

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50403370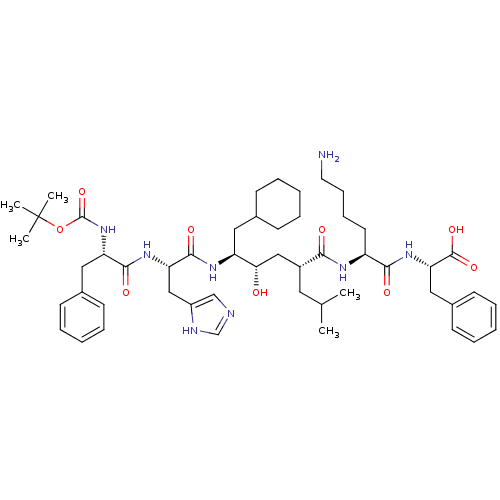 (CHEMBL2052021 | CP-71362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against dog plasma renin | Bioorg Med Chem Lett 4: 589-592 (1994) Article DOI: 10.1016/S0960-894X(01)80160-8 BindingDB Entry DOI: 10.7270/Q2KD1XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Rattus norvegicus) | BDBM50403370 (CHEMBL2052021 | CP-71362) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Rat plasma renin | Bioorg Med Chem Lett 4: 589-592 (1994) Article DOI: 10.1016/S0960-894X(01)80160-8 BindingDB Entry DOI: 10.7270/Q2KD1XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50126018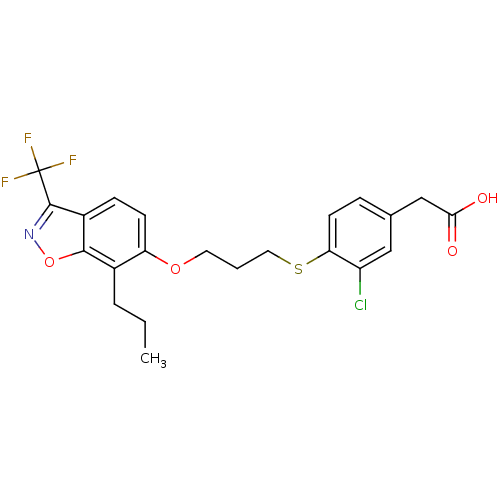 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PPAR delta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50184263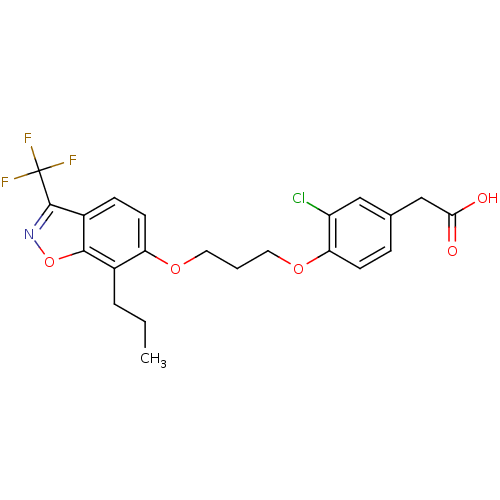 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PPAR delta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146206 (2-Benzyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146200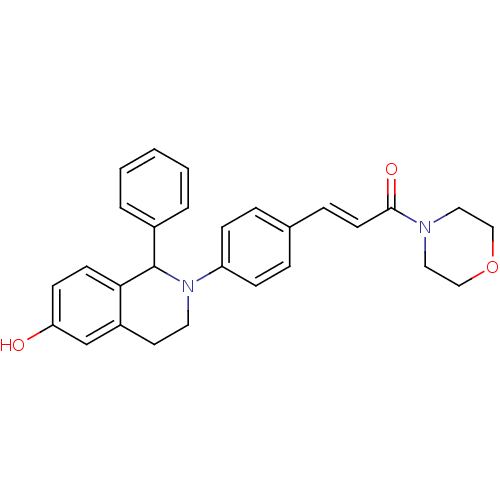 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146223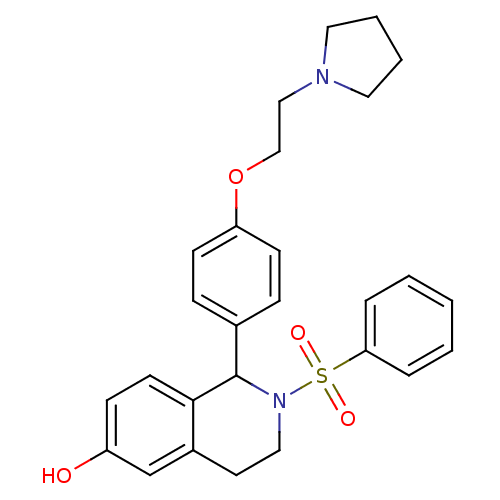 (2-Benzenesulfonyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146216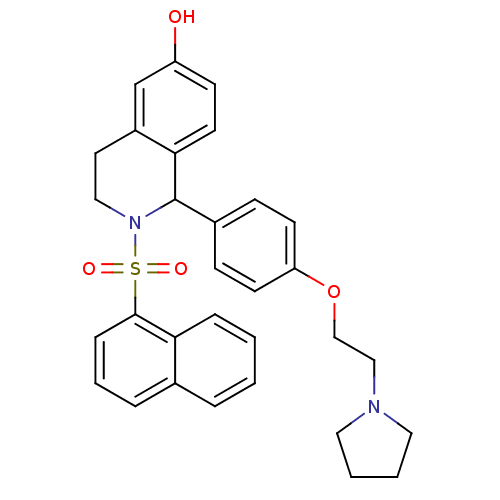 (2-(Naphthalene-1-sulfonyl)-1-[4-(2-pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146200 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184249 (3-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146222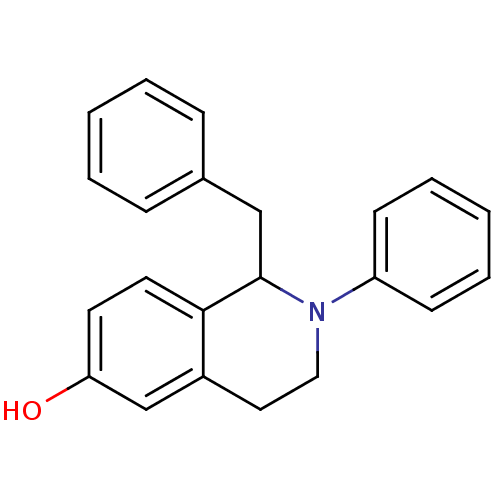 (1-Benzyl-2-phenyl-1,2,3,4-tetrahydro-isoquinolin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50184261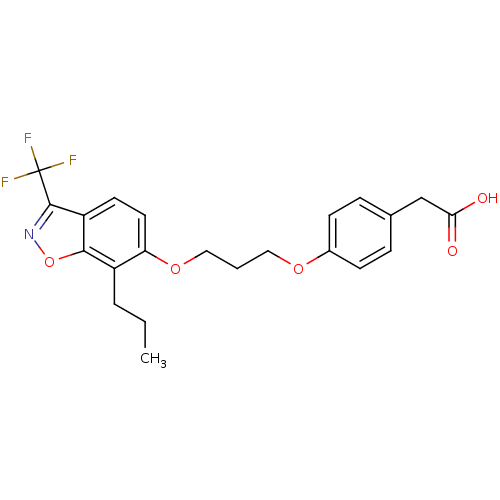 (2-(4-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PPAR delta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146199 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146199 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146220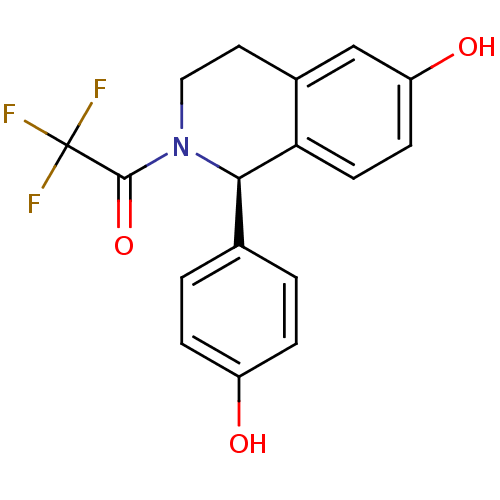 (2,2,2-Trifluoro-1-[(R)-6-hydroxy-1-(4-hydroxy-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146205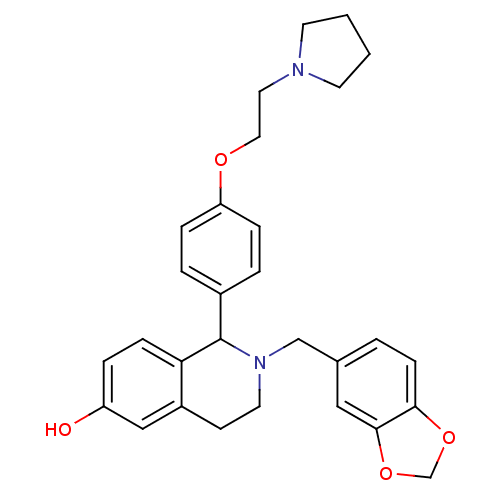 (2-Benzo[1,3]dioxol-5-ylmethyl-1-[4-(2-pyrrolidin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM50471710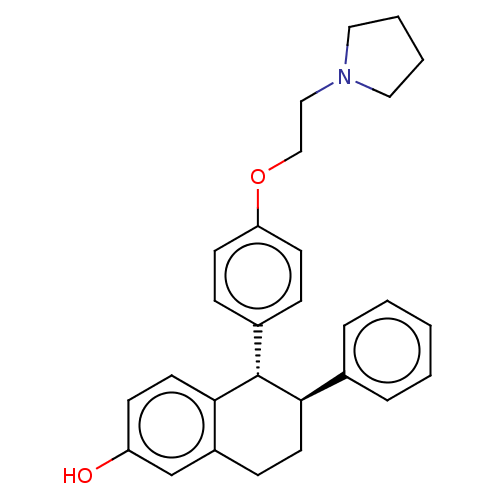 (CHEMBL317748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184259 (2-(2-chloro-4-(methyl(3-(7-propyl-3-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM50471711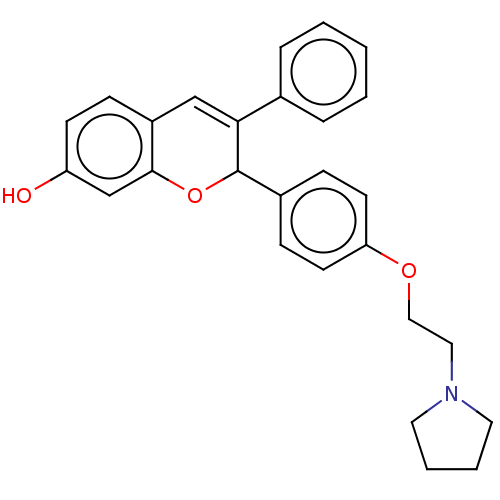 (CHEMBL98892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM20606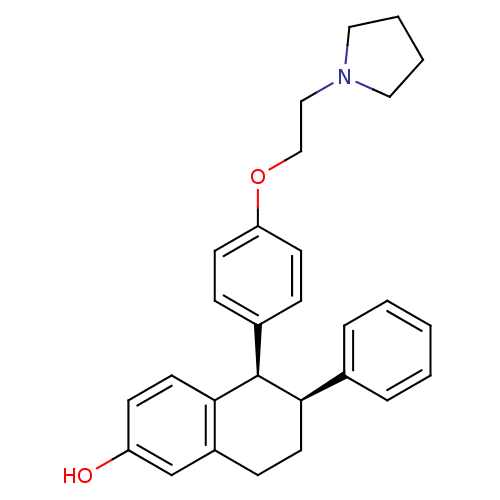 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146207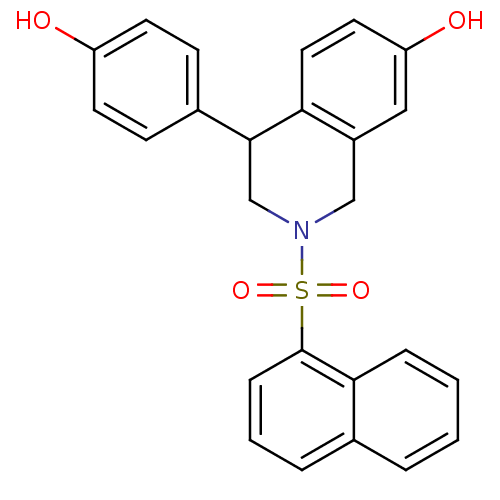 (4-(4-Hydroxy-phenyl)-2-(naphthalene-1-sulfonyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146211 (2,2,2-Trifluoro-1-[7-hydroxy-4-(4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM20606 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor | J Med Chem 41: 2928-31 (1998) Article DOI: 10.1021/jm980048b BindingDB Entry DOI: 10.7270/Q20C4ZGS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20585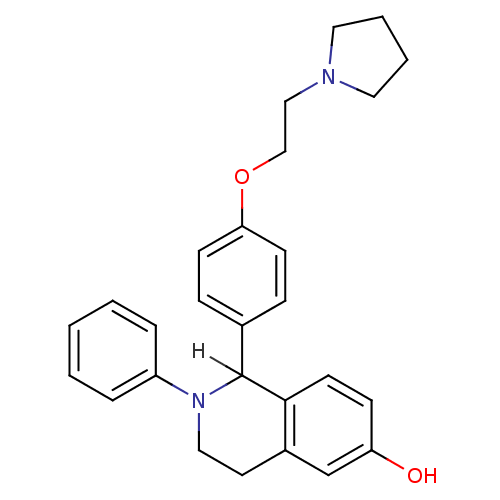 (2-phenyl-1-{4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50184259 (2-(2-chloro-4-(methyl(3-(7-propyl-3-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at LXR alpha as beta-lactamase transactivation in CHO cells | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146198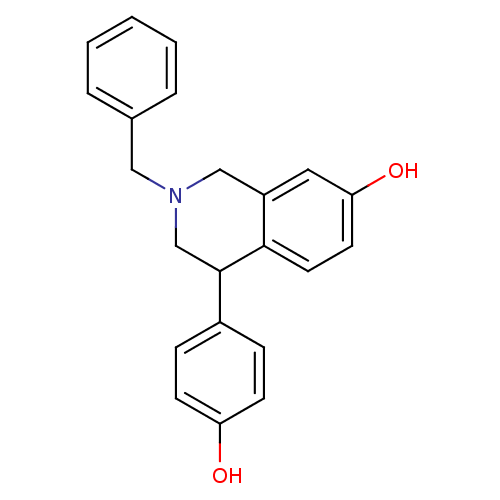 (2-Benzyl-4-(4-hydroxy-phenyl)-1,2,3,4-tetrahydro-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184257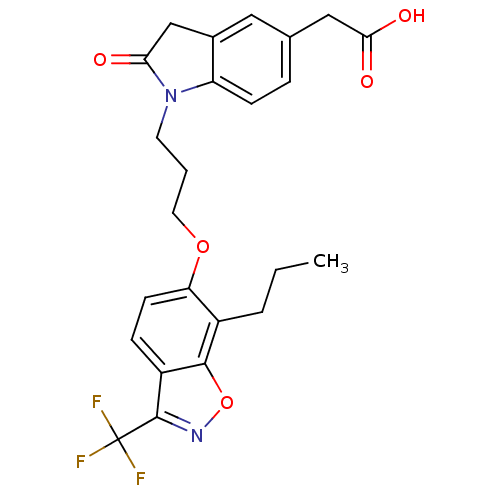 (2-(2-oxo-1-(3-(7-propyl-3-(trifluoromethyl)benzo[d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184255 (2-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50184274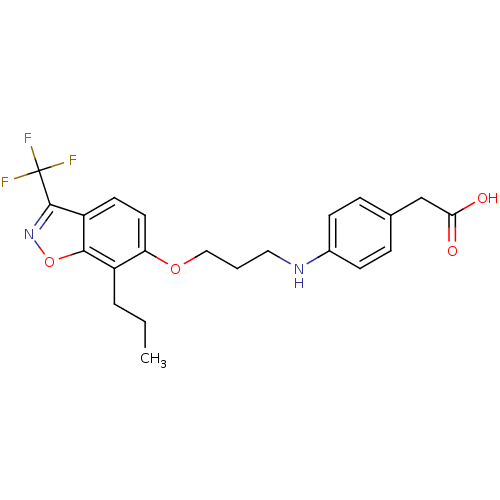 (2-(4-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PPAR alpha | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50184255 (2-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at