

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000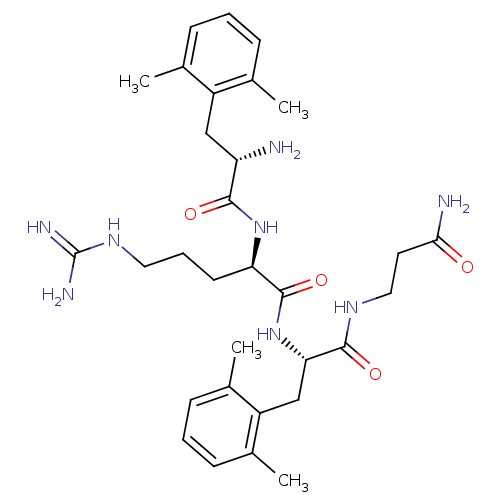 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126003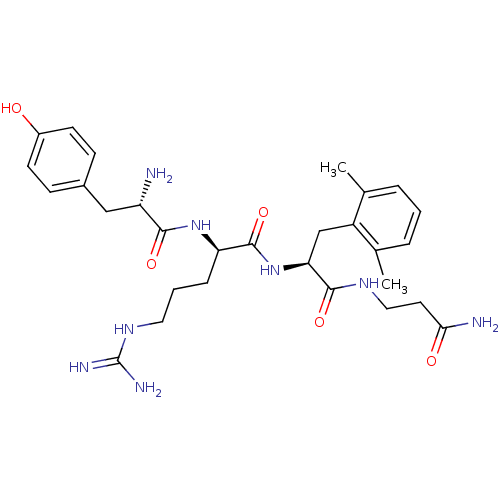 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125997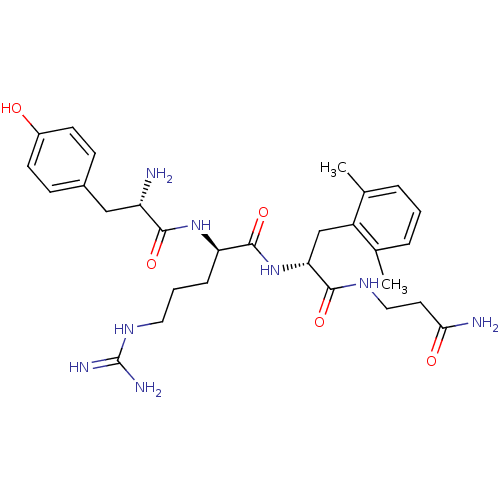 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0618 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126001 ((R)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126002 ((R)-2-[(R)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126003 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 544 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126002 ((R)-2-[(R)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125997 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126001 ((R)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9410 (N-[2-(1-benzylpiperidin-4-yl)ethyl]-N-ethyl-4-(phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9409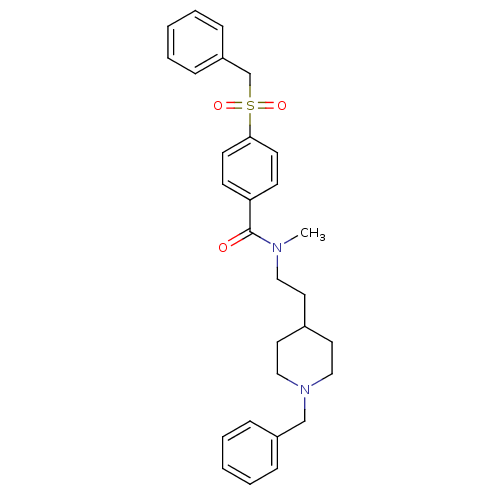 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9409 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9411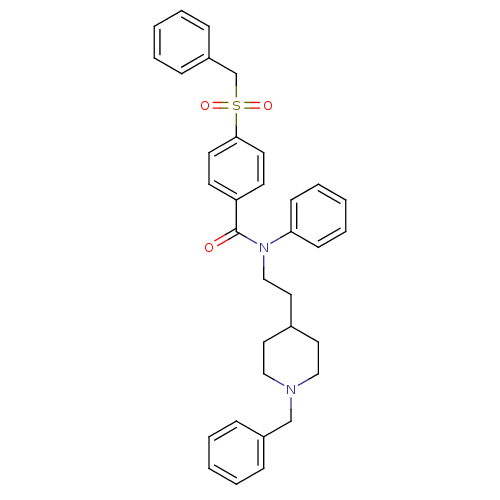 (CHEMBL54058 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004016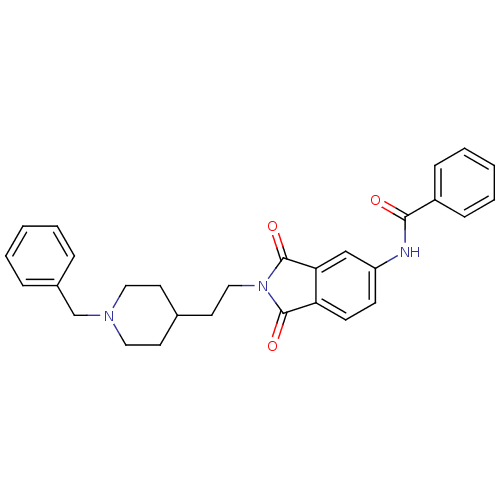 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50126003 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004007 