

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | Neuropsychopharmacology 8: 23-33 (1993) Article DOI: 10.1038/npp.1993.4 BindingDB Entry DOI: 10.7270/Q2XS5SXF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219966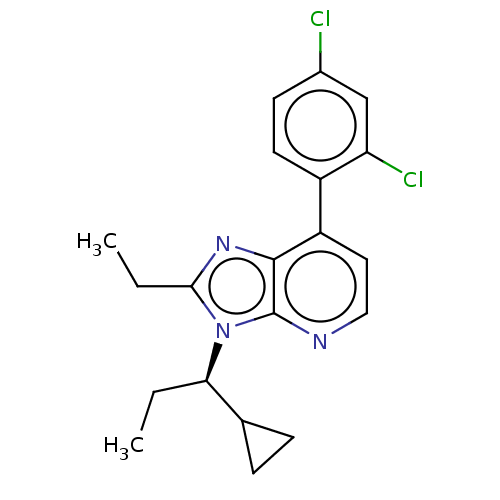 (CHEMBL23959) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125155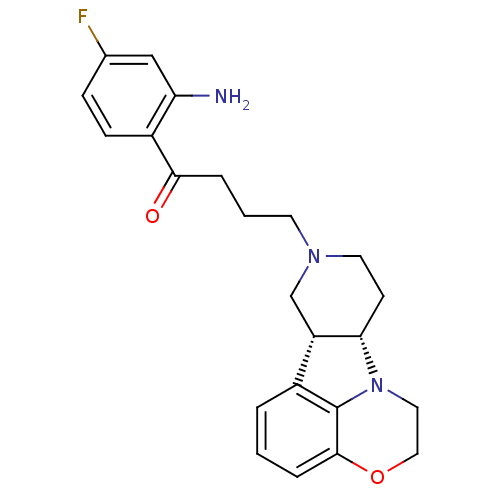 (1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125161 (1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125173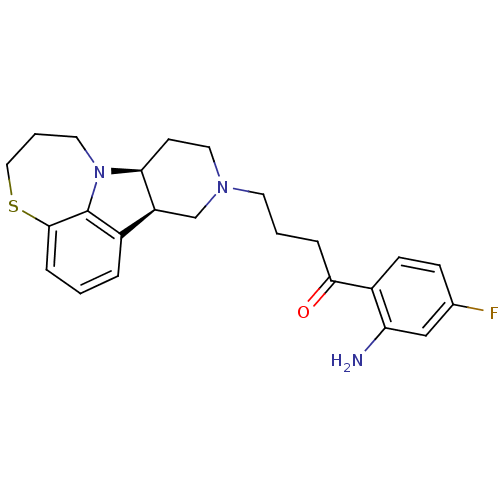 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219965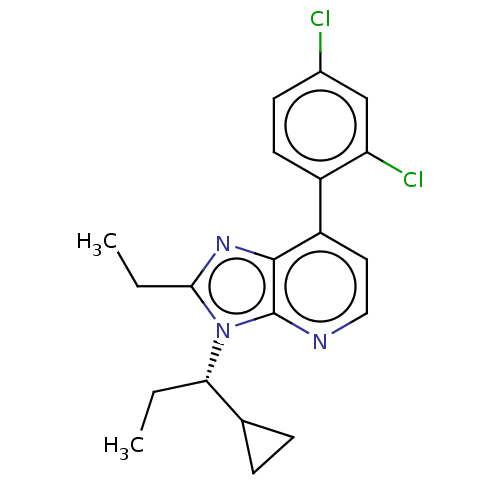 (CHEMBL430913) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description pKi value for inhibition of [3H]LY-278584 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 33: 3176-81 (1991) BindingDB Entry DOI: 10.7270/Q2TM7BQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor in rat cortical membranes using [3H]5-HT as a radioligand | J Med Chem 33: 3176-81 (1991) BindingDB Entry DOI: 10.7270/Q2TM7BQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50220479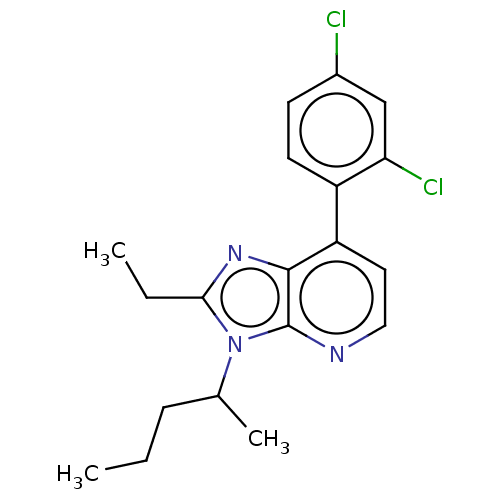 (CHEMBL23342) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50222124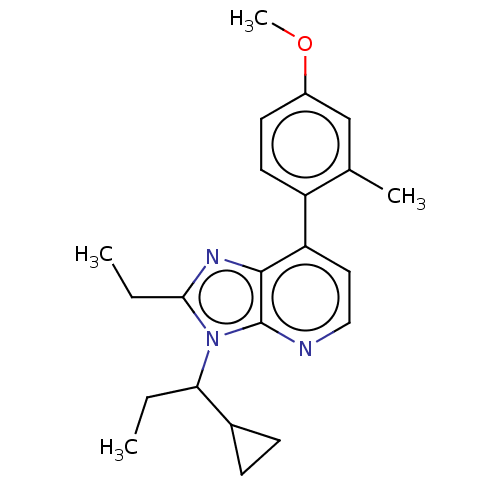 (CHEMBL97340) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity at Corticotropin releasing factor receptor of rat cortical homogenates. | Bioorg Med Chem Lett 13: 289-91 (2003) BindingDB Entry DOI: 10.7270/Q2765HJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125154 (1-(2-Amino-4-fluoro-phenyl)-4-(7aS,11aR)-5,6,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50228247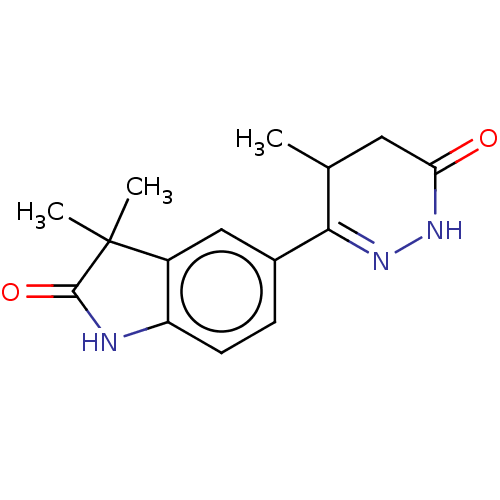 (CHEMBL38017 | LY-197055) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement [3H]LY-186,126 from phosphodiesterase 4 of myocardial vesicles | J Med Chem 32: 1476-80 (1989) BindingDB Entry DOI: 10.7270/Q2P271B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50220478 (CHEMBL22622) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219957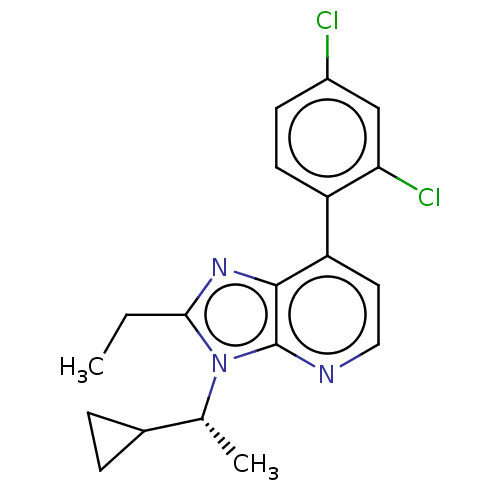 (CHEMBL3085294) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50222115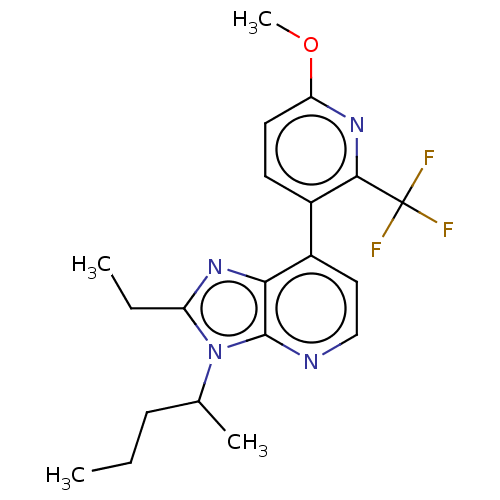 (CHEMBL100157) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity at Corticotropin releasing factor receptor of rat cortical homogenates. | Bioorg Med Chem