 Found 3798 hits with Last Name = 'seo' and Initial = 'h'
Found 3798 hits with Last Name = 'seo' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bromodomain-containing protein 3 [306-416]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [333-460]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [348-455]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Isoform C of Bromodomain-containing protein 4 (Short)
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194,348-455]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144,306-416]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168,333-460]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194,348-455]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [348-455]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [333-460]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Isoform C of Bromodomain-containing protein 4 (Short)
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [251-382]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [306-416]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137,251-382]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [251-382]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168,333-460]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144,306-416]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137,251-382]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50069447
(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50135168
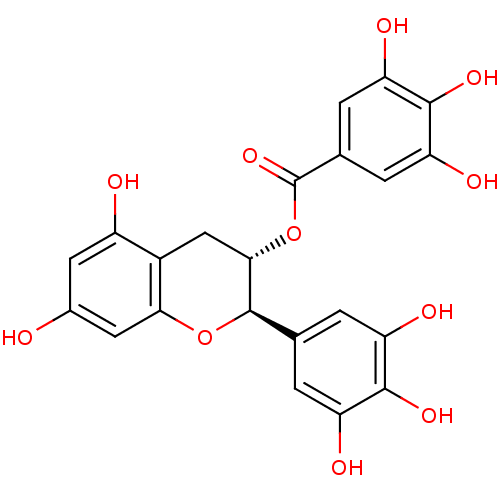
(3,4,5-Trihydroxy-benzoic acid (2R,3S)-5,7-dihydrox...)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of human Beta-secretase 1 |
Bioorg Med Chem Lett 13: 3905-8 (2003)
BindingDB Entry DOI: 10.7270/Q28K78HN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50135163
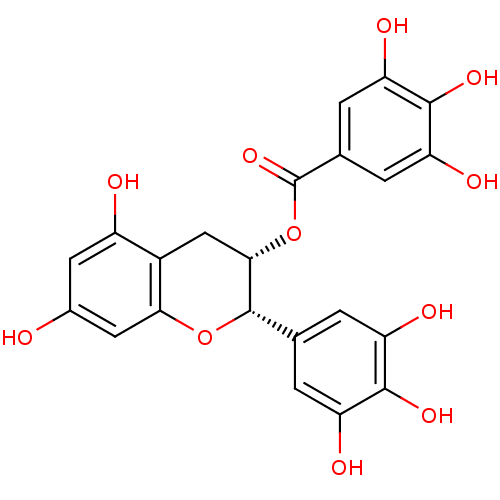
(3,4,5-Trihydroxy-benzoic acid (2S,3S)-5,7-dihydrox...)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of human Beta-secretase 1 |
Bioorg Med Chem Lett 13: 3905-8 (2003)
BindingDB Entry DOI: 10.7270/Q28K78HN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50069447

(1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...)Show SMILES CCc1nn(CCCN2CCN(CC2)c2cccc(Cl)c2)c(=O)n1CCOc1ccccc1 Show InChI InChI=1S/C25H32ClN5O2/c1-2-24-27-31(25(32)30(24)18-19-33-23-10-4-3-5-11-23)13-7-12-28-14-16-29(17-15-28)22-9-6-8-21(26)20-22/h3-6,8-11,20H,2,7,12-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to SERT |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