LXR alpha as beta-lactamase transactivation in CHO cells | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50184254 (2-(4-(methyl(3-(7-propyl-3-(trifluoromethyl)benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at LXR alpha as beta-lactamase transactivation in CHO cells | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50184249 (3-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at LXR alpha as beta-lactamase transactivation in CHO cells | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184250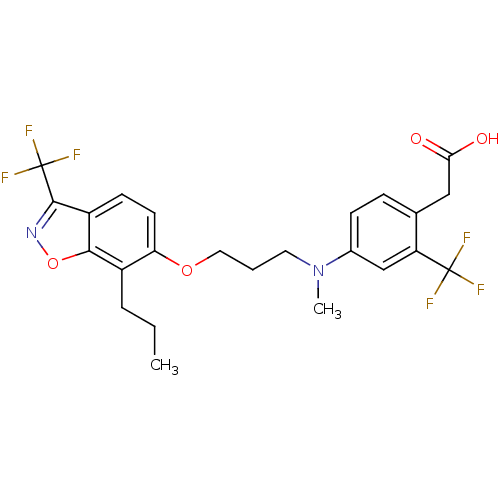 (2-(4-(methyl(3-(7-propyl-3-(trifluoromethyl)benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50184261 (2-(4-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PPAR alpha | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146223 (2-Benzenesulfonyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50184257 (2-(2-oxo-1-(3-(7-propyl-3-(trifluoromethyl)benzo[d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at LXR alpha as beta-lactamase transactivation in CHO cells | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50403370 (CHEMBL2052021 | CP-71362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against human plasma renin | Bioorg Med Chem Lett 4: 589-592 (1994) Article DOI: 10.1016/S0960-894X(01)80160-8 BindingDB Entry DOI: 