Lett 13: 289-91 (2003) BindingDB Entry DOI: 10.7270/Q2765HJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125174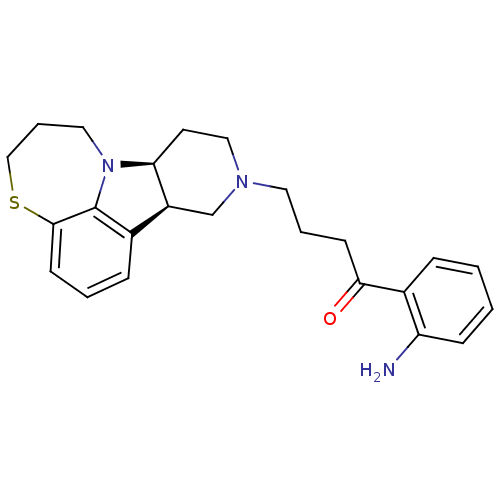 (1-(2-Amino-phenyl)-4-(7bS,11aR)-6,7,8,9,11,11a-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125170 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-4,5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125163 (1-(2-Amino-phenyl)-4-(7bS,11aR)-4,5,6,7,8,9,11,11a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50136685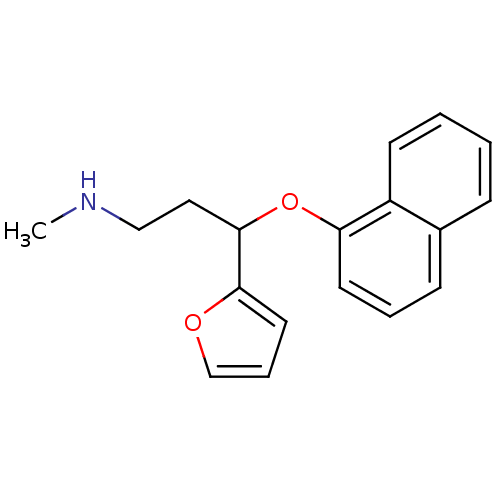 (CHEMBL337822 | [3-Furan-2-yl-3-(naphthalen-1-yloxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to serotonin transporter, using [3H]-citalopram as radioligand | Bioorg Med Chem Lett 13: 4477-80 (2003) BindingDB Entry DOI: 10.7270/Q24J0DJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50222119 (CHEMBL316624) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity at Corticotropin releasing factor receptor of rat cortical homogenates. | Bioorg Med Chem Lett 13: 289-91 (2003) BindingDB Entry DOI: 10.7270/Q2765HJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50222117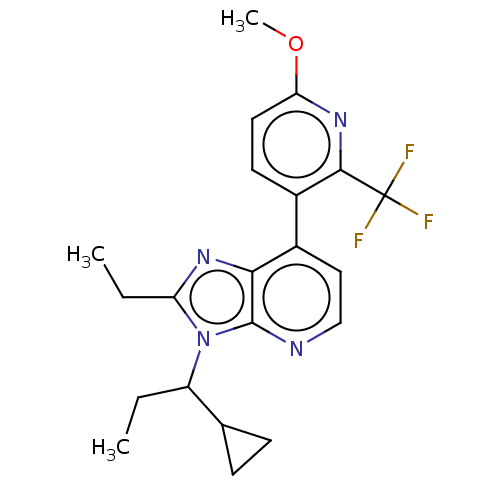 (CHEMBL328163) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity at Corticotropin releasing factor receptor of rat cortical homogenates. | Bioorg Med Chem Lett 13: 289-91 (2003) BindingDB Entry DOI: 10.7270/Q2765HJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125173 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards DA D2 receptor using [3H]-N-methyl-spiperone as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219954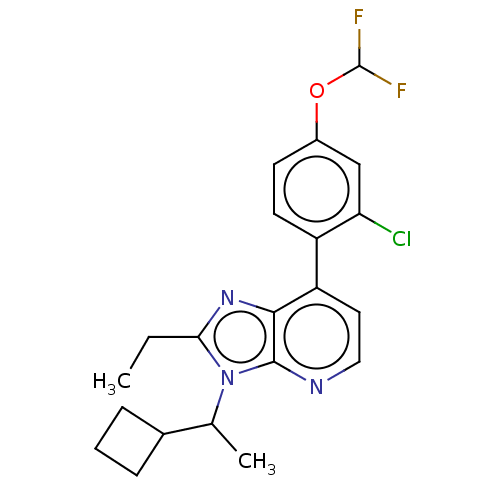 (CHEMBL283993) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity against Corticotropin releasing factor receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219969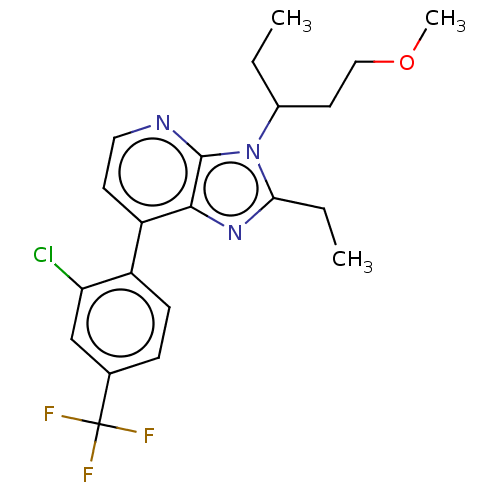 (CHEMBL23439) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219962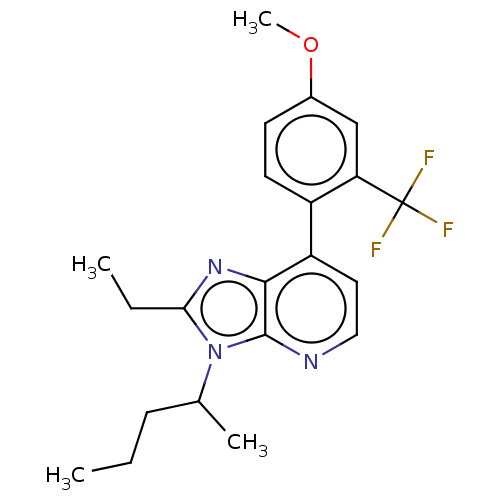 (CHEMBL431105) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity against Corticotropin releasing factor receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219962 (CHEMBL431105) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125169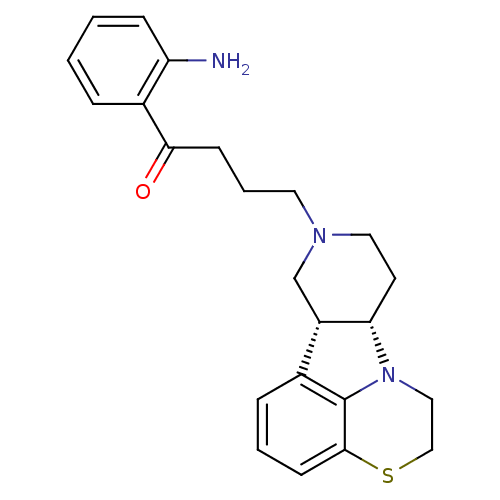 (1-(2-Amino-phenyl)-4-(6bR,10aS)-1,2,6b,9,10,10a-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit the reuptake of 5-HT at human serotonin transporter | Bioorg Med Chem Lett 13: 4477-80 (2003) BindingDB Entry DOI: 10.7270/Q24J0DJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50220480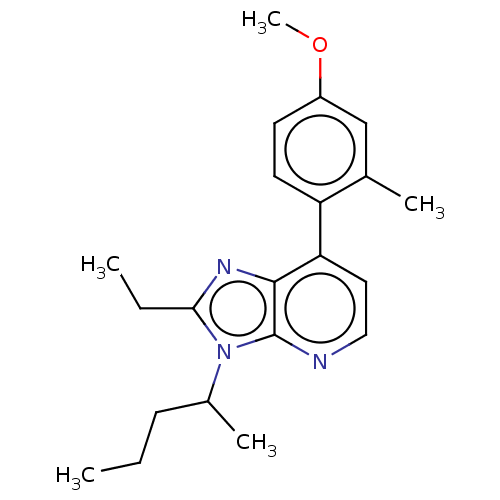 (CHEMBL22433) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125151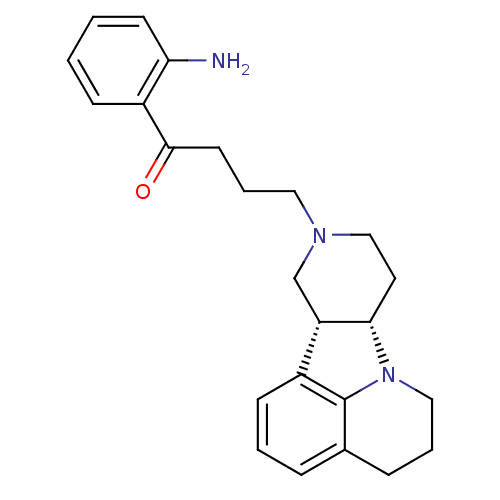 (1-(2-Amino-phenyl)-4-(7aS,11aR)-5,6,8,9,11,11a-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125177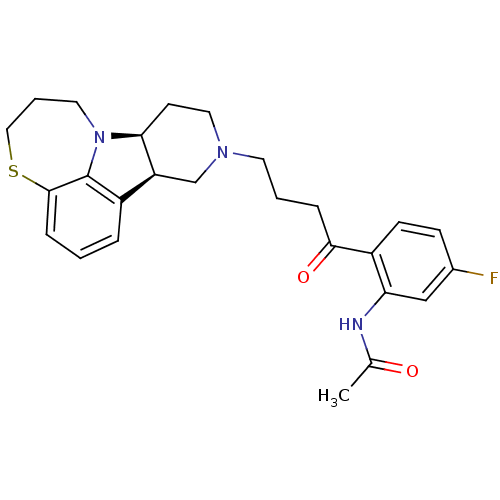 (CHEMBL162768 | N-[5-Fluoro-2-((7bS,11aR)-4-6,7,8,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125150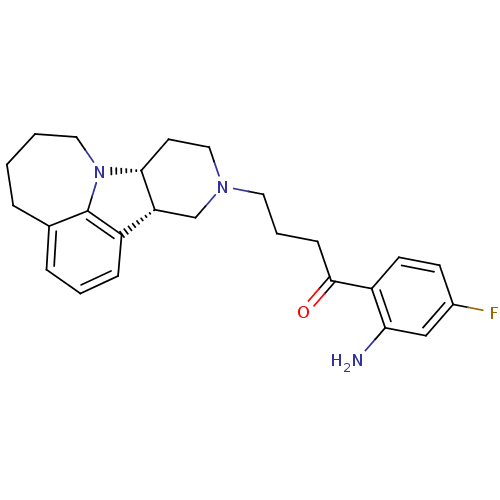 (1-(2-Amino-4-fluoro-phenyl)-4-(7bR,11aS)-4,5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476859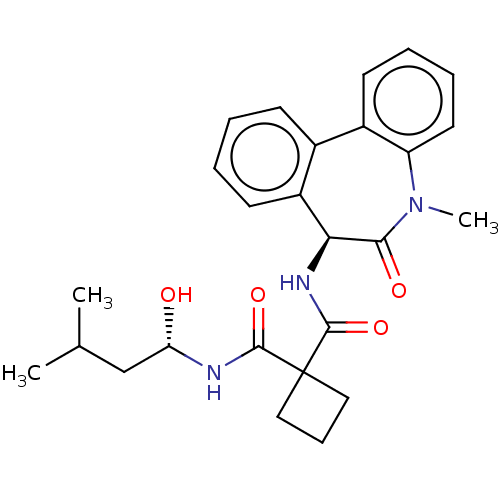 (CHEMBL232938) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50136672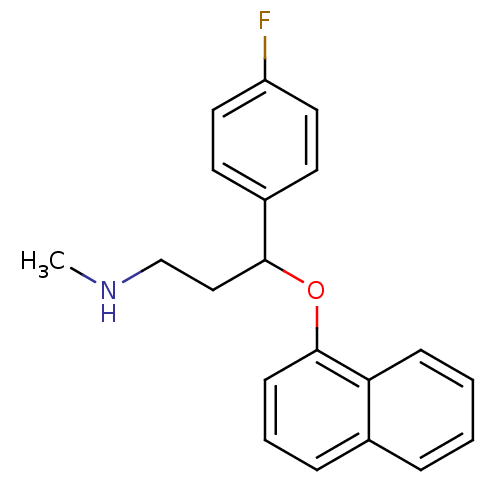 (CHEMBL342716 | [3-(4-Fluoro-phenyl)-3-(naphthalen-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to serotonin transporter, using [3H]-citalopram as radioligand | Bioorg Med Chem Lett 13: 4477-80 (2003) BindingDB Entry DOI: 