

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (MOUSE) | BDBM50240845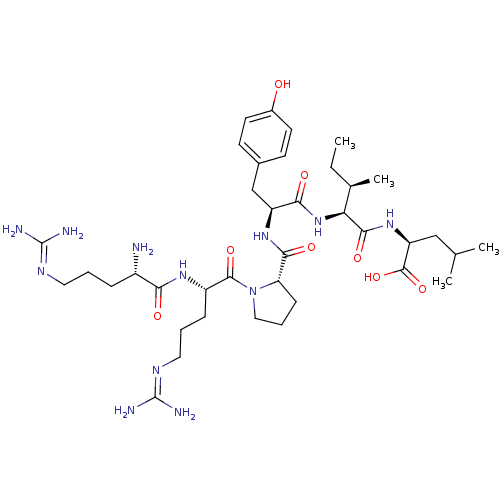 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Binding affinity to NTR1 in mouse brain membrane | Bioorg Med Chem Lett 18: 2013-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.110 BindingDB Entry DOI: 10.7270/Q2HM5994 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin (Homo sapiens (Human)) | BDBM50009575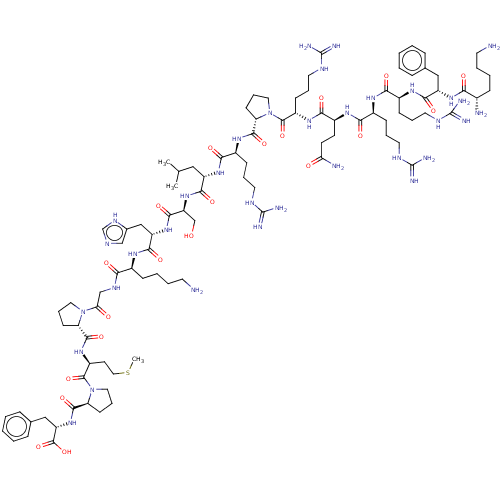 (CHEMBL3234446) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [125I]-pE13F from human apelin receptor expressed in CHO cell membranes after 1 hr by gamma counting analysis | J Med Chem 57: 2908-19 (2014) Article DOI: 10.1021/jm401789v BindingDB Entry DOI: 10.7270/Q21J9C9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0558 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay | Bioorg Med Chem Lett 16: 5493-7 (2006) Article DOI: 10.1016/j.bmcl.2006.08.049 BindingDB Entry DOI: 10.7270/Q2D79C7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183152 (CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50194072 (CHEMBL220476 | syn-7-(6-chloro-pyridin-3-yl)-2-aza...