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1,3-dioxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004001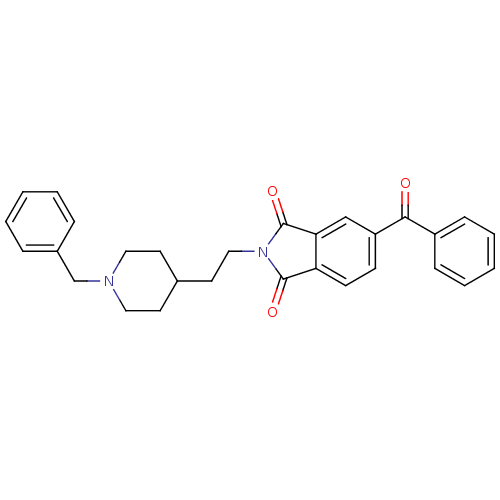 (5-Benzoyl-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50126000 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004035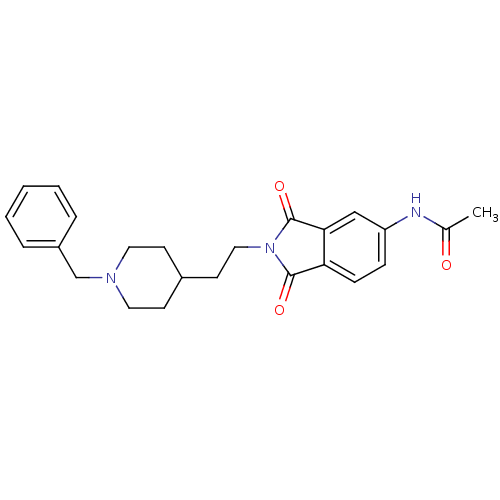 (CHEMBL433678 | N-(2-(2-(1-benzylpiperidin-4-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004018 (3-(2-(1-benzylpiperidin-4-yl)ethyl)quinazoline-2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003999 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-chloroquinaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003996 (CHEMBL126354 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004011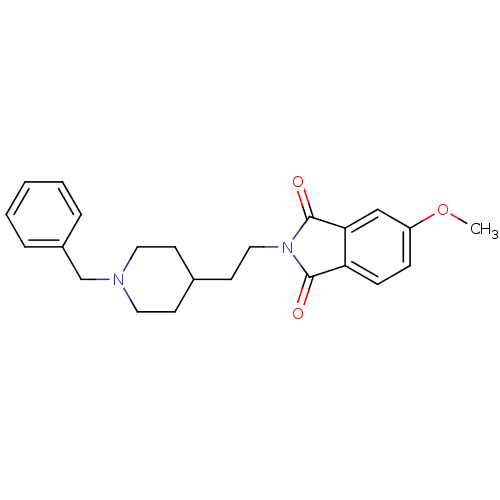 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxyisoin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against butyrylcholinesterase (BuChE) obtained from rat plasma | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003998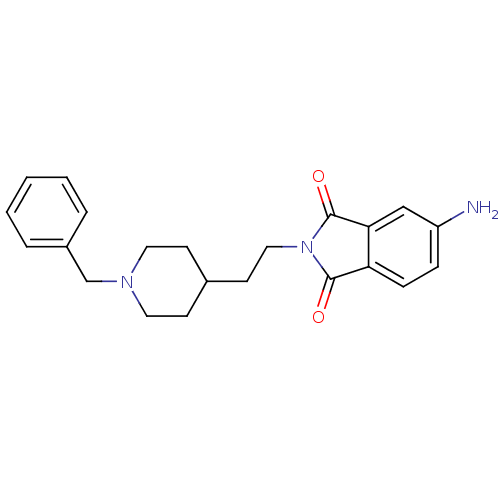 (5-Amino-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-isoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004009 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-4-nitroisoindo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50326129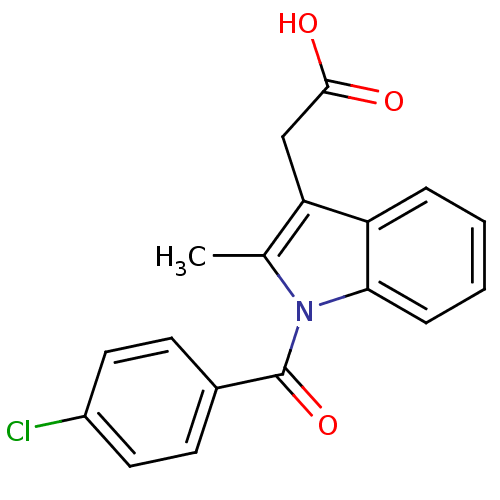 (Indometacin) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 by colorimetric assay | J Med Chem 55: 8152-63 (2012) Article DOI: 10.1021/jm301084z BindingDB Entry DOI: 