10.7270/Q24J0DJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50087713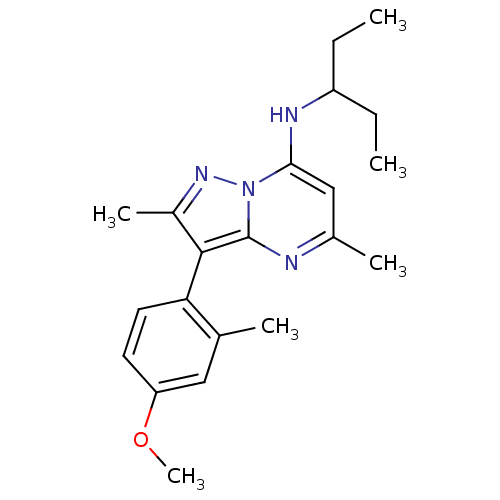 ((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Compound was tested for the binding affinity to human corticotropin releasing factor 1 (hCRF1) receptors | J Med Chem 43: 1641-60 (2000) BindingDB Entry DOI: 10.7270/Q29S1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219961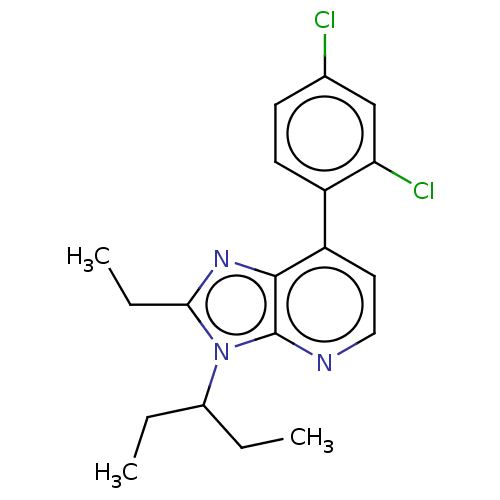 (CHEMBL23483) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50220485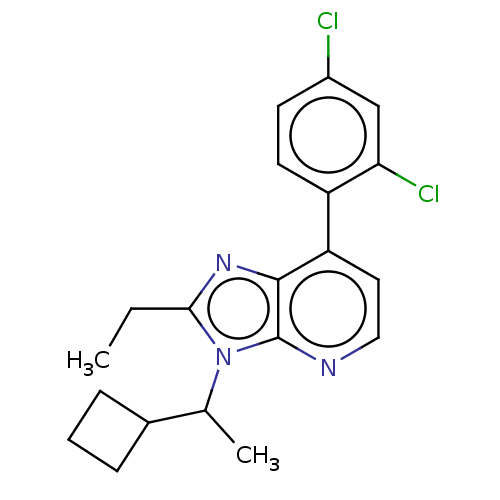 (CHEMBL23354) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219967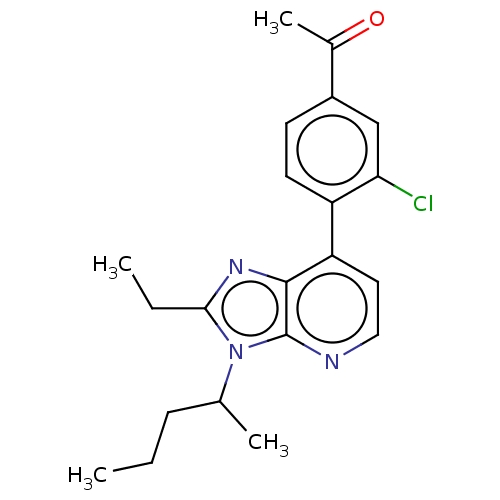 (CHEMBL423475) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50087706 (CHEMBL265847 | Urotensin-1-sucker fish(NDDPPISIDLT...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity of the receptor corticotropin releasing factor receptor with peptidic agonists | J Med Chem 43: 1641-60 (2000) BindingDB Entry DOI: 10.7270/Q29S1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219963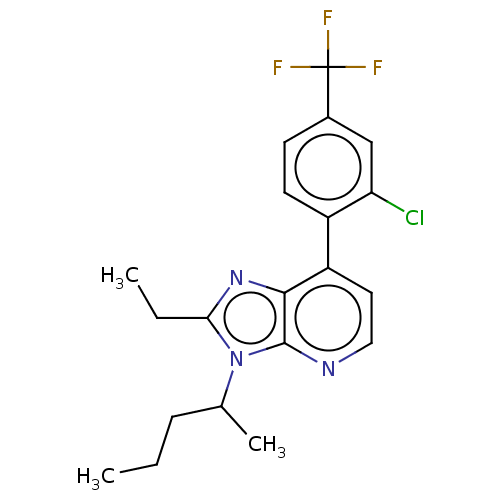 (CHEMBL276971) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity against Corticotropin releasing factor receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219963 (CHEMBL276971) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50222122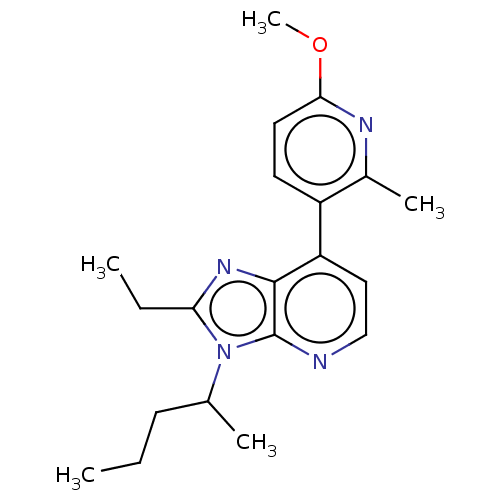 (CHEMBL99286) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity at Corticotropin releasing factor receptor of rat cortical homogenates. | Bioorg Med Chem Lett 13: 289-91 (2003) BindingDB Entry DOI: 10.7270/Q2765HJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50136683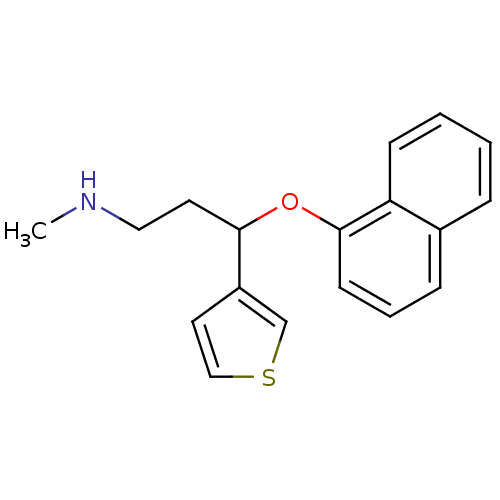 (CHEMBL142028 | Methyl-[3-(naphthalen-1-yloxy)-3-th...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to serotonin transporter, using [3H]-citalopram as radioligand | Bioorg Med Chem Lett 13: 4477-80 (2003) BindingDB Entry DOI: 10.7270/Q24J0DJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476878 (CHEMBL399204) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125164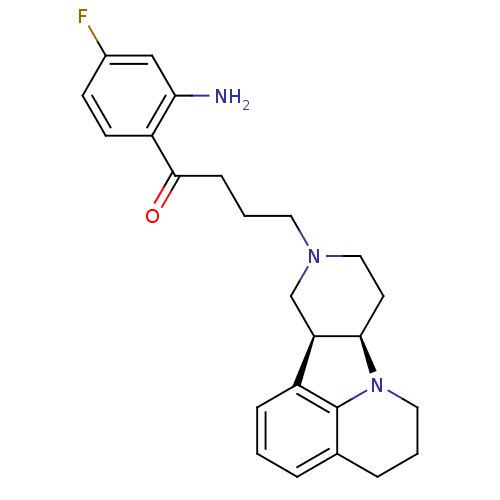 (1-(2-Amino-4-fluoro-phenyl)-4-(7aR,11aS)-5,6,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476861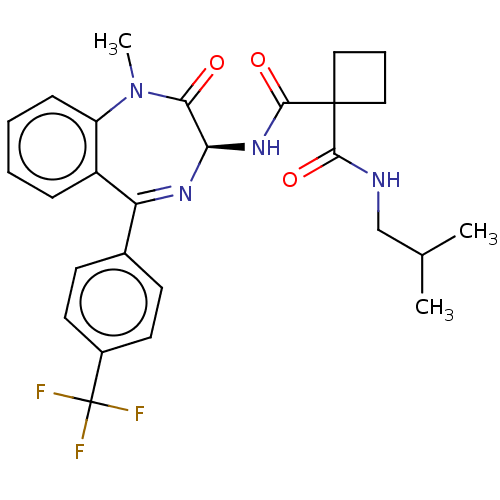 (CHEMBL401044) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 2 (Homo sapiens (Human)) | BDBM50087706 (CHEMBL265847 | Urotensin-1-sucker fish(NDDPPISIDLT...