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay | Bioorg Med Chem Lett 16: 5493-7 (2006) Article DOI: 10.1016/j.bmcl.2006.08.049 BindingDB Entry DOI: 10.7270/Q2D79C7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085734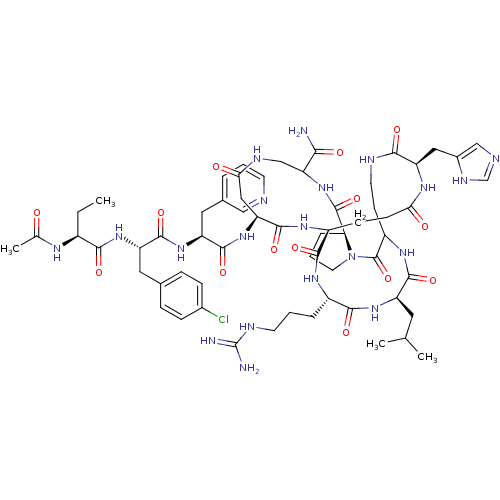 (CHEMBL407628 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis | J Med Chem 59: 2005-24 (2016) Article DOI: 10.1021/acs.jmedchem.5b01633 BindingDB Entry DOI: 10.7270/Q2KS6TDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085756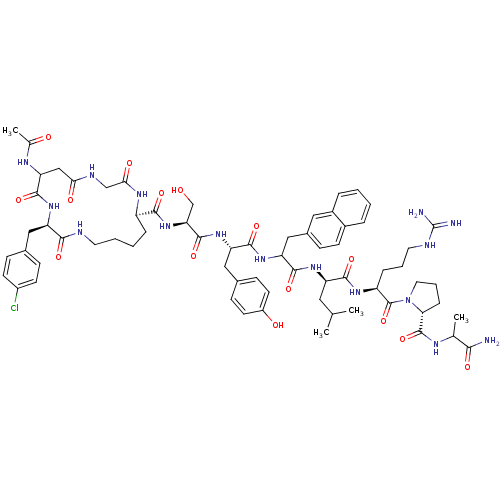 (CHEMBL439532 | cyclo(1,1'-3)Ac-D-Asp (Gly)-D-Cpa-D...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085715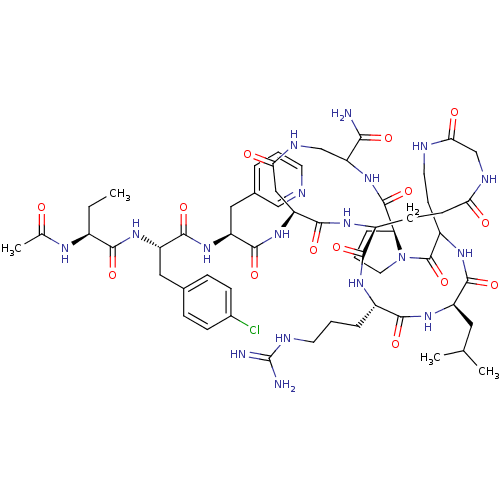 (CHEMBL266300 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391708 (CHEMBL1767136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085742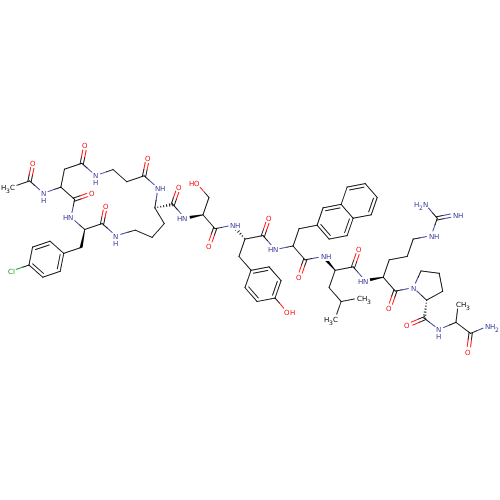 (CHEMBL414350 | cyclo(1,1'-3)Ac-D-Asp (beta-Ala)-D-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085761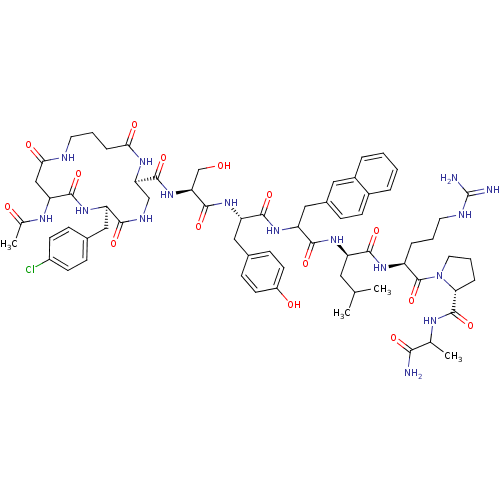 (CHEMBL437038 | cyclo(1,1'-3)Ac-D-Asp (Gaba)-D-Cpa-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085718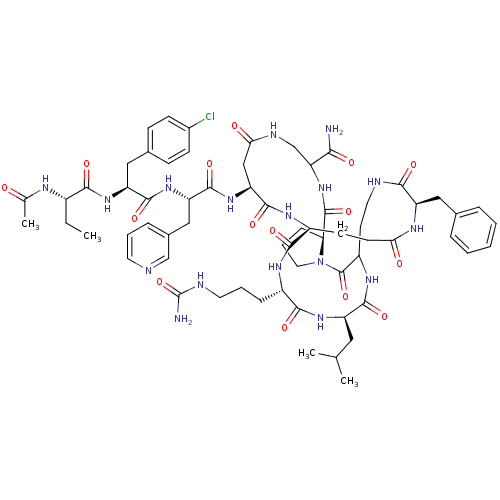 (CHEMBL410820 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085719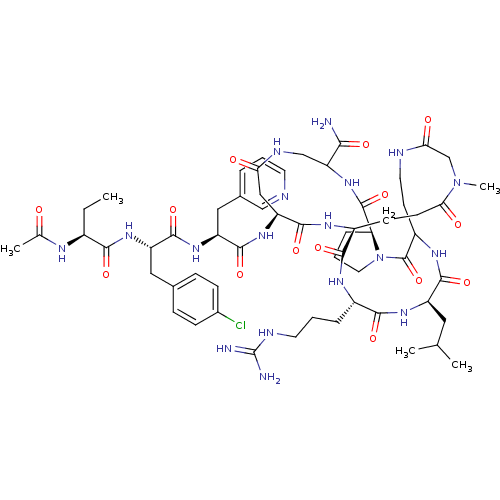 (CHEMBL438543 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085710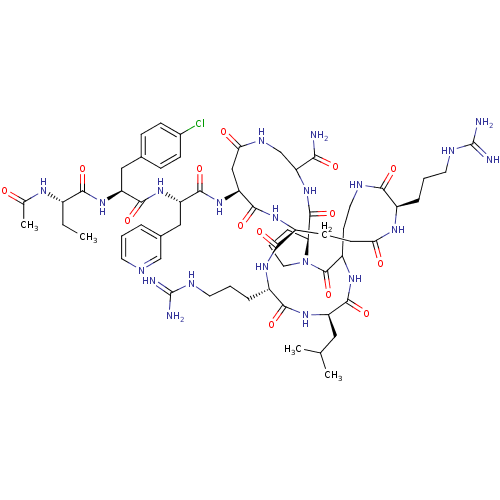 (CHEMBL414756 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085737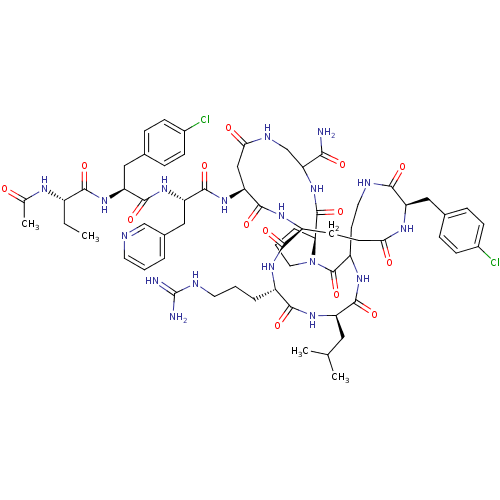 (CHEMBL438597 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085720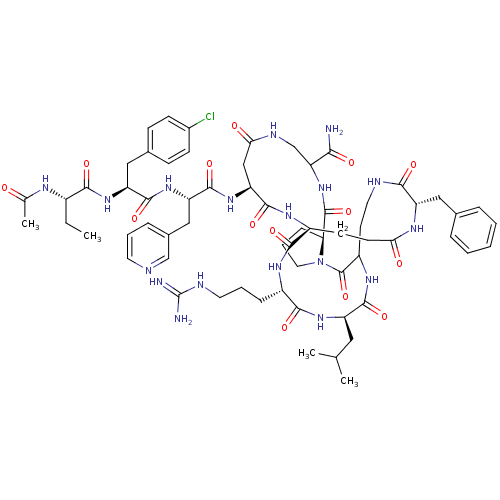 (CHEMBL404930 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50341447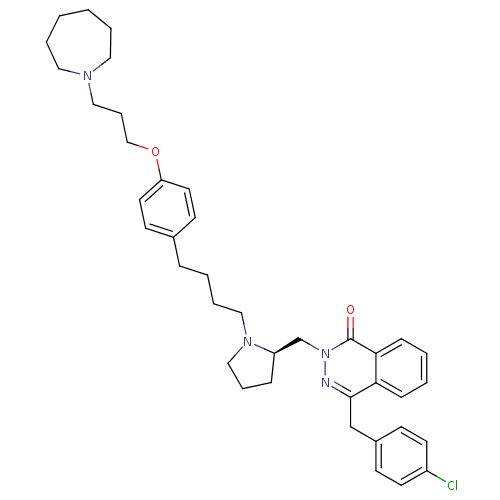 (4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox... | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391698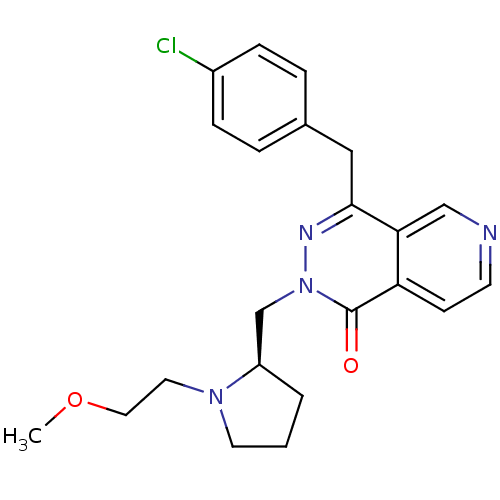 (CHEMBL2146801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183124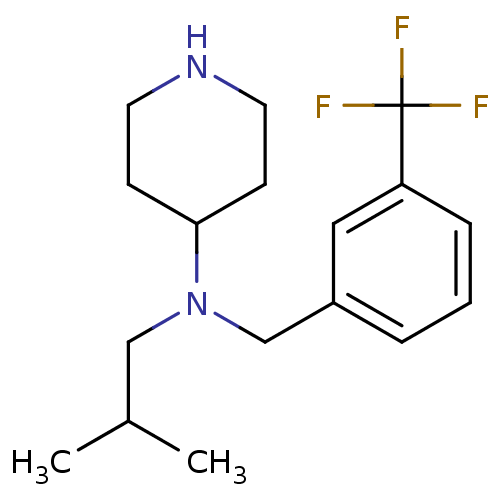 (CHEMBL381373 | N-(3-(trifluoromethyl)benzyl)-N-iso...