997
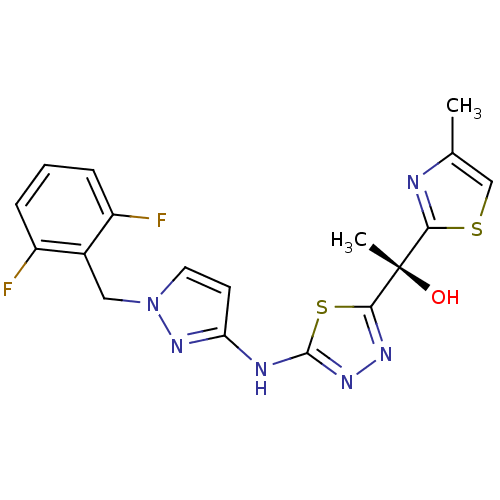
(CHEMBL3088172)Show SMILES Cc1csc(n1)[C@](C)(O)c1nnc(Nc2ccn(Cc3c(F)cccc3F)n2)s1 |r| Show InChI InChI=1S/C18H16F2N6OS2/c1-10-9-28-15(21-10)18(2,27)16-23-24-17(29-16)22-14-6-7-26(25-14)8-11-12(19)4-3-5-13(11)20/h3-7,9,27H,8H2,1-2H3,(H,22,24,25)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis full length inhA expressed in Escherichia coli BL21 preincubated for 15 mins by fluorescence based assay in ... |
J Med Chem 56: 8533-42 (2013)
Article DOI: 10.1021/jm4012033
BindingDB Entry DOI: 10.7270/Q2SB4769 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50446995

(CHEMBL3116058)Show SMILES C[C@H](Nc1nc(Nc2cn(C)cn2)c2ccccc2n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H17FN8/c1-11(16-20-7-12(19)8-21-16)23-18-24-14-6-4-3-5-13(14)17(26-18)25-15-9-27(2)10-22-15/h3-11H,1-2H3,(H2,23,24,25,26)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Jak2 (unknown origin) |
J Med Chem 57: 144-58 (2014)
Article DOI: 10.1021/jm401546n
BindingDB Entry DOI: 10.7270/Q2CR5VTM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50288372
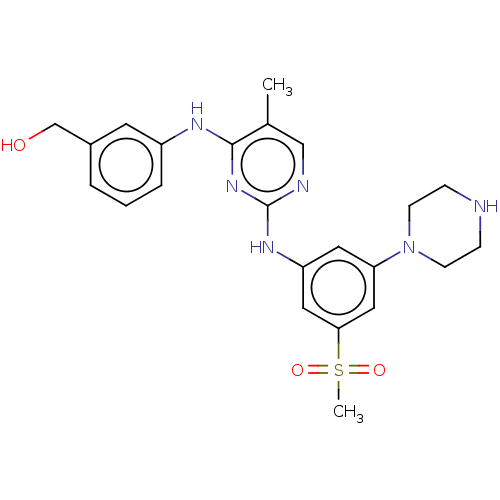
(CHEMBL4173429)Show SMILES Cc1cnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCNCC2)nc1Nc1cccc(CO)c1 Show InChI InChI=1S/C23H28N6O3S/c1-16-14-25-23(28-22(16)26-18-5-3-4-17(10-18)15-30)27-19-11-20(29-8-6-24-7-9-29)13-21(12-19)33(2,31)32/h3-5,10-14,24,30H,6-9,15H2,1-2H3,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50288378

(CHEMBL4162065)Show SMILES CN1CCN(CC1)c1cc(Nc2ncc(Br)c(Nc3cccc(CO)c3)n2)cc(c1)S(C)(=O)=O Show InChI InChI=1S/C23H27BrN6O3S/c1-29-6-8-30(9-7-29)19-11-18(12-20(13-19)34(2,32)33)27-23-25-14-21(24)22(28-23)26-17-5-3-4-16(10-17)15-31/h3-5,10-14,31H,6-9,15H2,1-2H3,(H2,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
GRAC: human IMPase 1 inhibitor |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50288437

(CHEMBL4165758)Show SMILES Cc1cnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)nc1Nc1cccc(CO)c1 Show InChI InChI=1S/C26H32N6O3/c1-19-17-27-26(30-25(19)28-21-4-2-3-20(13-21)18-33)29-22-14-23(31-5-9-34-10-6-31)16-24(15-22)32-7-11-35-12-8-32/h2-4,13-17,33H,5-12,18H2,1H3,(H2,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
GRAC: human PDE2A selective inhibitor |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50288438
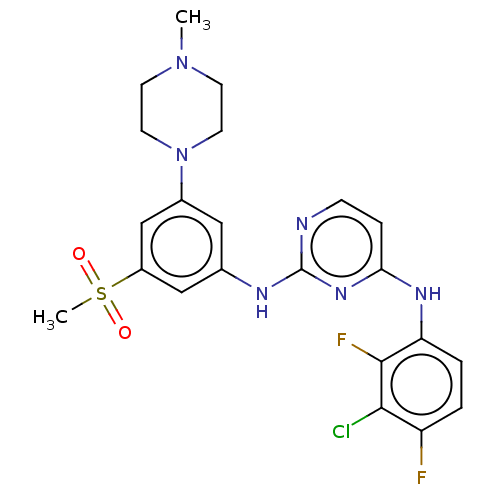
(CHEMBL4173676)Show SMILES CN1CCN(CC1)c1cc(Nc2nccc(Nc3ccc(F)c(Cl)c3F)n2)cc(c1)S(C)(=O)=O Show InChI InChI=1S/C22H23ClF2N6O2S/c1-30-7-9-31(10-8-30)15-11-14(12-16(13-15)34(2,32)33)27-22-26-6-5-19(29-22)28-18-4-3-17(24)20(23)21(18)25/h3-6,11-13H,7-10H2,1-2H3,(H2,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... |
J Med Chem 61: 5235-5244 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00076
BindingDB Entry DOI: 10.7270/Q2JH3PP4 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575300

(CHEMBL4851414)Show SMILES CN(C)C(=O)c1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575298
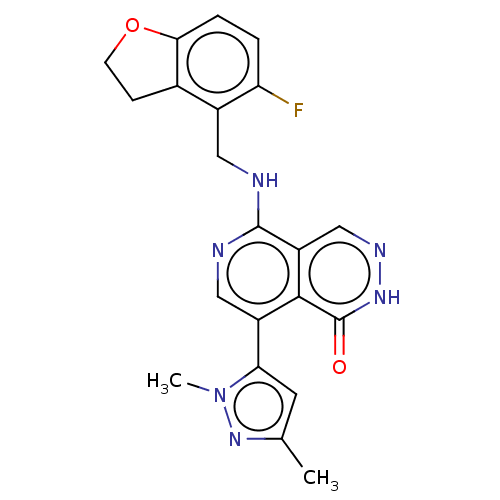
(CHEMBL4859723)Show SMILES Cc1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575298

(CHEMBL4859723)Show SMILES Cc1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data