10.7270/Q2KD1XVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146198 (2-Benzyl-4-(4-hydroxy-phenyl)-1,2,3,4-tetrahydro-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146216 (2-(Naphthalene-1-sulfonyl)-1-[4-(2-pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20585 (2-phenyl-1-{4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146204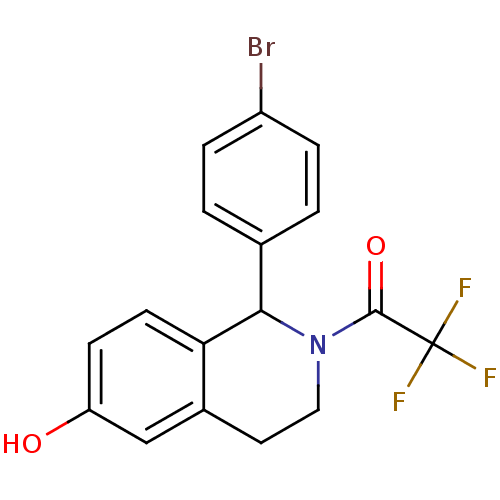 (1-[1-(4-Bromo-phenyl)-6-hydroxy-3,4-dihydro-1H-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50184248 (2-(1-(3-(7-propyl-3-(trifluoromethyl)benzo[d]isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PPAR delta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146208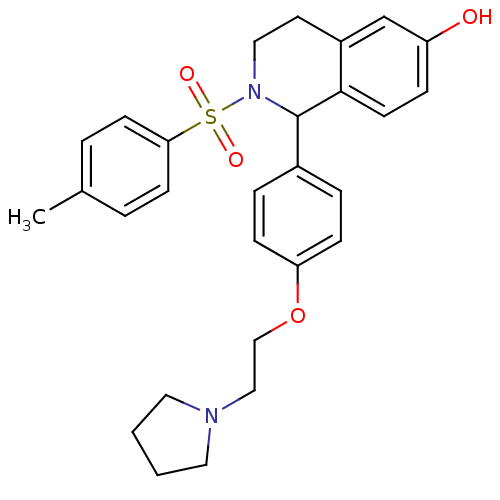 (1-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-2-(toluene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184254 (2-(4-(methyl(3-(7-propyl-3-(trifluoromethyl)benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50184256 (2-(2-methyl-4-(methyl(3-(7-propyl-3-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LXR beta | Bioorg Med Chem Lett 16: 3055-60 (2006) Article DOI: 10.1016/j.bmcl.2006.02.050 BindingDB Entry DOI: 10.7270/Q20V8CD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146221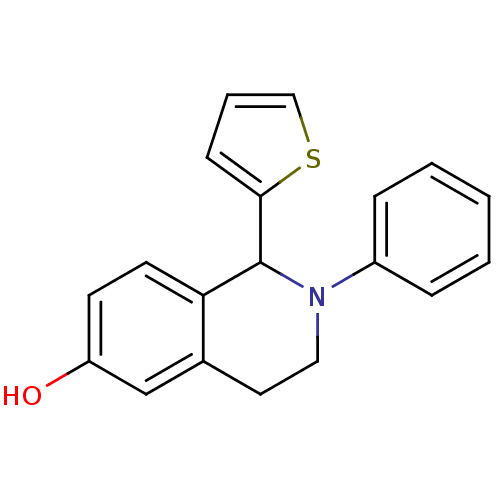 (2-Phenyl-1-thiophen-2-yl-1,2,3,4-tetrahydro-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 215 total ) | Next | Last >> |