

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50291312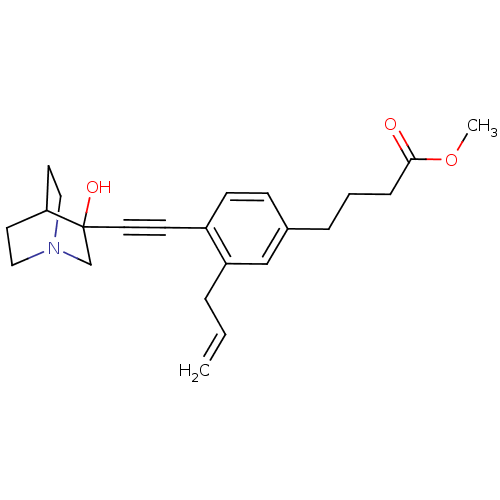 (4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719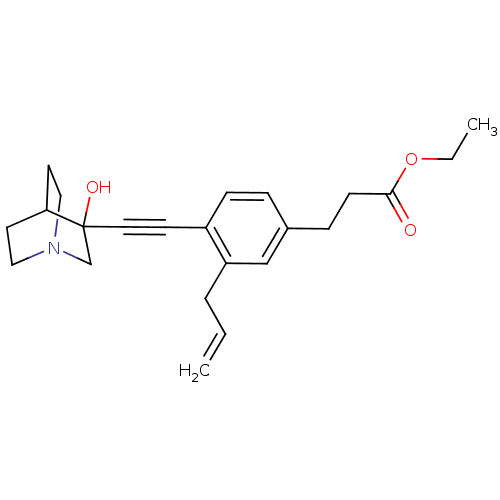 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291315 (5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291311 (6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291316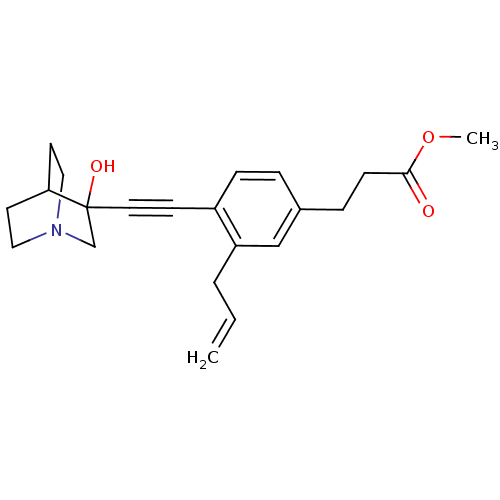 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291317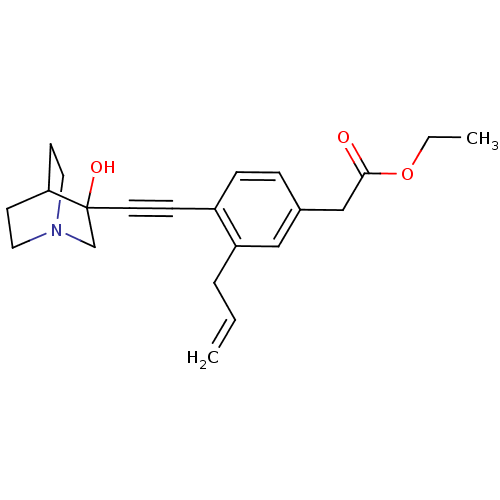 (CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291318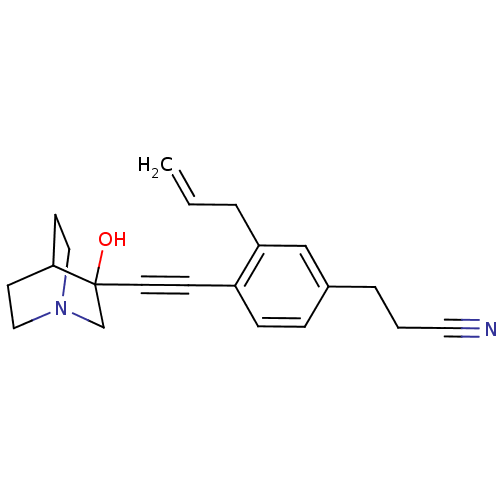 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291314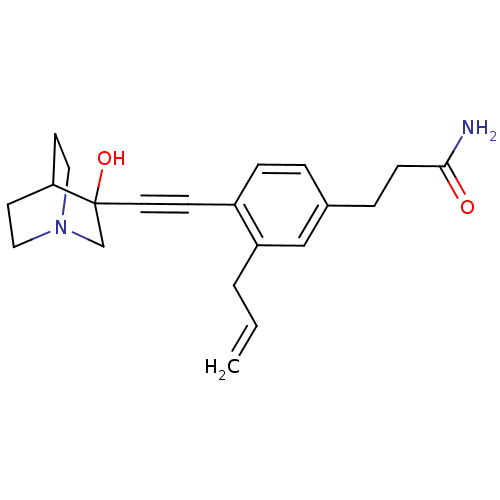 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291313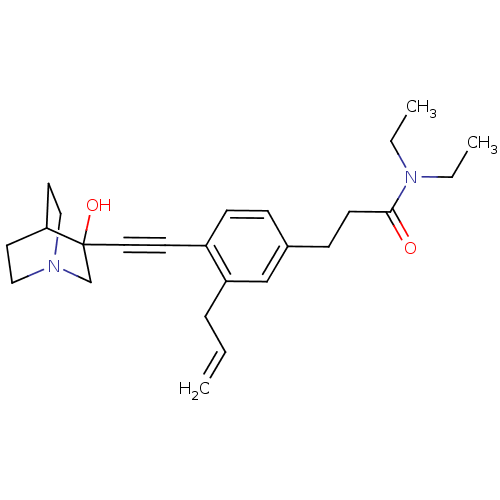 