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085732 (CHEMBL406094 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183122 (4-((isobutyl(piperidin-4-yl)amino)methyl)benzonitr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085735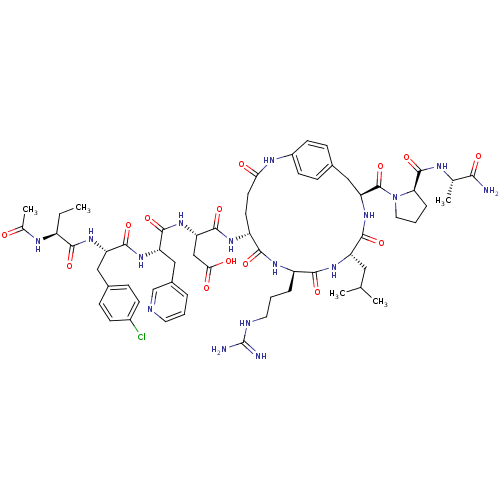 (CHEMBL412681 | Cyclo (5-8) [Ac-D Nal, D Cpa, D Pal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183121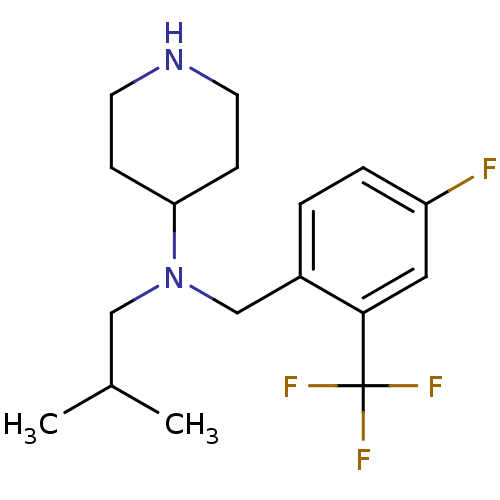 (CHEMBL441358 | N-(4-fluoro-2-(trifluoromethyl)benz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085753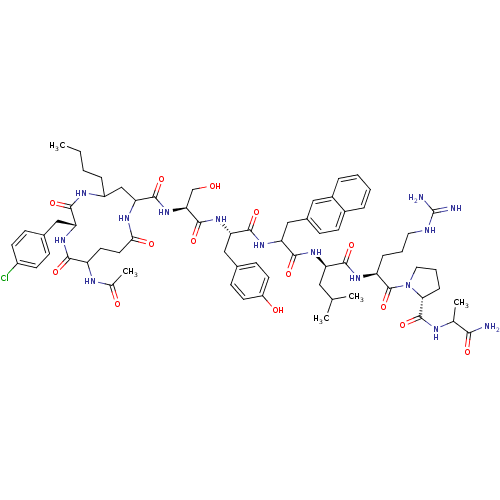 (CHEMBL409996 | cyclo(1-3)Ac-D-Glu-D-Cpa-D-Dbu-Ser-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391702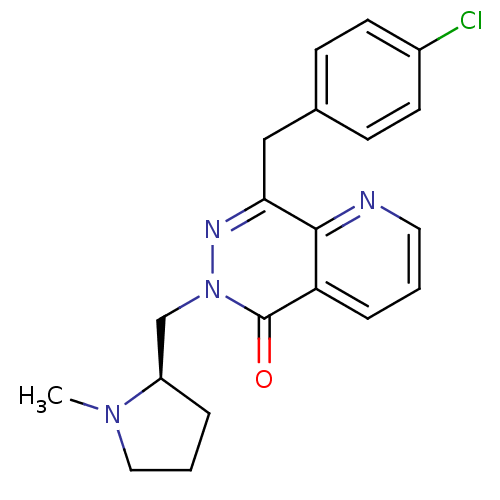 (CHEMBL2146809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085757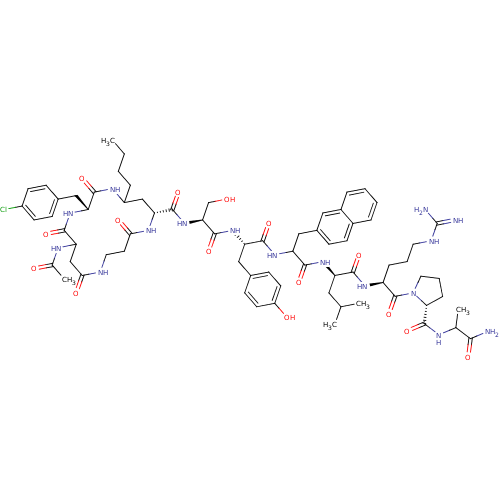 (CHEMBL427745 | cyclo(1,1'-3)Ac-D-Asp (beta-Ala)-D-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085711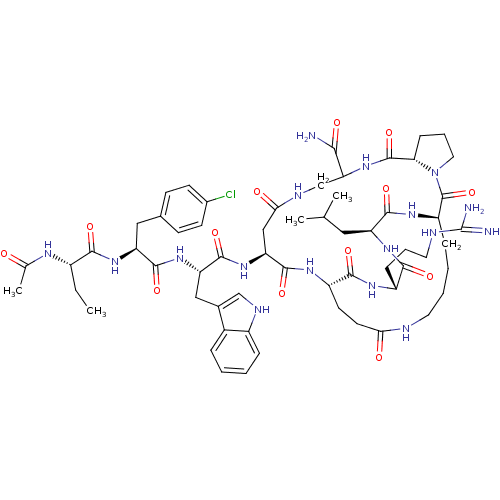 (CHEMBL412682 | DiCyclo (4-10/5-8) [Ac-D Nal, D Cpa...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085704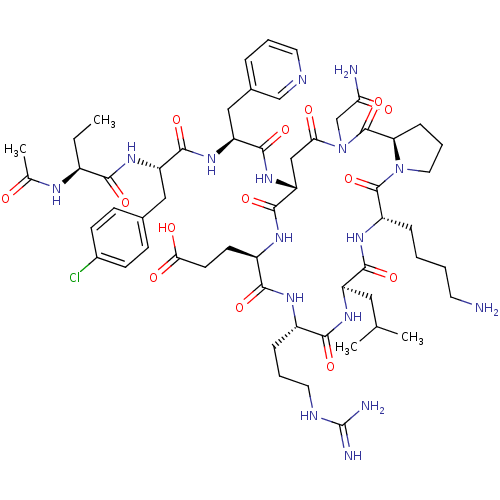 (CHEMBL440436 | Cyclo (4-10) [Ac-D Nal, D Cpa, D Pa...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085746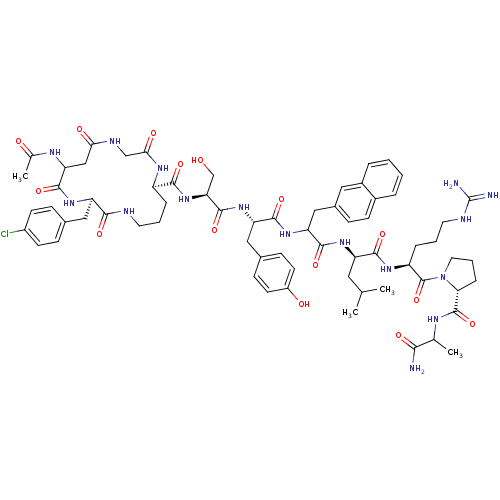 (CHEMBL405223 | cyclo(1,1'-3)Ac-D-Asp (Gly)-D-Cpa-D...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50194070 (6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay | Bioorg Med Chem Lett 16: 5493-7 (2006) Article DOI: 10.1016/j.bmcl.2006.08.049 BindingDB Entry DOI: 10.7270/Q2D79C7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50194070 (6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from human recombinant alpha4beta2 nAChR in HEK293 cells by SPA assay | Bioorg Med Chem Lett 16: 5493-7 (2006) Article DOI: 10.1016/j.bmcl.2006.08.049 BindingDB Entry DOI: 