 Found 384 hits with Last Name = 'schaefer' and Initial = 'm'
Found 384 hits with Last Name = 'schaefer' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50555575
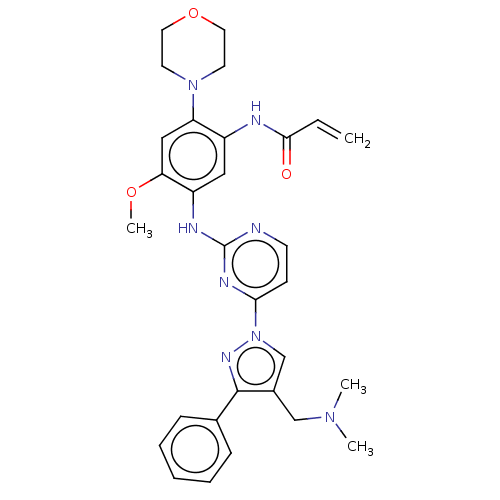
(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402859
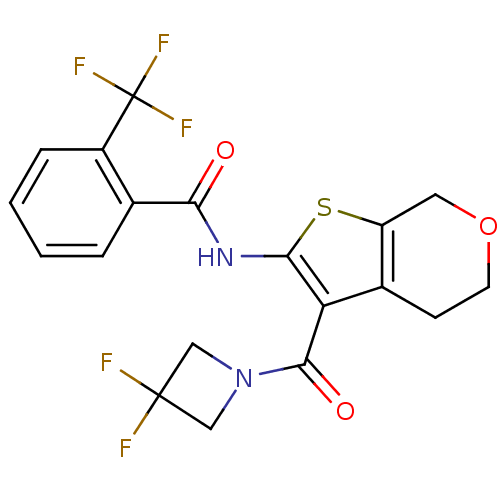
(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402864
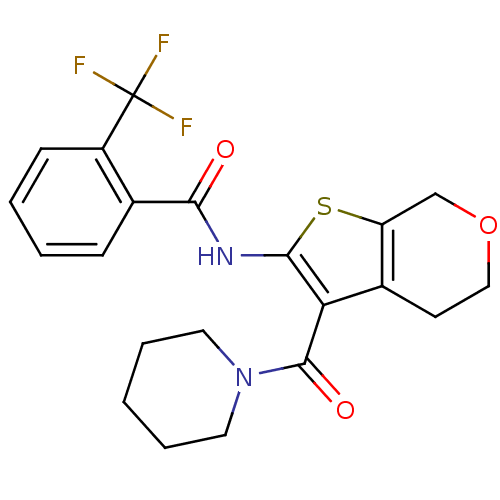
(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50555575

(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402863
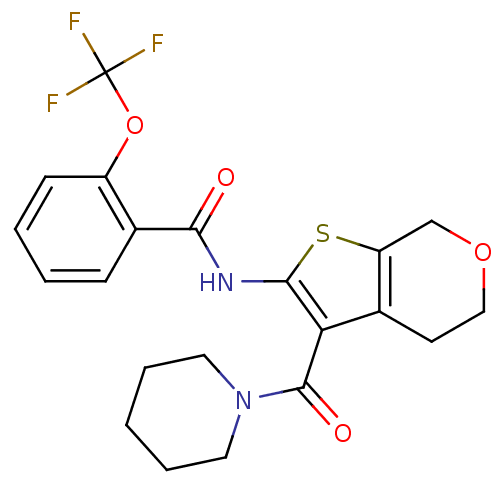
(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 434 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50555575

(C-18112003-G | GNS-1480 | GNS1480 | JNJ-73841937-A...)Show SMILES COc1cc(N2CCOCC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-n1cc(CN(C)C)c(n1)-c1ccccc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00213
BindingDB Entry DOI: 10.7270/Q2BG2T07 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 899 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402861
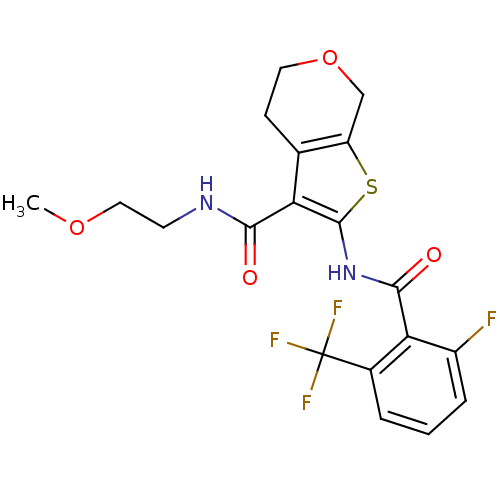
(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 904 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402864

(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402861

(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402863

(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402864

(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402864

(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402863

(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402863

(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402861

(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402861

(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
3-oxoacyl-[acyl-carrier-protein] synthase 1 [C171Q]
(Mycobacterium tuberculosis) | BDBM214777
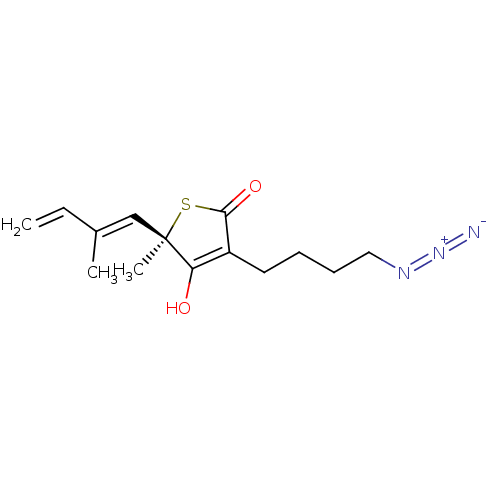
(TLM18)Show SMILES C\C(C=C)=C/[C@@]1(C)SC(=O)C(CCCCN=[N+]=[N-])=C1O |r,c:17| Show InChI InChI=1S/C14H19N3O2S/c1-4-10(2)9-14(3)12(18)11(13(19)20-14)7-5-6-8-16-17-15/h4,9,18H,1,5-8H2,2-3H3/b10-9+/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
University of Wuerzburg
| Assay Description
Binding of TLM18 to wild-type and mutant KasA was quantified by monitoring changes in the intrinsic tryptophan fluorescence of the enzyme at waveleng... |
J Biol Chem 288: 34190-204 (2013)
Article DOI: 10.1074/jbc.M113.511436
BindingDB Entry DOI: 10.7270/Q22F7M8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM254887
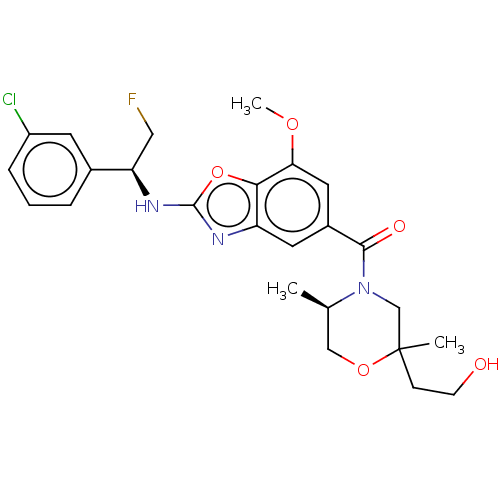
(US9493472, 10)Show SMILES COc1cc(cc2nc(N[C@H](CF)c3cccc(Cl)c3)oc12)C(=O)N1CC(C)(CCO)OC[C@H]1C |r| Show InChI InChI=1S/C25H29ClFN3O5/c1-15-13-34-25(2,7-8-31)14-30(15)23(32)17-10-19-22(21(11-17)33-3)35-24(28-19)29-20(12-27)16-5-4-6-18(26)9-16/h4-6,9-11,15,20,31H,7-8,12-14H2,1-3H3,(H,28,29)/t15-,20-,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01035
BindingDB Entry DOI: 10.7270/Q2Z60SND |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50547821
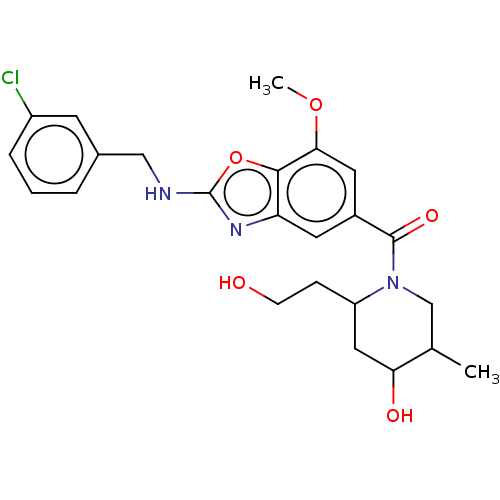
(CHEMBL4761137)Show SMILES COc1cc(cc2nc(NCc3cccc(Cl)c3)oc12)C(=O)N1CC(C)C(O)CC1CCO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01035
BindingDB Entry DOI: 10.7270/Q2Z60SND |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM254899
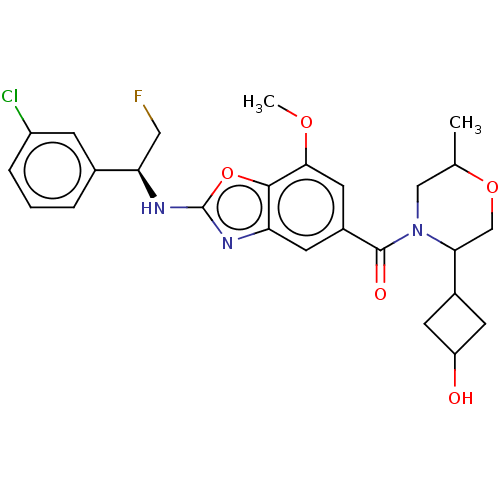
(US9493472, 21)Show SMILES COc1cc(cc2nc(N[C@H](CF)c3cccc(Cl)c3)oc12)C(=O)N1CC(C)OCC1C1CC(O)C1 |r,wU:10.9,(-3.32,5.32,;-1.98,4.55,;-1.98,3.01,;-3.32,2.24,;-3.32,.7,;-1.98,-.07,;-.65,.7,;.82,.23,;1.72,1.47,;3.26,1.47,;4.03,.14,;3.26,-1.19,;4.03,-2.53,;5.57,.14,;6.34,1.47,;7.88,1.47,;8.65,.14,;7.88,-1.19,;8.65,-2.53,;6.34,-1.19,;.82,2.72,;-.65,2.24,;-4.65,-.07,;-4.65,-1.61,;-5.98,.7,;-5.98,2.24,;-7.32,3.01,;-7.32,4.55,;-8.65,2.24,;-8.65,.7,;-7.32,-.07,;-7.32,-1.61,;-8.41,-2.69,;-7.32,-3.78,;-7.32,-5.32,;-6.23,-2.69,)| Show InChI InChI=1S/C26H29ClFN3O5/c1-14-12-31(22(13-35-14)16-7-19(32)8-16)25(33)17-9-20-24(23(10-17)34-2)36-26(29-20)30-21(11-28)15-4-3-5-18(27)6-15/h3-6,9-10,14,16,19,21-22,32H,7-8,11-13H2,1-2H3,(H,29,30)/t14?,16?,19?,21-,22?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01035
BindingDB Entry DOI: 10.7270/Q2Z60SND |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50547825
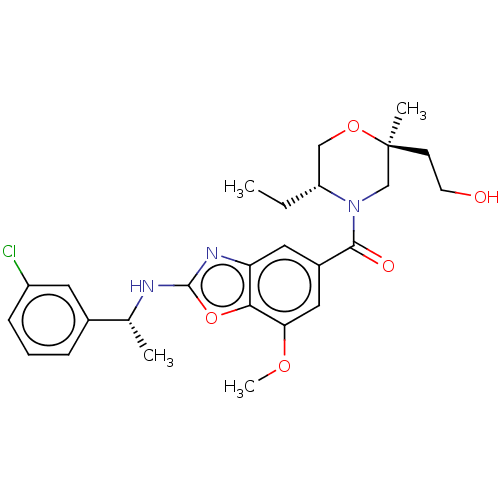
(CHEMBL4749829)Show SMILES CC[C@@H]1CO[C@@](C)(CCO)CN1C(=O)c1cc(OC)c2oc(N[C@H](C)c3cccc(Cl)c3)nc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01035
BindingDB Entry DOI: 10.7270/Q2Z60SND |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50547824
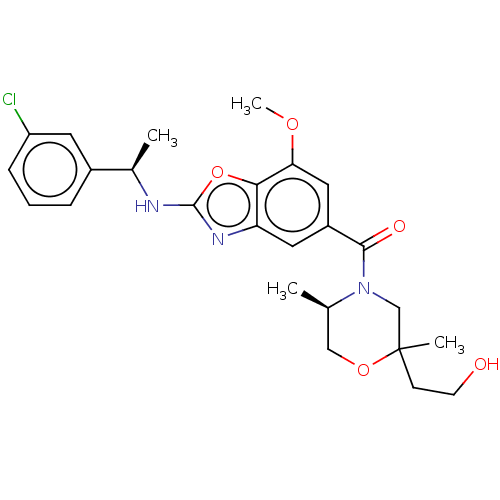
(CHEMBL4754786)Show SMILES COc1cc(cc2nc(N[C@H](C)c3cccc(Cl)c3)oc12)C(=O)N1CC(C)(CCO)OC[C@H]1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01035
BindingDB Entry DOI: 10.7270/Q2Z60SND |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104934
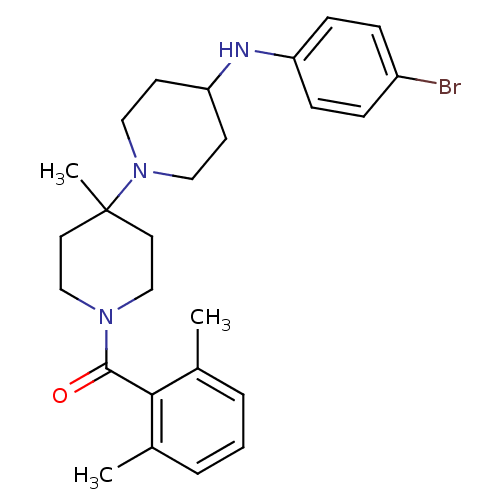
(CHEMBL292625 | [4-(4-Bromo-phenylamino)-4'-methyl-...)Show SMILES Cc1cccc(C)c1C(=O)N1CCC(C)(CC1)N1CCC(CC1)Nc1ccc(Br)cc1 Show InChI InChI=1S/C26H34BrN3O/c1-19-5-4-6-20(2)24(19)25(31)29-17-13-26(3,14-18-29)30-15-11-23(12-16-30)28-22-9-7-21(27)8-10-22/h4-10,23,28H,11-18H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity against monkey C-C chemokine receptor type 5. |
J Med Chem 47: 1939-55 (2004)
Article DOI: 10.1021/jm031046g
BindingDB Entry DOI: 10.7270/Q2V12478 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50143734
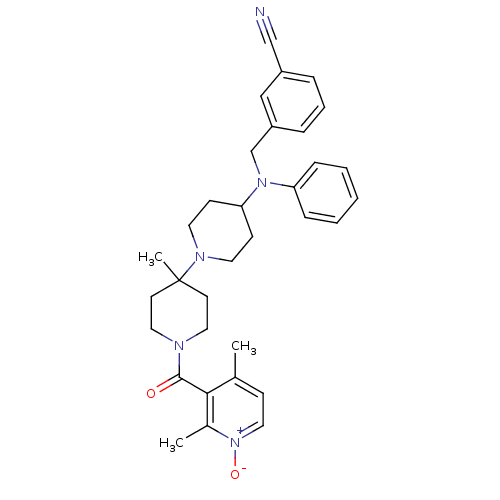
(3-({[1'-(2,4-Dimethyl-1-oxy-pyridine-3-carbonyl)-4...)Show SMILES Cc1cc[n+]([O-])c(C)c1C(=O)N1CCC(C)(CC1)N1CCC(CC1)N(Cc1cccc(c1)C#N)c1ccccc1 Show InChI InChI=1S/C33H39N5O2/c1-25-12-19-38(40)26(2)31(25)32(39)35-20-15-33(3,16-21-35)36-17-13-30(14-18-36)37(29-10-5-4-6-11-29)24-28-9-7-8-27(22-28)23-34/h4-12,19,22,30H,13-18,20-21,24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity against human C-C chemokine receptor type 5. |
J Med Chem 47: 1939-55 (2004)
Article DOI: 10.1021/jm031046g
BindingDB Entry DOI: 10.7270/Q2V12478 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50143736
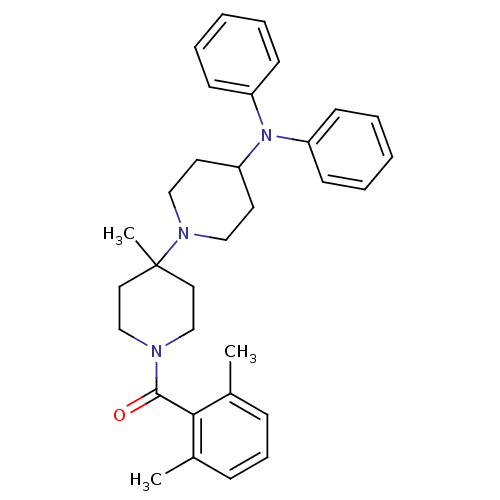
((2,6-Dimethyl-phenyl)-(4-diphenylamino-4'-methyl-[...)Show SMILES Cc1cccc(C)c1C(=O)N1CCC(C)(CC1)N1CCC(CC1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39N3O/c1-25-11-10-12-26(2)30(25)31(36)33-23-19-32(3,20-24-33)34-21-17-29(18-22-34)35(27-13-6-4-7-14-27)28-15-8-5-9-16-28/h4-16,29H,17-24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity against monkey C-C chemokine receptor type 5. |
J Med Chem 47: 1939-55 (2004)
Article DOI: 10.1021/jm031046g
BindingDB Entry DOI: 10.7270/Q2V12478 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50547836
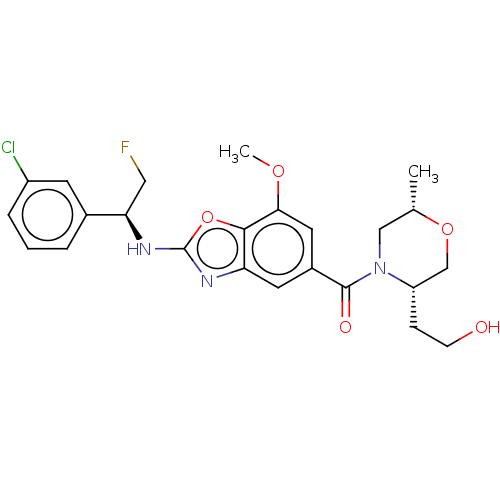
(CHEMBL4763593)Show SMILES COc1cc(cc2nc(N[C@H](CF)c3cccc(Cl)c3)oc12)C(=O)N1C[C@H](C)OC[C@@H]1CCO |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01035
BindingDB Entry DOI: 10.7270/Q2Z60SND |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data