10.7270/Q2BC40NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004015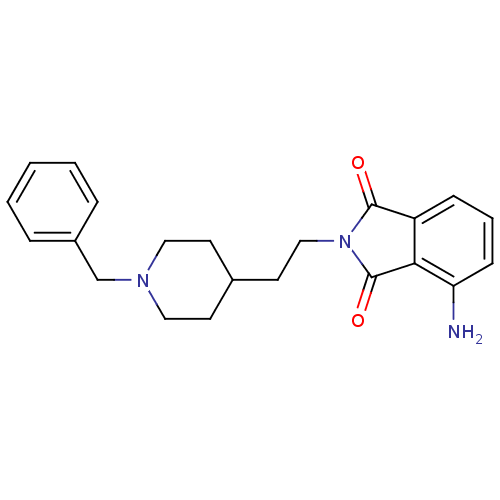 (4-Amino-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-isoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004033 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004040 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-5-nitroisoindo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004013 (6-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-pyrrolo[3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004026 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-1,2-dihydroiso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004037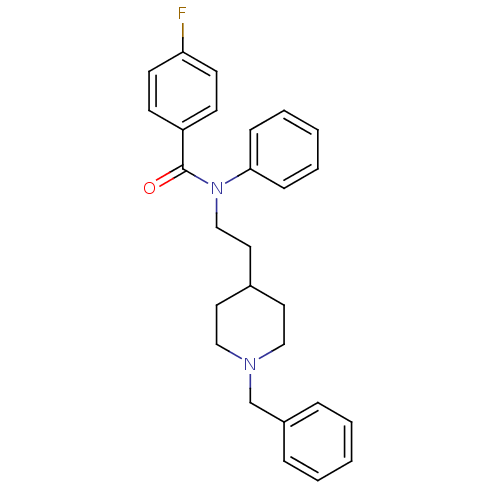 (CHEMBL140737 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125997 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004012 (2-(2-(1-benzylpiperidin-4-yl)ethyl)isoquinoline-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50126003 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9397 (CHEMBL59583 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Tsukuba Research Laboratories | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... | J Med Chem 33: 1880-7 (1990) Article DOI: 10.1021/jm00169a008 BindingDB Entry DOI: 10.7270/Q20Z71H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004020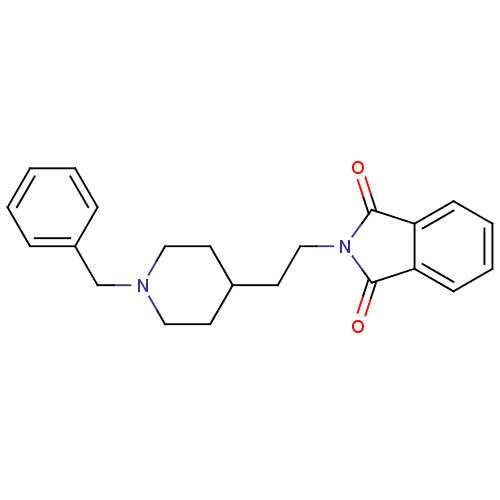 (2-(2-(1-benzylpiperidin-4-yl)ethyl)isoindoline-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 179 total ) | Next | Last >> |