10.7270/Q2D79C7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391707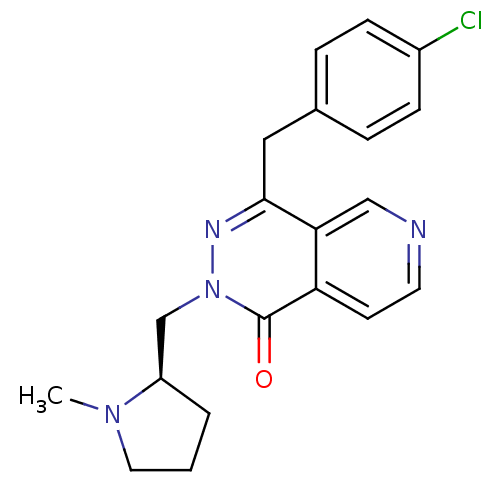 (CHEMBL2146805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50294115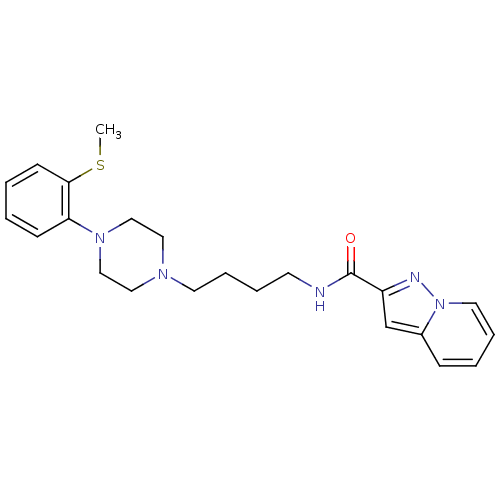 (CHEMBL550222 | N-(4-(4-(2-(methylthio)phenyl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in CHO cells | J Med Chem 52: 4923-35 (2009) Article DOI: 10.1021/jm900690y BindingDB Entry DOI: 10.7270/Q2PK0G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085751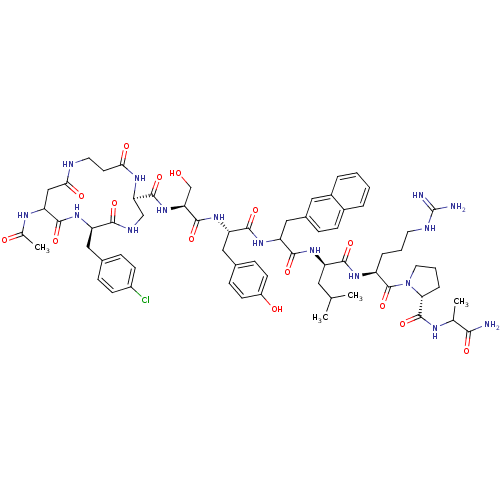 (CHEMBL437230 | cyclo(1,1'-3)Ac-D-Asp (beta-Ala)-D-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085722 (CHEMBL413896 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391699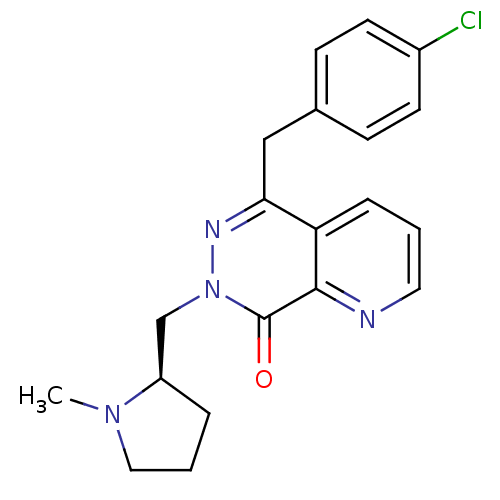 (CHEMBL2146484) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085730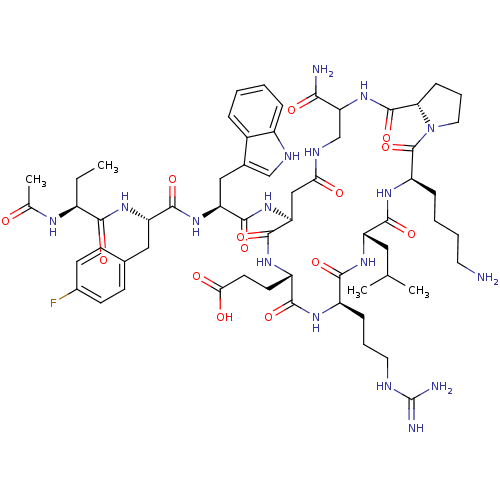 (CHEMBL407074 | Cyclo (4-10) [Ac-D Nal, D Fpa, D Tr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085729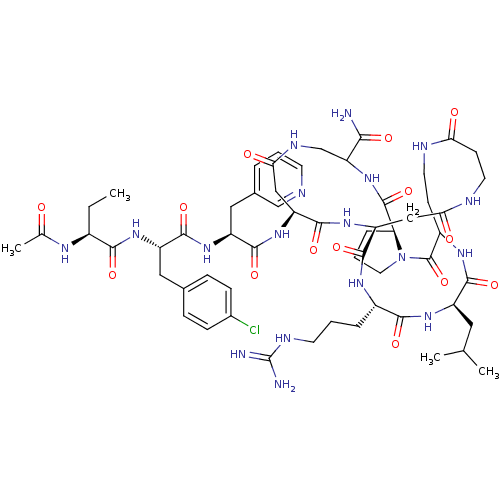 (CHEMBL438902 | DiCyclo (4-10/5,5'-8) [Ac-D