

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175609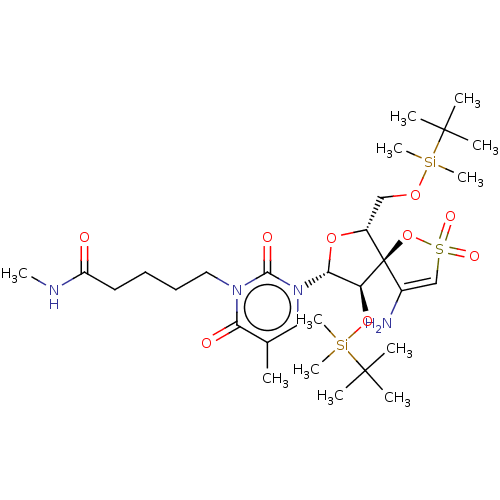 (5-{3-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490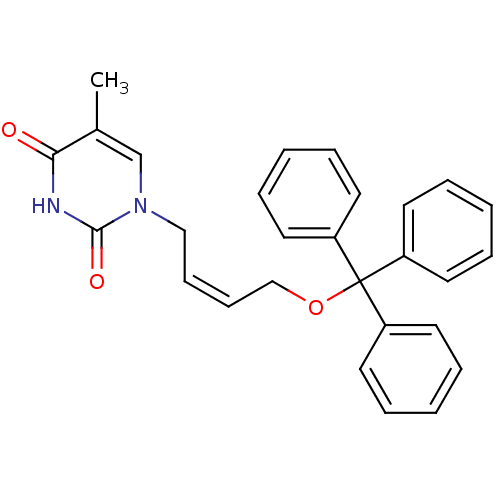 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132253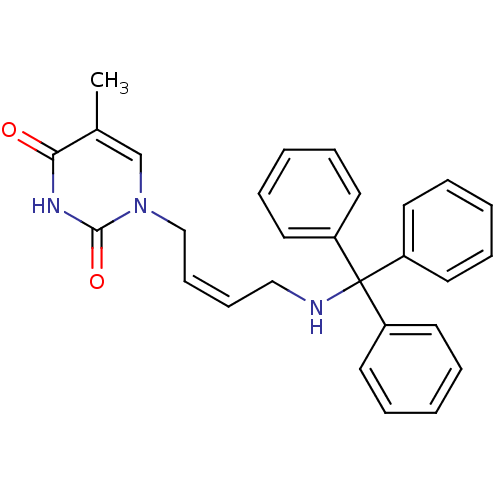 (5-Methyl-1-[4-(trityl-amino)-but-2-enyl]-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175620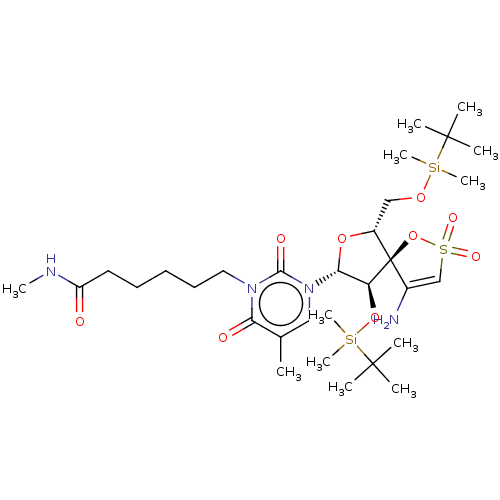 (6-{3-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175627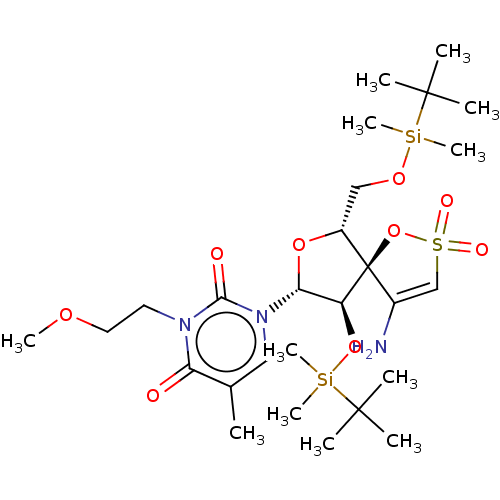 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50100110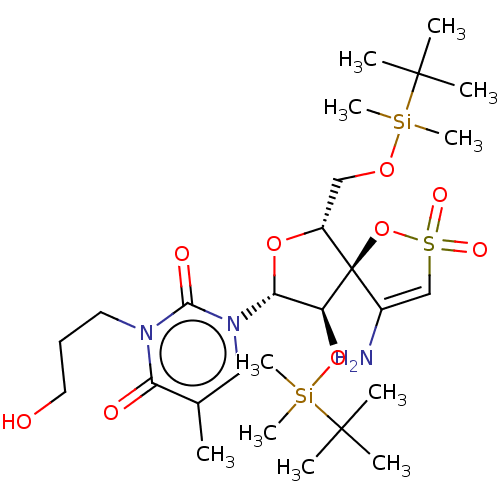 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175621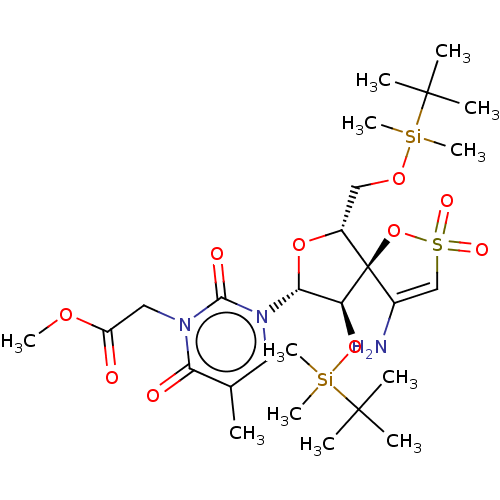 (CHEMBL3143760 | {3-[4-Amino-9-(tert-butyl-dimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175602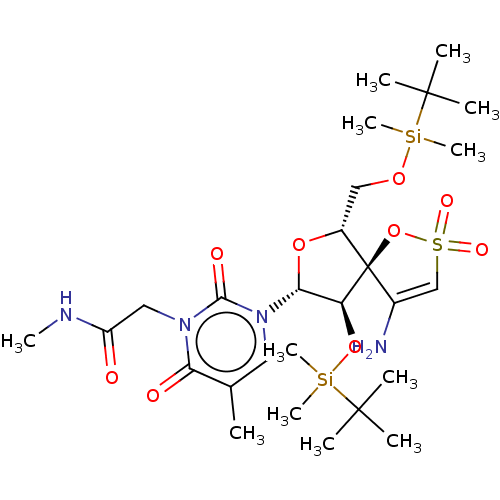 (2-{3-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175618 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175604 (2-{3-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132251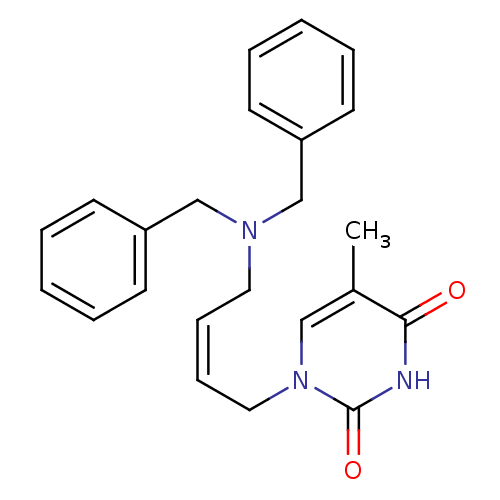 (1-(4-Dibenzylamino-but-2-enyl)-5-methyl-1H-pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 1 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50169035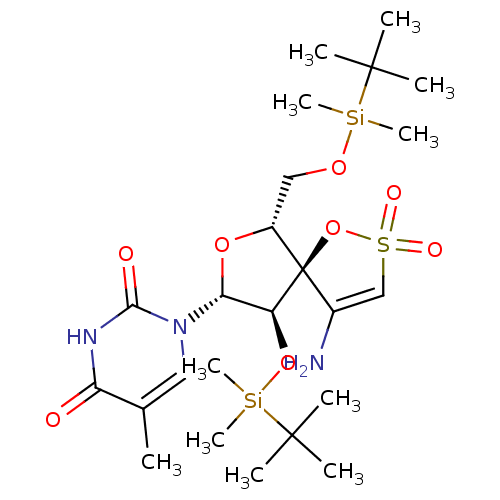 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193562 (CHEMBL263515 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193556 (CHEMBL213257 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132252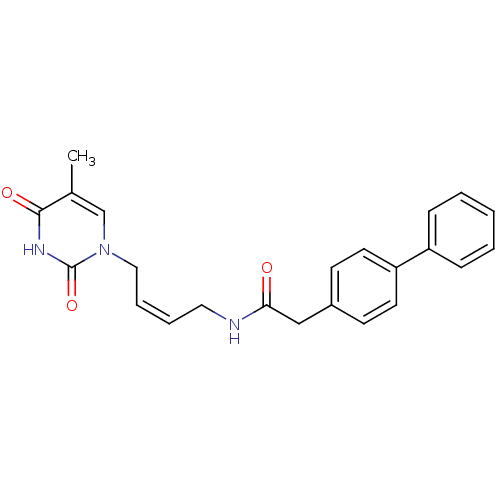 (2-Biphenyl-4-yl-N-[4-(5-methyl-2,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193557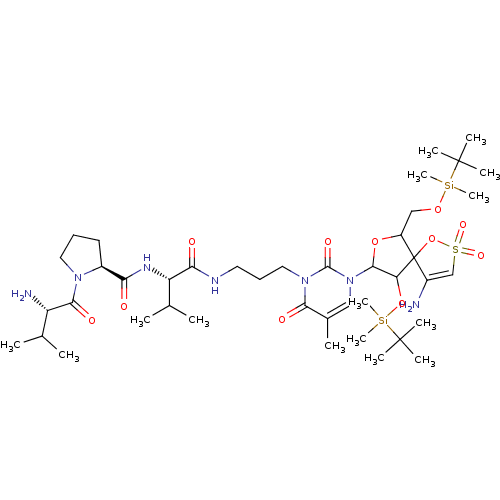 (CHEMBL212339 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175607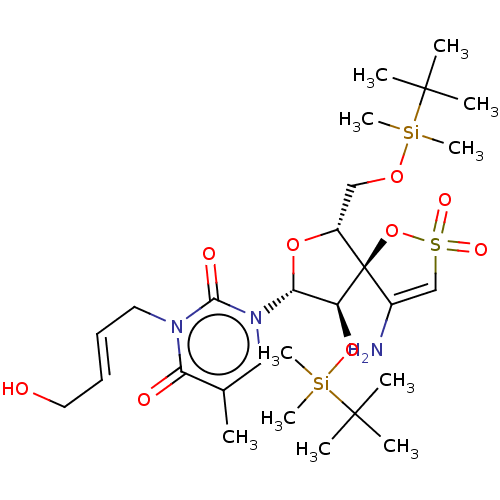 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193563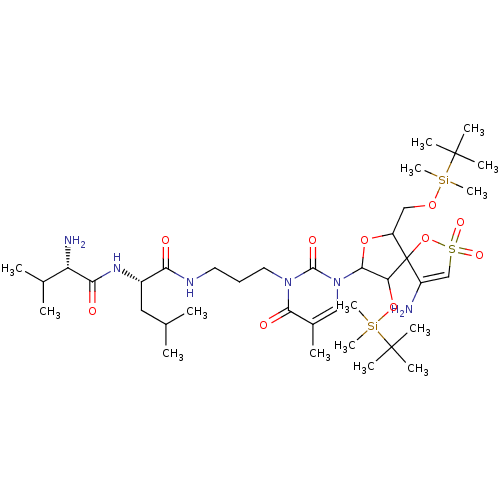 (CHEMBL378037 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193560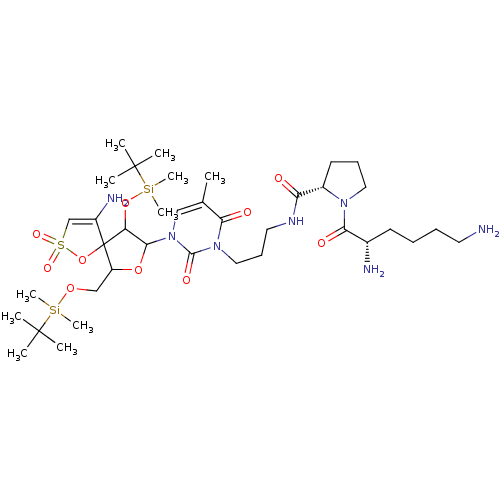 (CHEMBL380211 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193559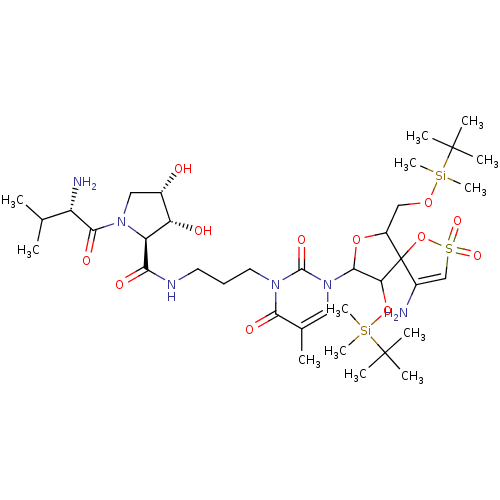 (CHEMBL214795 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193558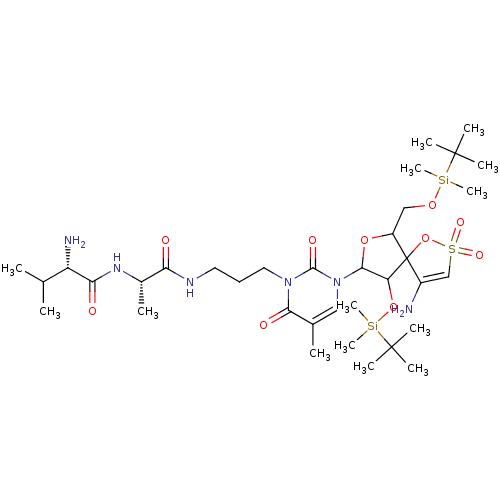 (CHEMBL383894 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175623 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193554 (CHEMBL438175 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193564 (CHEMBL385844 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193565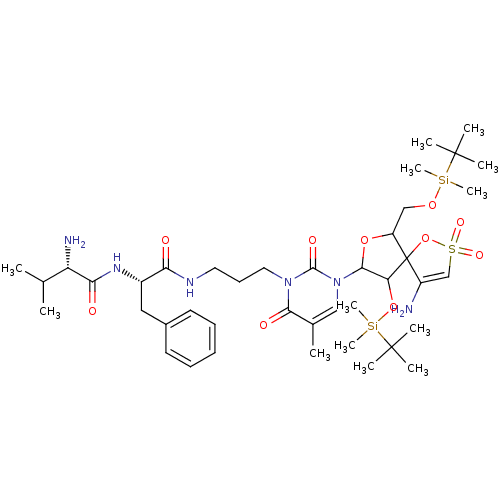 (CHEMBL445417 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193551 (CHEMBL405279 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50192301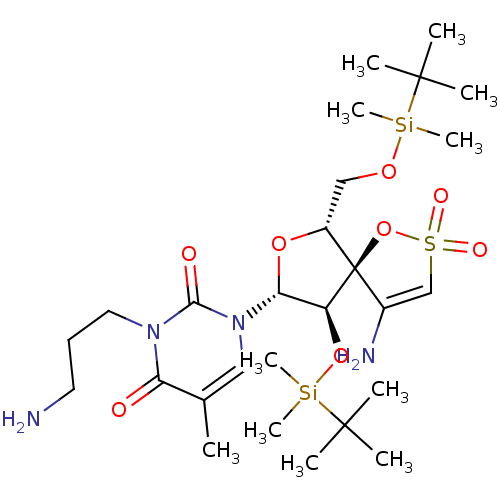 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50192301 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193552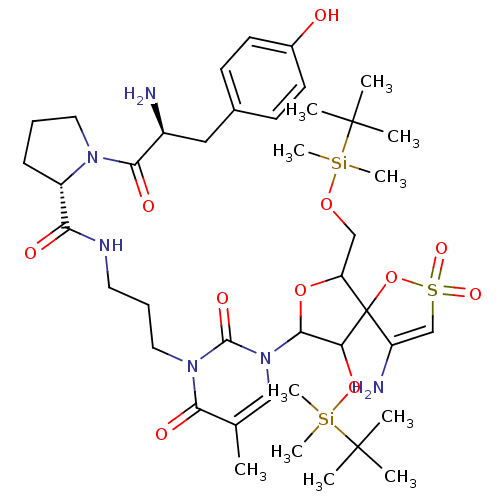 (CHEMBL379994 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175622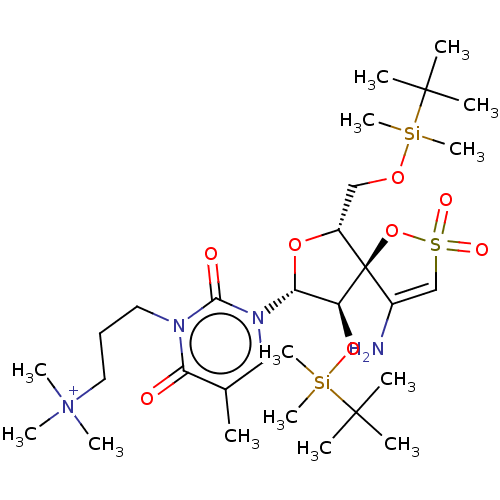 ((3-{3-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193550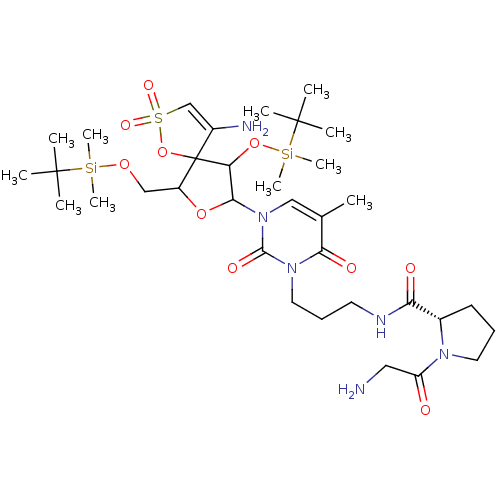 (CHEMBL213365 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50024403 (CHEMBL3143799) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175628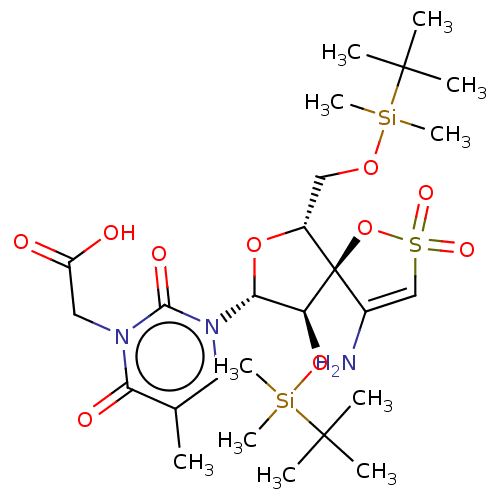 (BDBM50192299 | CHEMBL379599 | {3-[4-Amino-9-(tert-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193553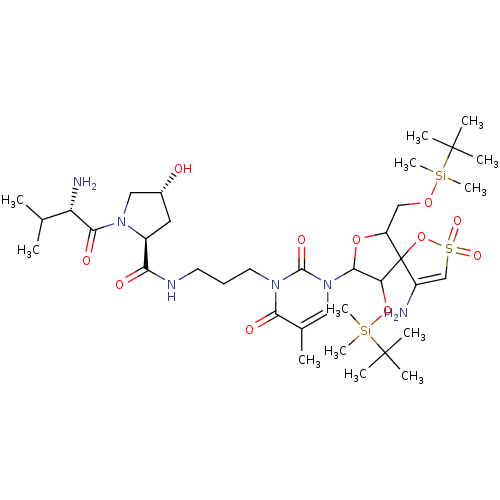 (CHEMBL379485 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193555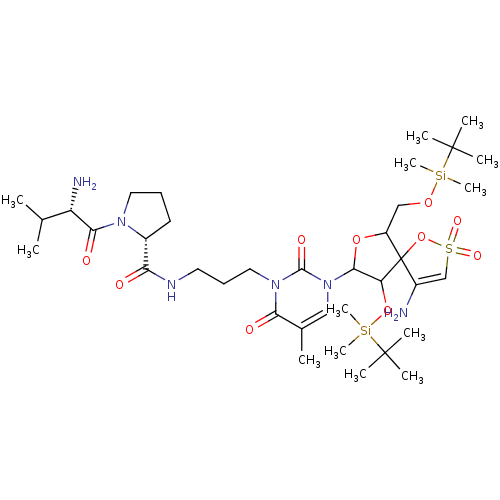 (CHEMBL408333 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132249 (2-(4-Chloro-phenyl)-N-[4-(5-methyl-2,4-dioxo-3,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132253 (5-Methyl-1-[4-(trityl-amino)-but-2-enyl]-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132256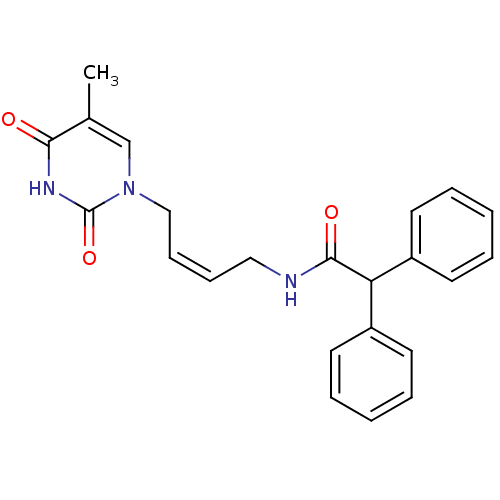 (CHEMBL102986 | N-[4-(5-Methyl-2,4-dioxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50193566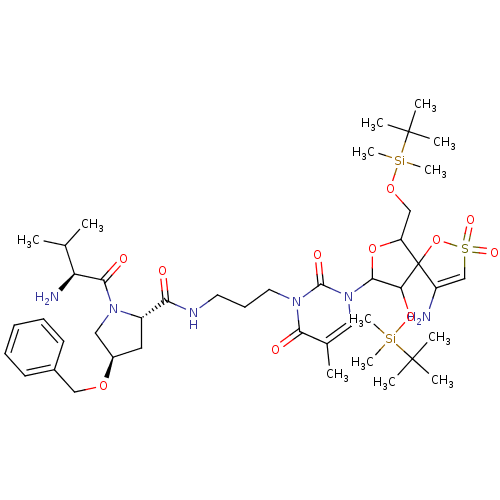 (CHEMBL378646 | [1-[2',5'-bis-O-(tert-butyldimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase | J Med Chem 49: 5339-51 (2006) Article DOI: 10.1021/jm0606490 BindingDB