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity of the receptor h-CRF2-alpha with peptidic agonists | J Med Chem 43: 1641-60 (2000) BindingDB Entry DOI: 10.7270/Q29S1RRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description pKi value for inhibition of [3H]LY-278584 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 33: 3176-81 (1991) BindingDB Entry DOI: 10.7270/Q2TM7BQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1/2 (Rattus norvegicus (rat)-RAT) | BDBM50219970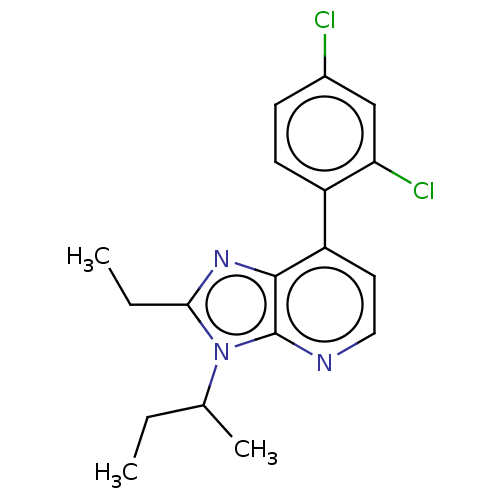 (CHEMBL23950) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description In vitro binding affinity to the CRF receptor in rat cortical homogenates | Bioorg Med Chem Lett 13: 125-8 (2003) BindingDB Entry DOI: 10.7270/Q2JM2CV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476880 (CHEMBL450279) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]succinamide from gamma secretase in THP1 cell membranes | Bioorg Med Chem Lett 17: 3910-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.102 BindingDB Entry DOI: 10.7270/Q2M90CFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2286 total ) | Next | Last >> |