Nal, D ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay, in human HEK-293 cells stably transfected with the rat Gonadotropin-releasing hormone receptor | J Med Chem 43: 784-96 (2000) BindingDB Entry DOI: 10.7270/Q2J38RS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183126 (CHEMBL207374 | N-(2,4-dimethylbenzyl)-N-isobutylpi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391697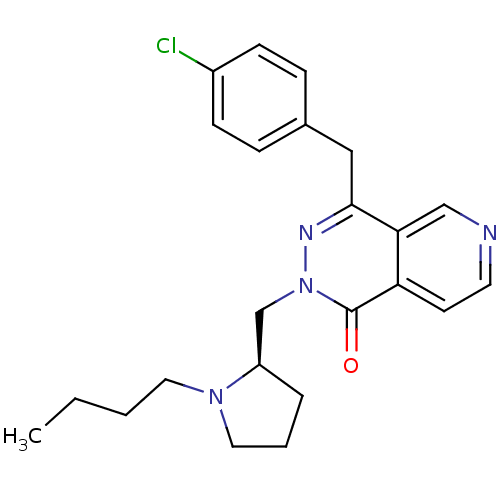 (CHEMBL2146806) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183154 (CHEMBL207067 | CHEMBL207214 | N-(4-fluoro-2-(trifl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183177 (4-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183141 (3-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50085748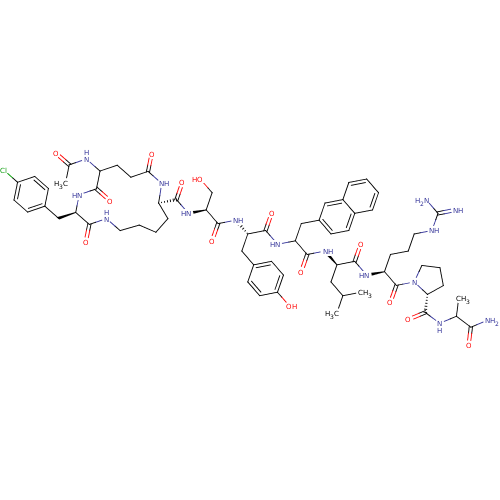 (CHEMBL407186 | cyclo(1-3)Ac-D-Glu-D-Cpa-D-Lys-Ser-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Salk Institute Curated by ChEMBL | Assay Description Competitive radioligand binding assay for rat Gonadotropin-releasing hormone receptor cells stably transfected in human HEK-293 | J Med Chem 43: 797-806 (2000) BindingDB Entry DOI: 10.7270/Q2DF6QD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183136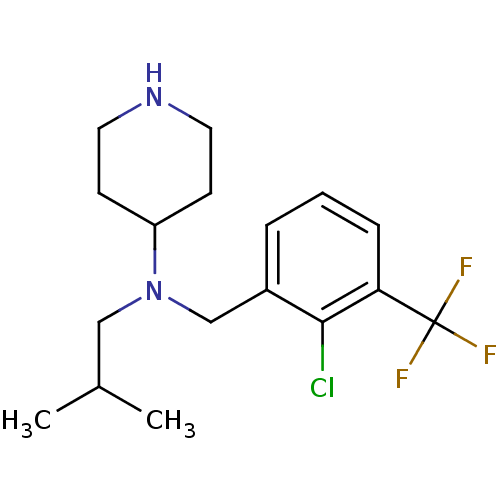 (CHEMBL208533 | N-(2-chloro-3-(trifluoromethyl)benz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183137 (1-(2-cyclohexyl-4-methylpentyl)-3-ethynylbenzene f...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50263386 (CHEMBL443967 | H-Arg-N-Me-Arg-Pro-Tyr-Ile-Leu-OH) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]NT(8-13) from wild type human NTR1 expressed in HEK293 cells | Bioorg Med Chem 16: 9359-68 (2008) Article DOI: 10.1016/j.bmc.2008.08.051 BindingDB Entry DOI: 10.7270/Q2VT1T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50391710 (CHEMBL2146803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assay | Bioorg Med Chem 20: 6097-108 (2012) Article DOI: 10.1016/j.bmc.2012.08.032 BindingDB Entry DOI: 10.7270/Q2NG4RQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6199 total ) | Next | Last >> |