Entry DOI: 10.7270/Q2M32VDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132255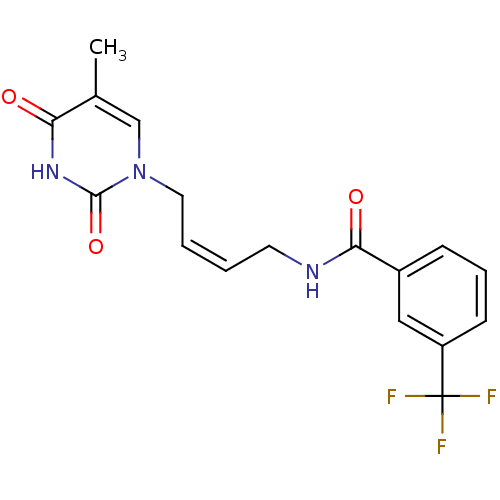 (CHEMBL431234 | N-[4-(5-Methyl-2,4-dioxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50175616 (4-{3-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit recombinant HIV-1 reverse transcriptase | J Med Chem 48: 6653-60 (2005) Article DOI: 10.1021/jm050437n BindingDB Entry DOI: 10.7270/Q2VT1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132250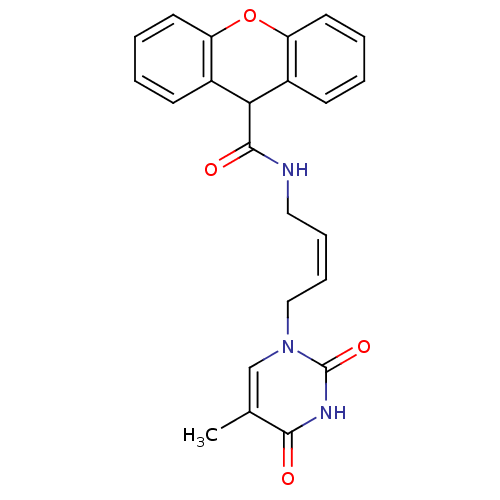 (9H-Xanthene-9-carboxylic acid [4-(5-methyl-2,4-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106919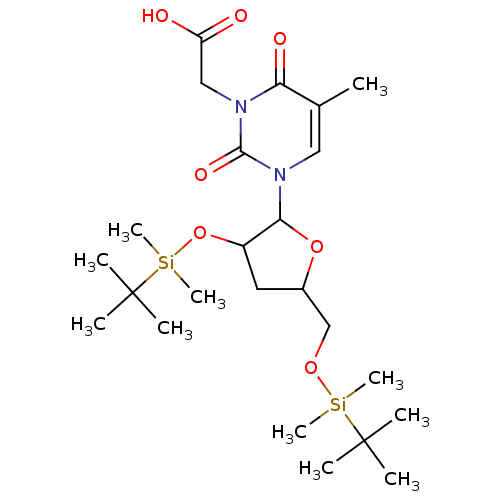 (CHEMBL105018 | {3-[3-(tert-Butyl-dimethyl-silanylo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50106919 (CHEMBL105018 | {3-[3-(tert-Butyl-dimethyl-silanylo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV-1 wild types GluL38-Lys recombinant reverse transcriptase | Bioorg Med Chem Lett 11: 3085-8 (2001) BindingDB Entry DOI: 10.7270/Q2QC02S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132257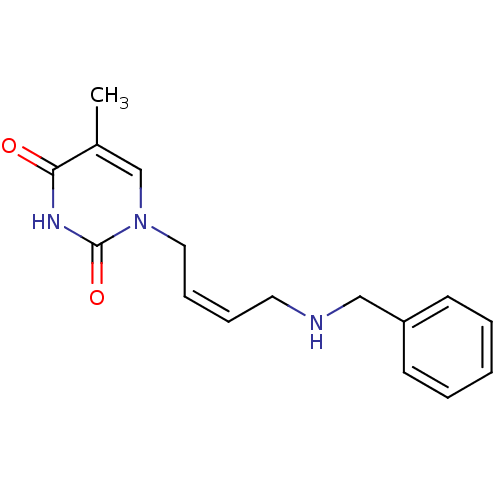 (1-(4-Benzylamino-but-2-enyl)-5-methyl-1H-pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132255 (CHEMBL431234 | N-[4-(5-Methyl-2,4-dioxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132256 (CHEMBL102986 | N-[4-(5-Methyl-2,4-dioxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 1 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132252 (2-Biphenyl-4-yl-N-[4-(5-methyl-2,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132250 (9H-Xanthene-9-carboxylic acid [4-(5-methyl-2,4-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 99 total ) | Next | Last >> |