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291319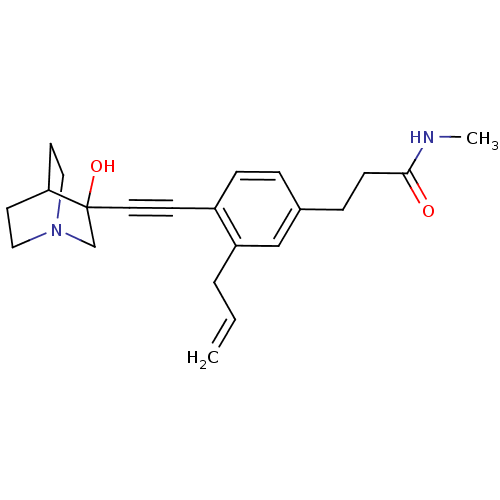 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052343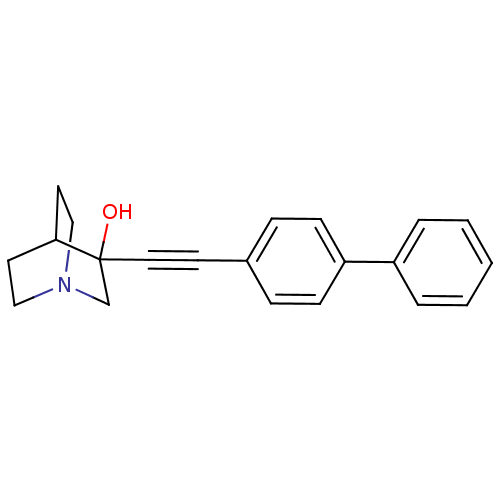 (3-Biphenyl-4-ylethynyl-1-aza-bicyclo[2.2.2]octan-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052350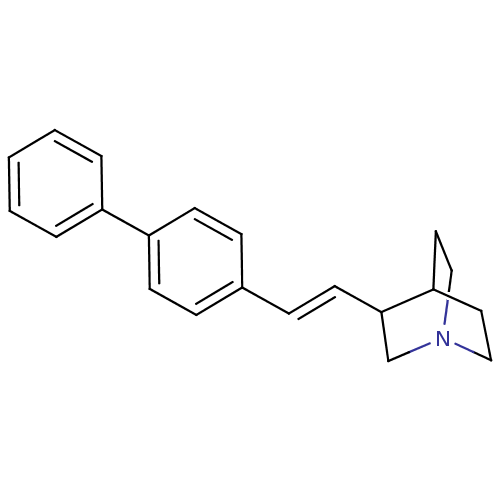 (3-((E)-2-Biphenyl-4-yl-vinyl)-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052342 (3-(2-Biphenyl-4-yl-ethyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052353 (3-(4-Benzothiazol-2-yl-phenyl)-1-aza-bicyclo[2.2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052355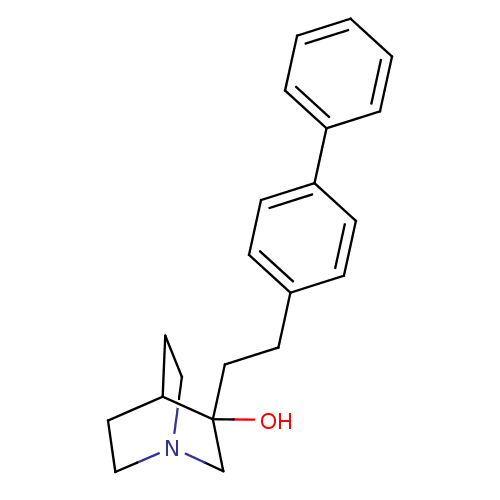 (3-(2-Biphenyl-4-yl-ethyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052377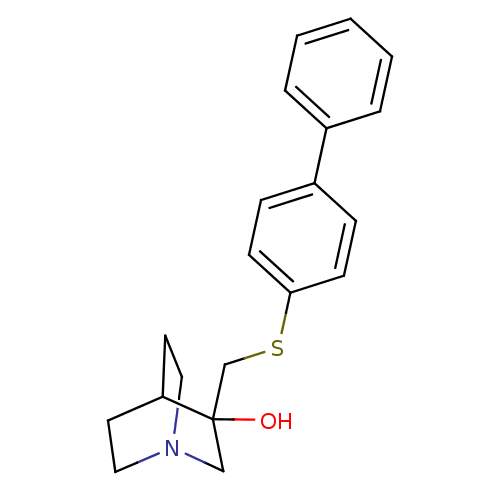 (3-(Biphenyl-4-ylsulfanylmethyl)-1-aza-bicyclo[2.2....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052376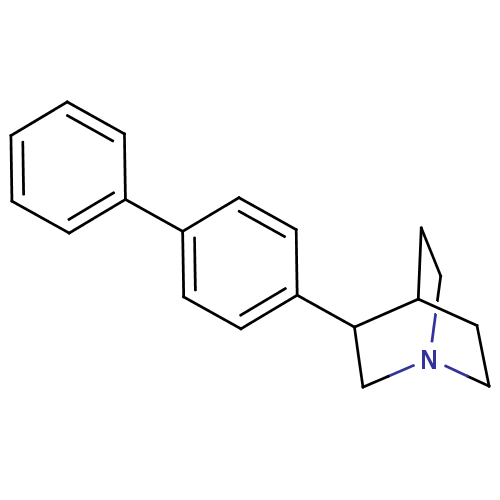 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octane | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052358 (3-(Biphenyl-4-yloxymethyl)-1-aza-bicyclo[2.2.2]oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052351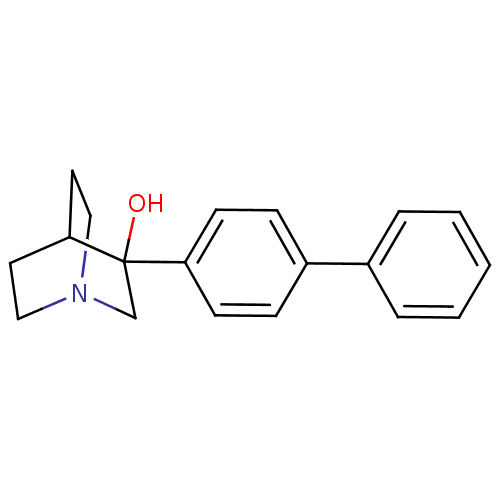 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052352 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]oct-2-ene | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052366 (3-(4'-Fluoro-biphenyl-4-yl)-1-aza-bicyclo[2.2.2]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052367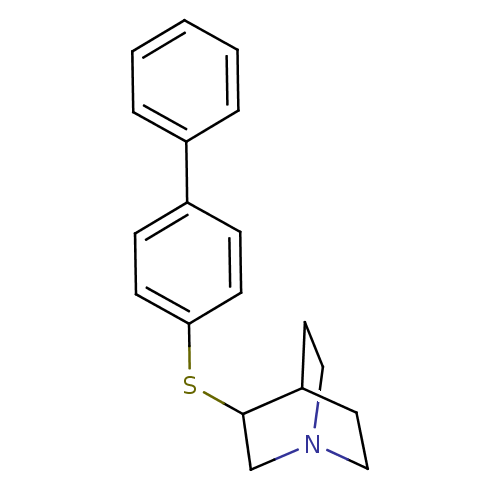 (3-(Biphenyl-4-ylsulfanyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052357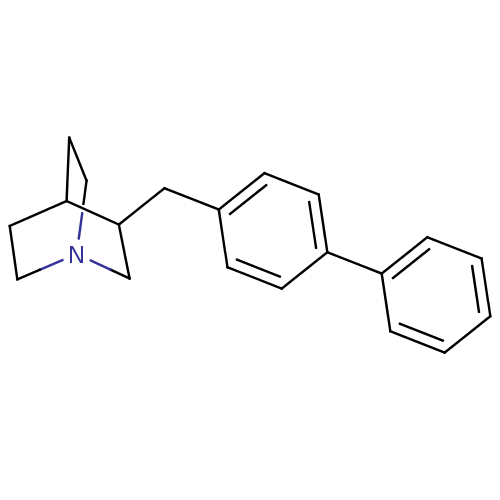 (3-Biphenyl-4-ylmethyl-1-aza-bicyclo[2.2.2]octane |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052341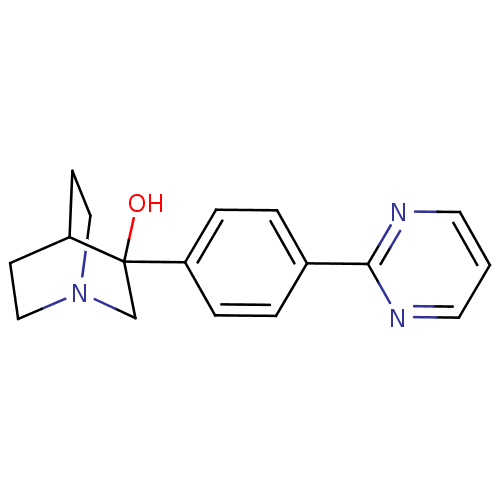 (3-(4-Pyrimidin-2-yl-phenyl)-1-aza-bicyclo[2.2.2]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052372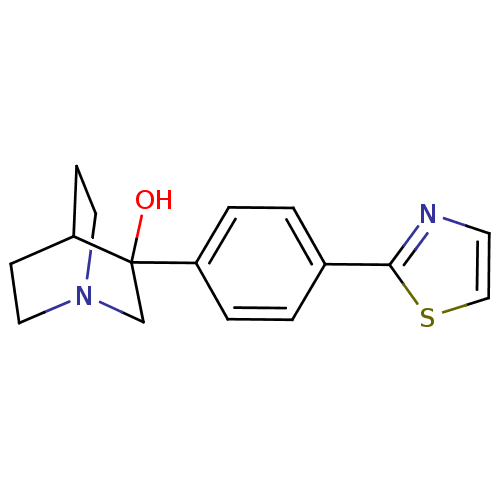 (3-(4-Thiazol-2-yl-phenyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052364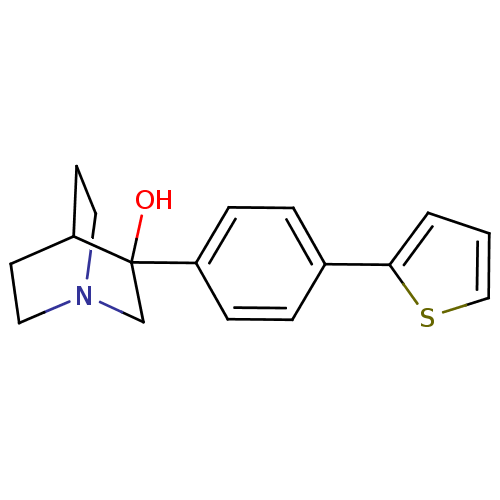 (3-(4-Thiophen-2-yl-phenyl)-1-aza-bicyclo[2.2.2]oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052344 (3-Biphenyl-3-yl-1-aza-bicyclo[2.2.2]octan-3-ol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052362 (3-(2-Phenyl-thiazol-5-yl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052373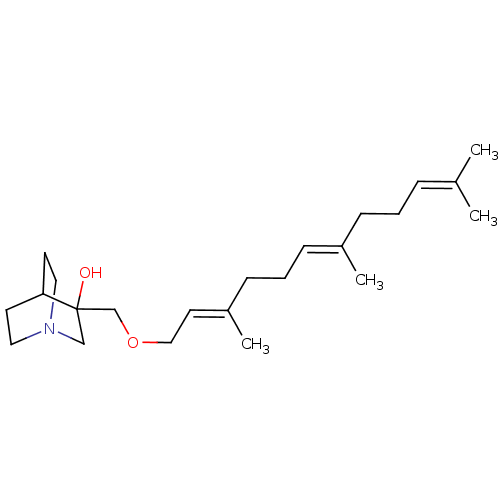 (3-((2E,6E)-3,7,11-Trimethyl-dodeca-2,6,10-trienylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052361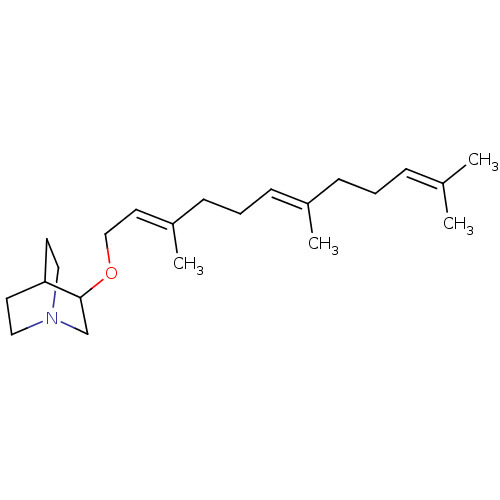 (3-((2E,6E)-3,7,11-Trimethyl-dodeca-2,6,10-trienylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052351 (3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052359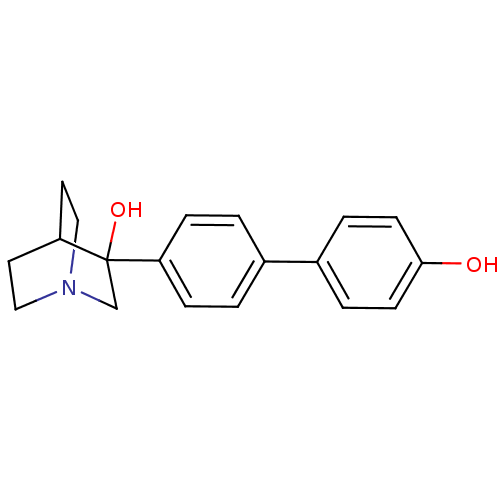 (3-(4'-Hydroxy-biphenyl-4-yl)-1-aza-bicyclo[2.2.2]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052354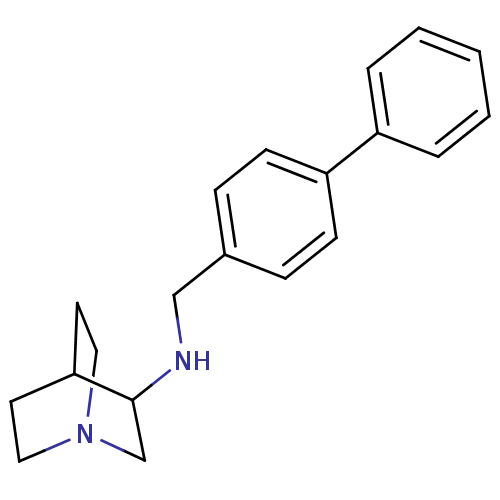 ((1-Aza-bicyclo[2.2.2]oct-3-yl)-biphenyl-4-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052345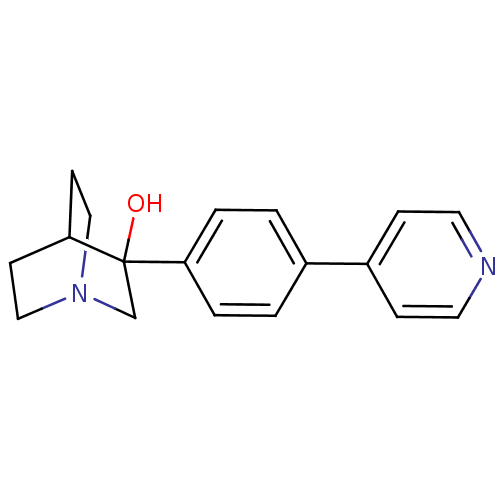 (3-(4-Pyridin-4-yl-phenyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052356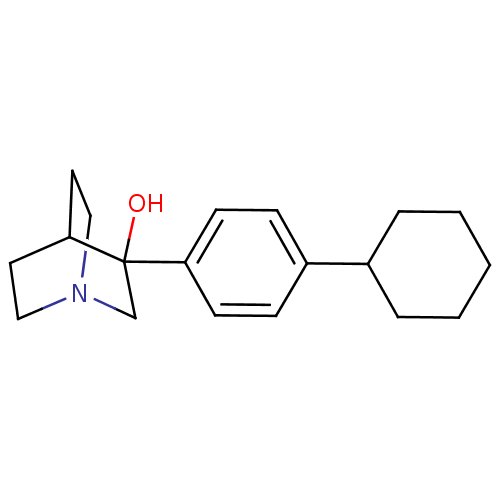 (3-(4-Cyclohexyl-phenyl)-1-aza-bicyclo[2.2.2]octan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052360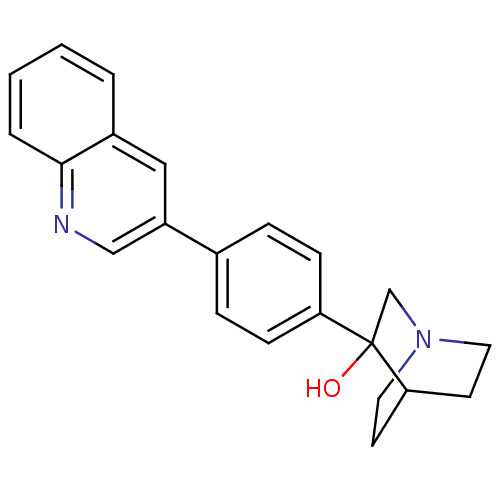 (3-(4-Quinolin-3-yl-phenyl)-1-aza-bicyclo[2.2.2]oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052378 (3-(4-Pyridin-2-yl-phenyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052374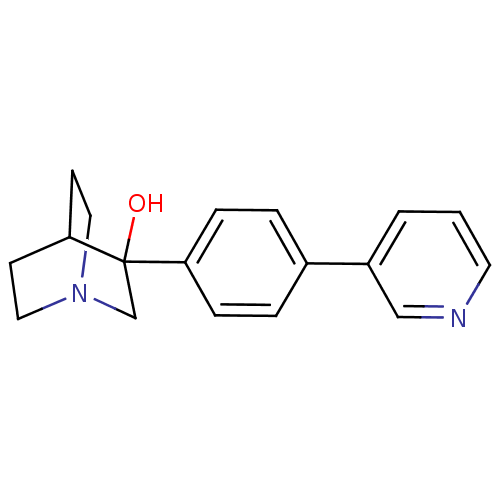 (3-(4-Pyridin-3-yl-phenyl)-1-aza-bicyclo[2.2.2]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052349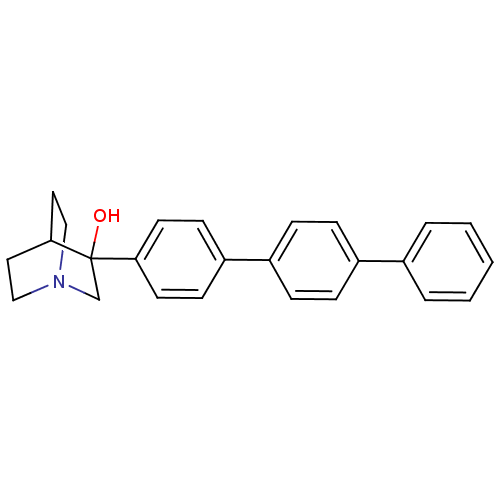 (3-[1,1';4',1'']Terphenyl-4-yl-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052346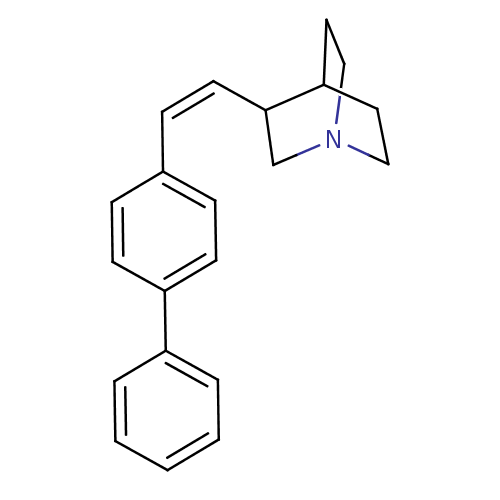 (3-((Z)-2-Biphenyl-4-yl-vinyl)-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052371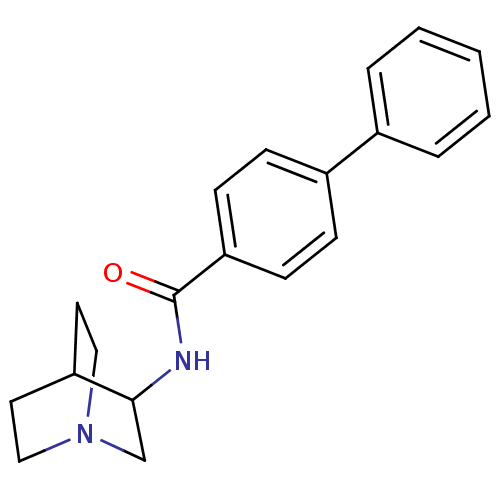 (Biphenyl-4-carboxylic acid (1-aza-bicyclo[2.2.2]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052340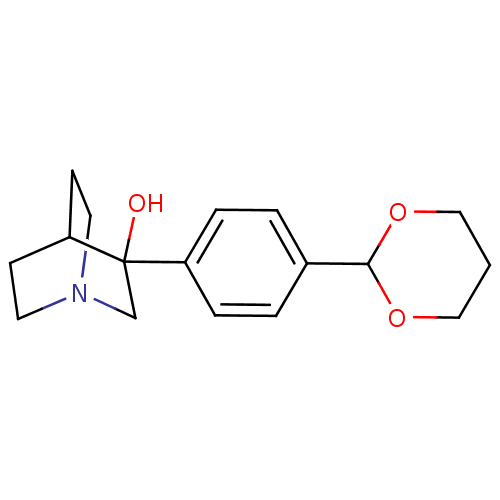 (3-(4-[1,3]Dioxan-2-yl-phenyl)-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052347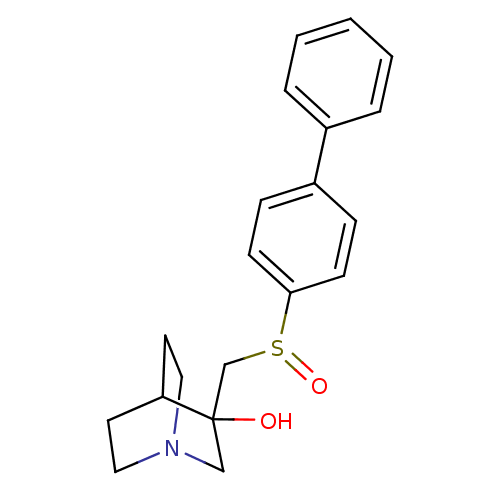 (3-(Biphenyl-4-sulfinylmethyl)-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052348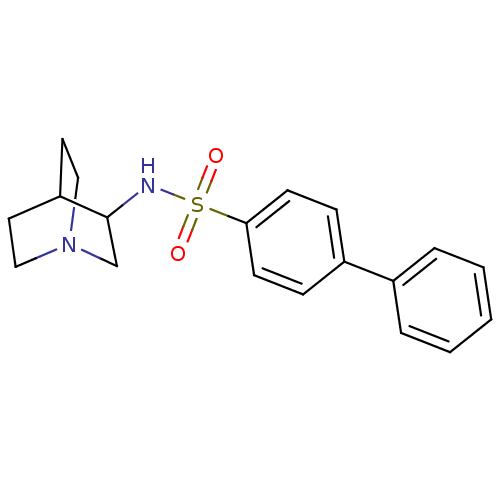 (Biphenyl-4-sulfonic acid (1-aza-bicyclo[2.2.2]oct-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052369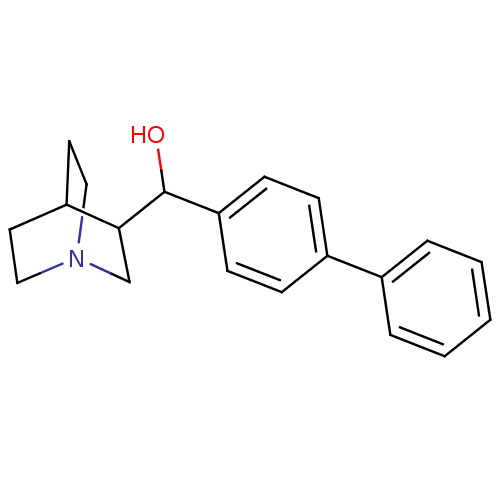 ((1-Aza-bicyclo[2.2.2]oct-3-yl)-biphenyl-4-yl-metha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052375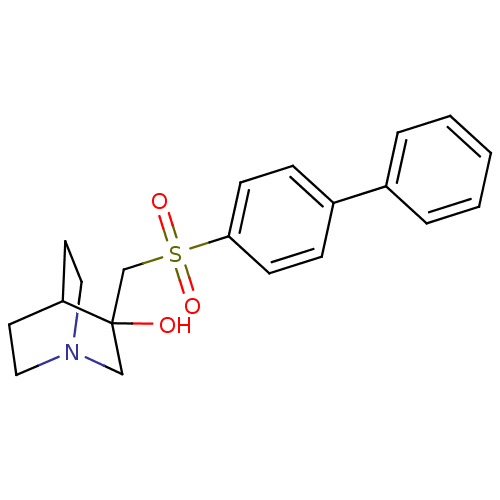 (3-(Biphenyl-4-sulfonylmethyl)-1-aza-bicyclo[2.2.2]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052369 ((1-Aza-bicyclo[2.2.2]oct-3-yl)-biphenyl-4-yl-metha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052370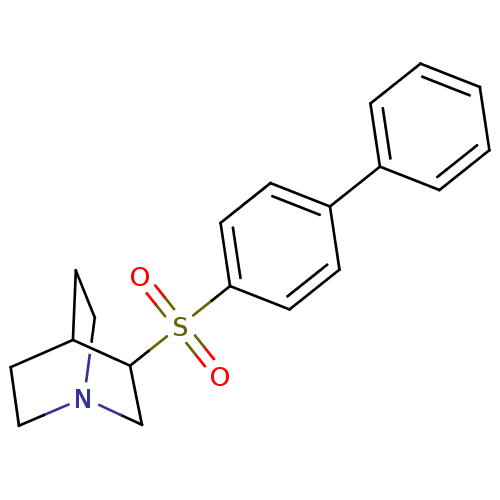 (3-(Biphenyl-4-sulfonyl)-1-aza-bicyclo[2.2.2]octane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50052363 (3-Phenyl-1-aza-bicyclo[2.2.2]octan-3-ol | CHEMBL10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition against rat microsomal squalene synthase (SS) | J Med Chem 39: 2971-9 (1996) Article DOI: 10.1021/jm950907l BindingDB Entry DOI: 10.7270/Q24J0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |