

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290280 ((E)-Hepta-2,6-dienoic acid {2-[4-(2,4-dioxo-thiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290271 (CHEMBL432747 | Nonanoic acid {2-[4-(2,4-dioxo-thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290283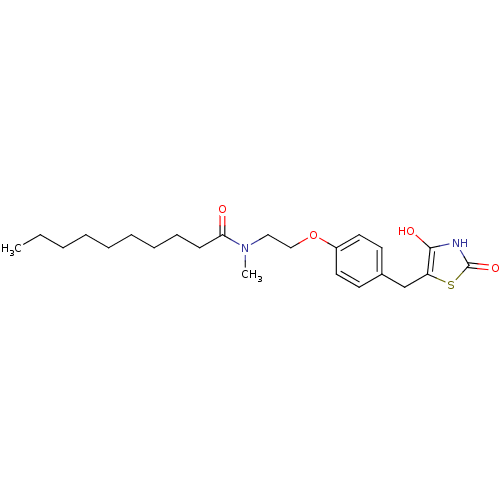 (CHEMBL82041 | Decanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290275 (CHEMBL83875 | Octanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290281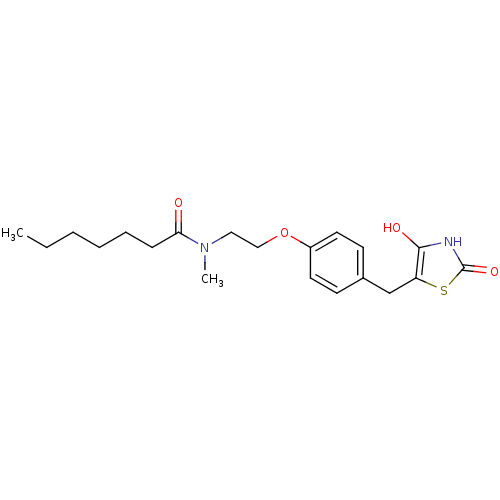 (CHEMBL84181 | Heptanoic acid {2-[4-(2,4-dioxo-thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290282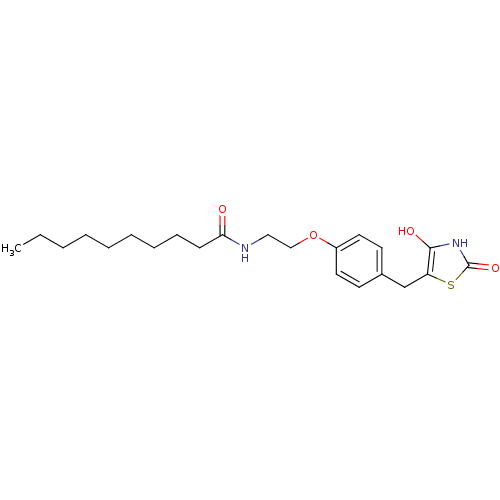 (CHEMBL84491 | Decanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290276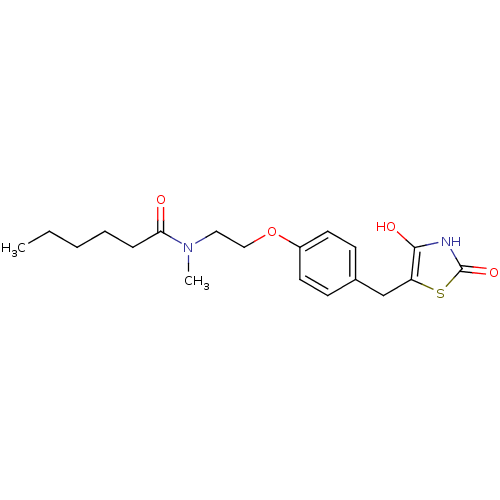 (CHEMBL81985 | Hexanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290285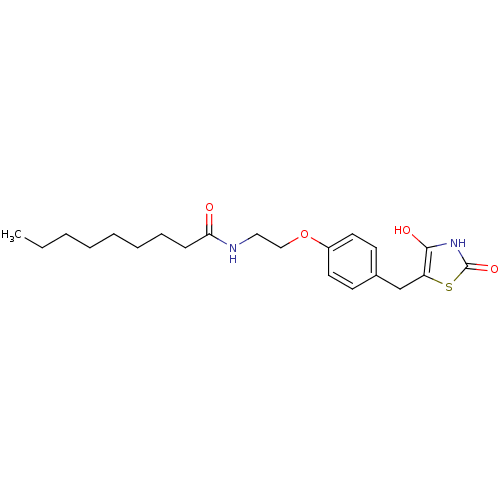 (CHEMBL83924 | Nonanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290284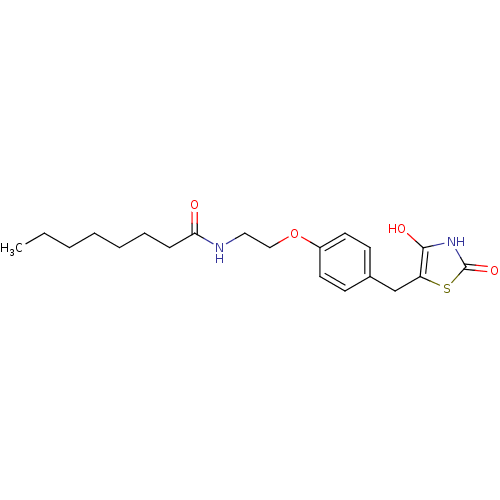 (CHEMBL79359 | Octanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290274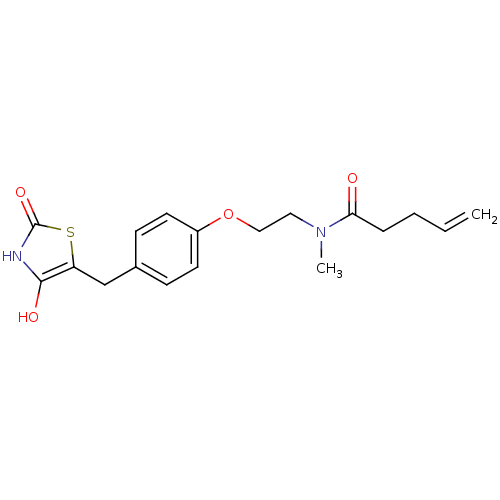 (CHEMBL420401 | Pent-4-enoic acid {2-[4-(2,4-dioxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290270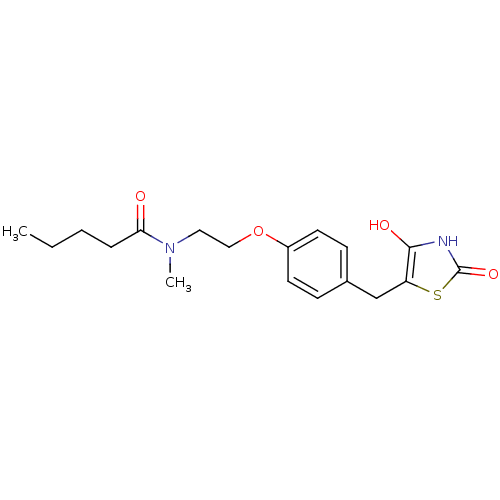 (CHEMBL83059 | Pentanoic acid {2-[4-(2,4-dioxo-thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290273 ((E)-Pent-2-enoic acid {2-[4-(2,4-dioxo-thiazolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290278 ((E)-Pent-3-enoic acid {2-[4-(2,4-dioxo-thiazolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290269 (CHEMBL83677 | Heptanoic acid {2-[4-(2,4-dioxo-thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290287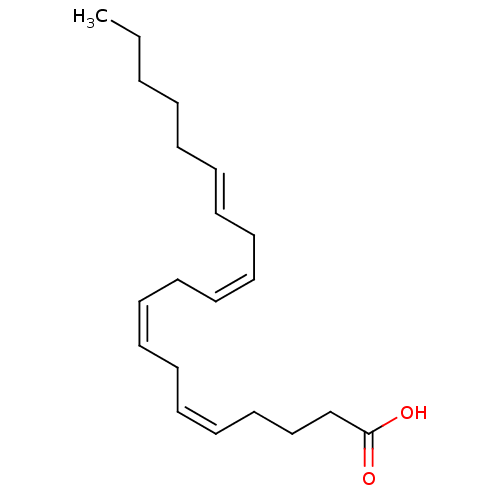 ((5Z,8Z,11Z,14E)-Icosa-5,8,11,14-tetraenoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290268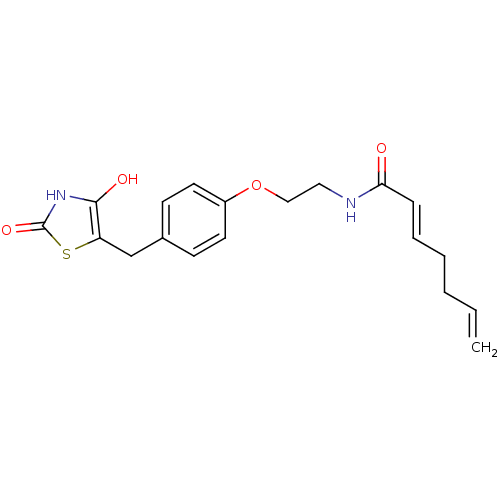 ((E)-Hepta-2,6-dienoic acid {2-[4-(2,4-dioxo-thiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290277 ((E)-Pent-2-enoic acid {2-[4-(2,4-dioxo-thiazolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290279 (CHEMBL84422 | Pent-4-enoic acid {2-[4-(2,4-dioxo-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290286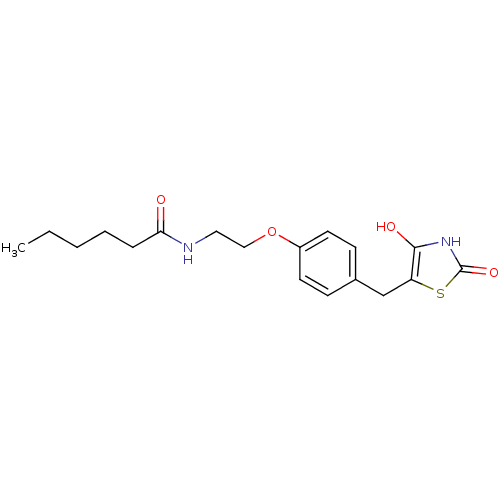 (CHEMBL83275 | Hexanoic acid {2-[4-(2,4-dioxo-thiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human Peroxisome proliferator activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290288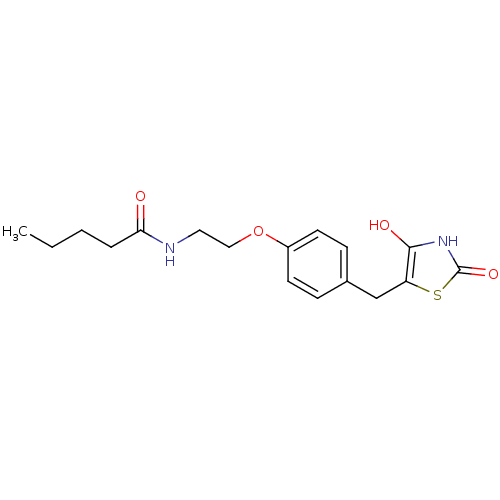 (CHEMBL84826 | Pentanoic acid {2-[4-(2,4-dioxo-thia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50290272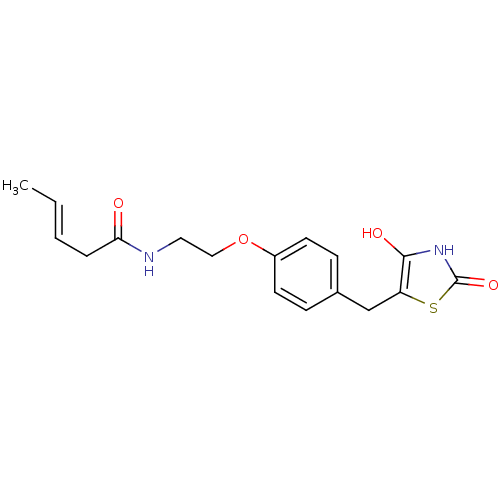 ((E)-Pent-3-enoic acid {2-[4-(2,4-dioxo-thiazolidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of 20 nM [3H]- thiazolidinedione from 4 nM biotinylated human peroxisome proliferator-activated recep... | Bioorg Med Chem Lett 7: 2491-2496 (1997) Article DOI: 10.1016/S0960-894X(97)10017-8 BindingDB Entry DOI: 10.7270/Q2DB81VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032181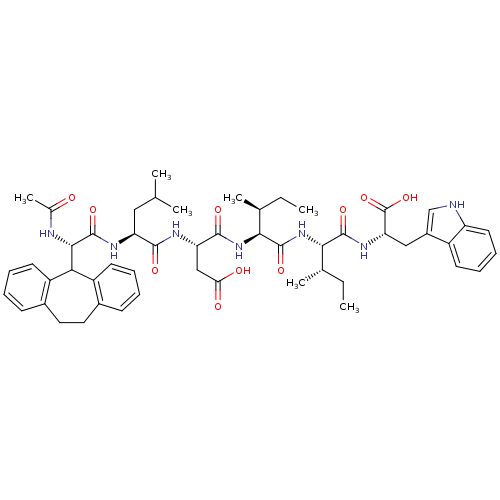 (2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032175 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032181 (2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in rabbit renal vascular smooth muscle cells | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032175 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032216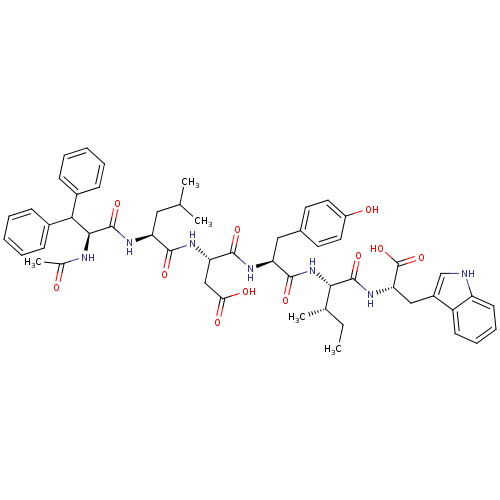 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032193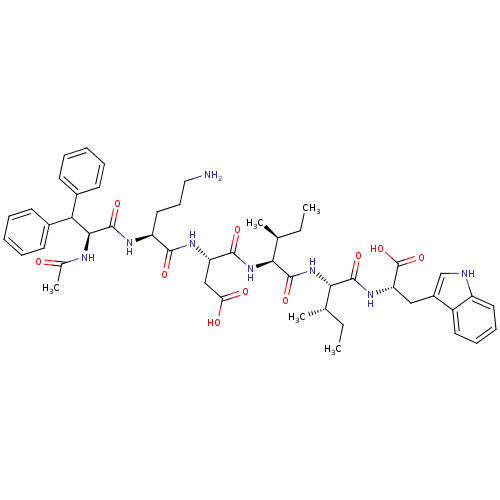 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032175 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in rabbit renal vascular smooth muscle cells | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032181 (2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032202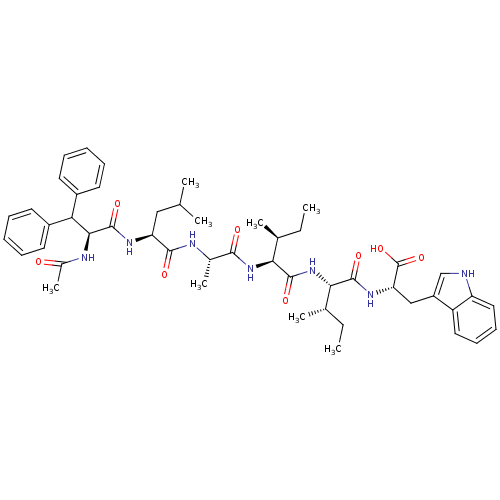 ((S)-2-[(2S,3S)-2-((2S,3S)-2-{(S)-2-[(S)-2-((S)-2-A...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032207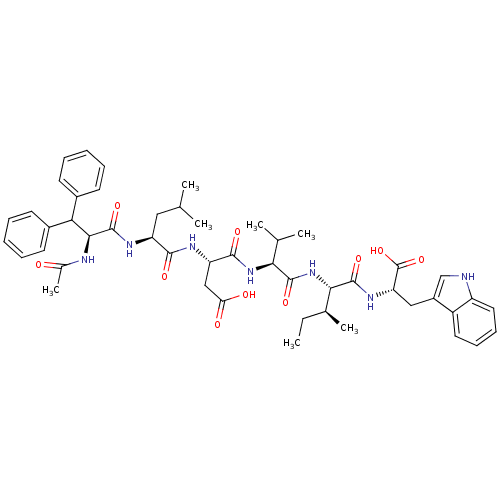 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032166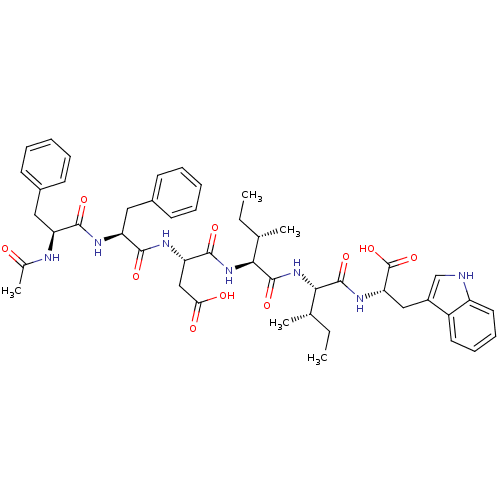 ((S)-3-[(S)-2-((S)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032172 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in CHO cells expressing rat ET- B receptors | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032178 ((S)-N-((1S,2S)-1-{(1S,2S)-1-[(S)-1-Carboxy-2-(1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in CHO cells expressing rat ET- B receptors | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032191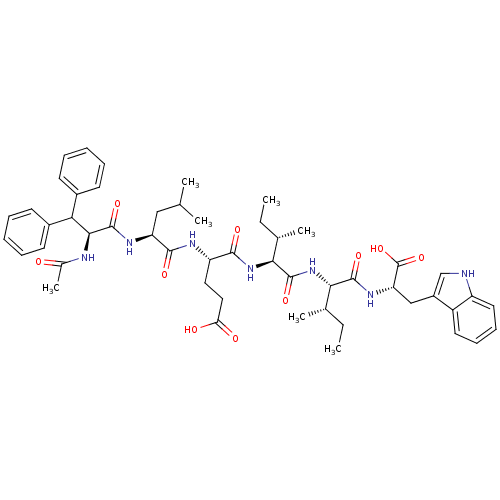 ((S)-4-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032171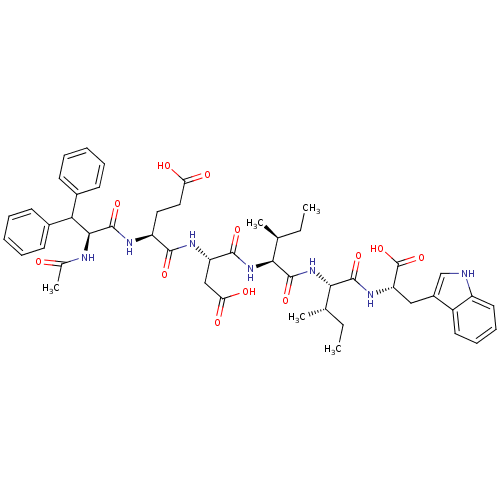 ((S)-4-((S)-2-Acetylamino-3,3-diphenyl-propionylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032207 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in rabbit renal vascular smooth muscle cells | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032193 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in rabbit renal vascular smooth muscle cells | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032173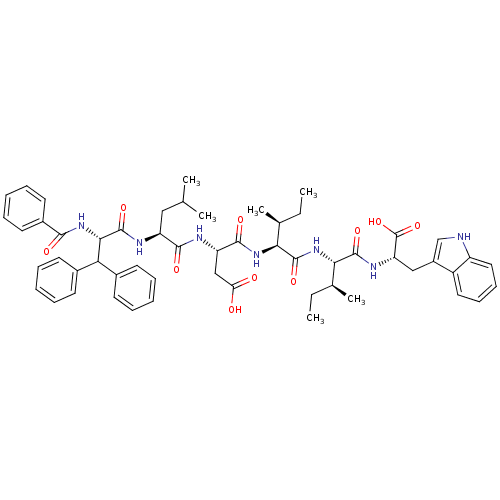 ((S)-3-[(S)-2-((S)-2-Benzoylamino-3,3-diphenyl-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032180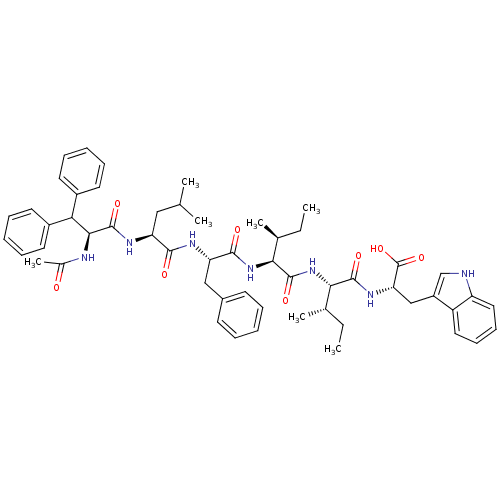 ((S)-2-[(2S,3S)-2-((2S,3S)-2-{(S)-2-[(S)-2-((S)-2-A...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032202 ((S)-2-[(2S,3S)-2-((2S,3S)-2-{(S)-2-[(S)-2-((S)-2-A...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032198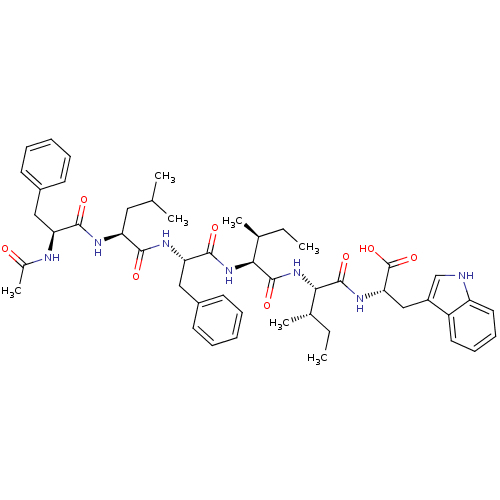 ((S)-2-[(2S,3S)-2-((2S,3S)-2-{(S)-2-[(S)-2-((S)-2-A...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50032198 ((S)-2-[(2S,3S)-2-((2S,3S)-2-{(S)-2-[(S)-2-((S)-2-A...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity measured in CHO cells stably transfected with the human endothelin B receptor | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032172 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032207 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032171 ((S)-4-((S)-2-Acetylamino-3,3-diphenyl-propionylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032208 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50032172 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50032191 ((S)-4-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 177 total ) | Next | Last >> |