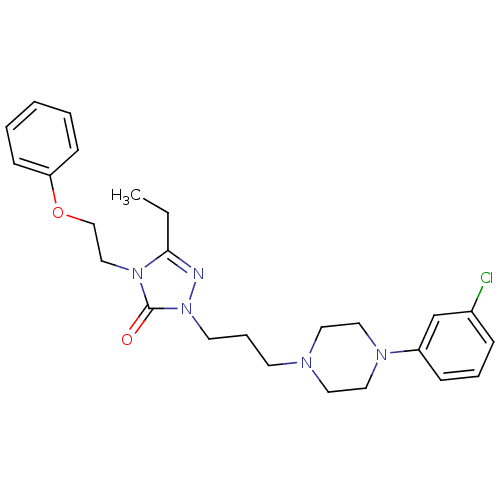
Proteinase-activated receptor 2
(Homo sapiens (Human)) | BDBM50134199
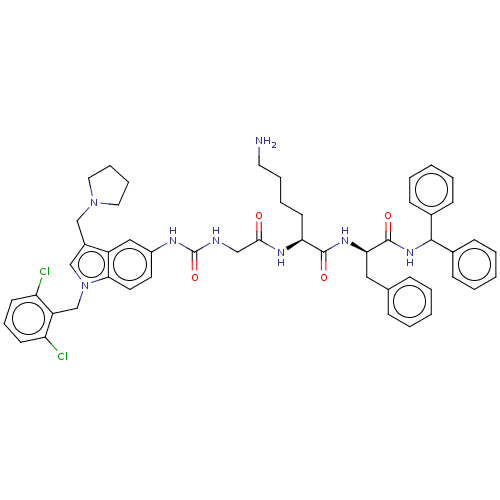
(CHEMBL3735057)Show SMILES NCCCC[C@H](NC(=O)CNC(=O)Nc1ccc2n(Cc3c(Cl)cccc3Cl)cc(CN3CCCC3)c2c1)C(=O)N[C@H](Cc1ccccc1)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C51H56Cl2N8O4/c52-42-21-14-22-43(53)41(42)34-61-33-38(32-60-27-12-13-28-60)40-30-39(24-25-46(40)61)56-51(65)55-31-47(62)57-44(23-10-11-26-54)49(63)58-45(29-35-15-4-1-5-16-35)50(64)59-48(36-17-6-2-7-18-36)37-19-8-3-9-20-37/h1-9,14-22,24-25,30,33,44-45,48H,10-13,23,26-29,31-32,34,54H2,(H,57,62)(H,58,63)(H,59,64)(H2,55,56,65)/t44-,45+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells |
Bioorg Med Chem 23: 7717-27 (2015)
Article DOI: 10.1016/j.bmc.2015.11.016
BindingDB Entry DOI: 10.7270/Q2VH5QP3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267969

(Sargahydroquinoic Acid)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#8])cc(-[#6])c1-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C27H38O4/c1-19(2)9-6-13-23(27(30)31)14-8-12-20(3)10-7-11-21(4)15-16-24-18-25(28)17-22(5)26(24)29/h9-10,14-15,17-18,28-29H,6-8,11-13,16H2,1-5H3,(H,30,31)/b20-10+,21-15+,23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM246524

(Psoralidin (5))Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2c3oc4cc(-[#8])ccc4c3c(=O)oc2cc1-[#8] Show InChI InChI=1S/C20H16O5/c1-10(2)3-4-11-7-14-17(9-15(11)22)25-20(23)18-13-6-5-12(21)8-16(13)24-19(14)18/h3,5-9,21-22H,4H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Gyeongsang National University
| Assay Description
Reactions were performed in a total volume of 200 mL, which contained thefollowing components: 20mM Tris-buffer, pH 8.0, 10mM DTT, 30 mM Z-RLRGG-AMC,... |
J Enzyme Inhib Med Chem 29: 59-63 (2014)
Article DOI: 10.3109/14756366.2012.753591
BindingDB Entry DOI: 10.7270/Q21835FQ |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 2
(Homo sapiens (Human)) | BDBM50134200
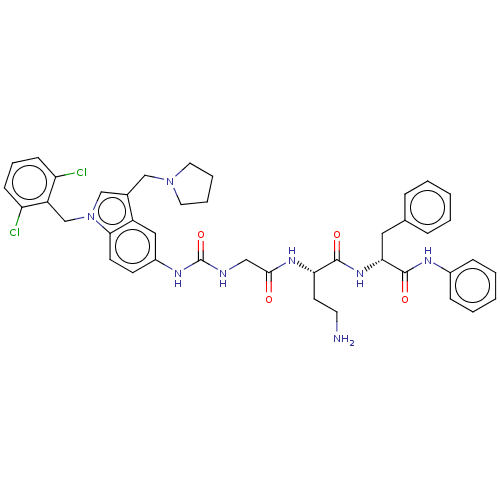
(CHEMBL3735405)Show SMILES NCC[C@H](NC(=O)CNC(=O)Nc1ccc2n(Cc3c(Cl)cccc3Cl)cc(CN3CCCC3)c2c1)C(=O)N[C@H](Cc1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C42H46Cl2N8O4/c43-34-14-9-15-35(44)33(34)27-52-26-29(25-51-20-7-8-21-51)32-23-31(16-17-38(32)52)48-42(56)46-24-39(53)49-36(18-19-45)40(54)50-37(22-28-10-3-1-4-11-28)41(55)47-30-12-5-2-6-13-30/h1-6,9-17,23,26,36-37H,7-8,18-22,24-25,27,45H2,(H,47,55)(H,49,53)(H,50,54)(H2,46,48,56)/t36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells |
Bioorg Med Chem 23: 7717-27 (2015)
Article DOI: 10.1016/j.bmc.2015.11.016
BindingDB Entry DOI: 10.7270/Q2VH5QP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241345
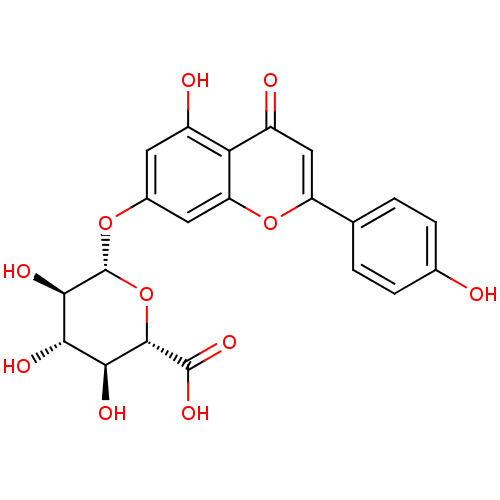
(Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...)Show SMILES O[C@H]1[C@H](Oc2cc(O)c3c(c2)oc(cc3=O)-c2ccc(O)cc2)O[C@@H]([C@@H](O)[C@@H]1O)C(O)=O |r| Show InChI InChI=1S/C21H18O11/c22-9-3-1-8(2-4-9)13-7-12(24)15-11(23)5-10(6-14(15)31-13)30-21-18(27)16(25)17(26)19(32-21)20(28)29/h1-7,16-19,21-23,25-27H,(H,28,29)/t16-,17-,18+,19-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
| Assay Description
The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... |
Bioorg Chem 72: 293-300 (2017)
BindingDB Entry DOI: 10.7270/Q2Q52NH6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM4078
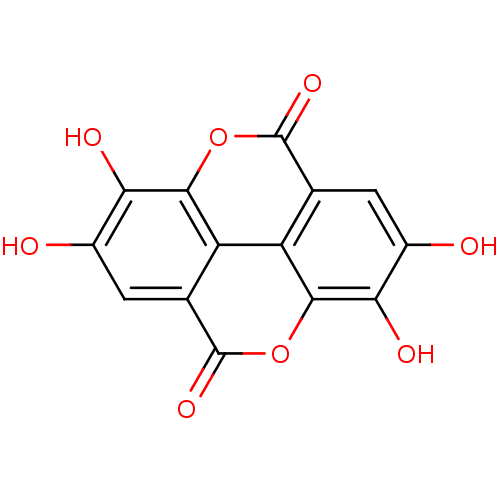
(6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...)Show InChI InChI=1S/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
| Assay Description
The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... |
Bioorg Chem 72: 293-300 (2017)
BindingDB Entry DOI: 10.7270/Q2Q52NH6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50494304
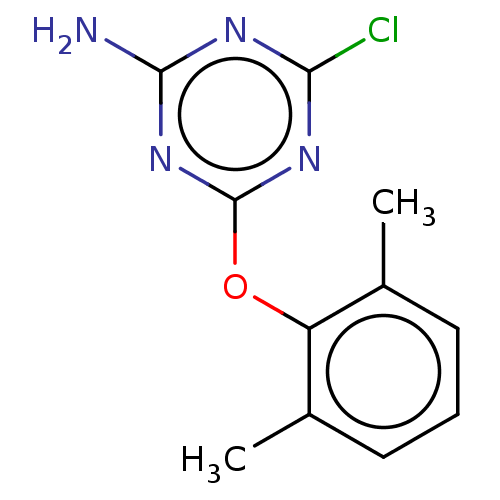
(CHEMBL3086710)Show InChI InChI=1S/C11H11ClN4O/c1-6-4-3-5-7(2)8(6)17-11-15-9(12)14-10(13)16-11/h3-5H,1-2H3,(H2,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Hsp90alpha ATP-binding pocket of N-terminal domain after 4 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 6427-31 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.050
BindingDB Entry DOI: 10.7270/Q29Z97VK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267968
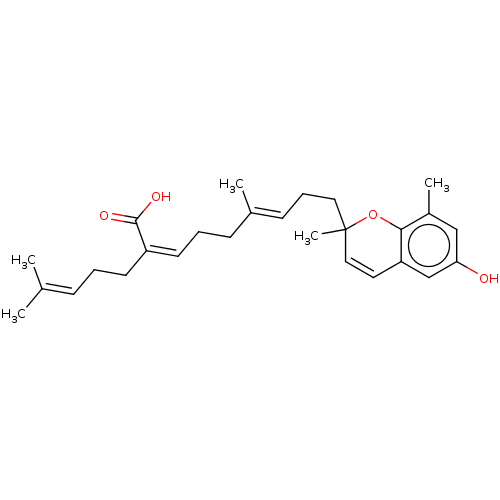
(CHEMBL4085945)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]C1([#6])[#8]-c2c(-[#6])cc(-[#8])cc2-[#6]=[#6]1)-[#6](-[#8])=O |c:27| Show InChI InChI=1S/C27H36O4/c1-19(2)9-6-12-22(26(29)30)13-7-10-20(3)11-8-15-27(5)16-14-23-18-24(28)17-21(4)25(23)31-27/h9,11,13-14,16-18,28H,6-8,10,12,15H2,1-5H3,(H,29,30)/b20-11+,22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM226157

(PTP1B spring 7 (7))Show SMILES COc1ccc(-c2ccc(O)cc2)c(C(c2ccc(O)cc2)c2ccc(O)cc2)c1C#Cc1ccc(O)cc1 Show InChI InChI=1S/C34H26O5/c1-39-32-21-20-30(23-5-13-27(36)14-6-23)34(31(32)19-4-22-2-11-26(35)12-3-22)33(24-7-15-28(37)16-8-24)25-9-17-29(38)18-10-25/h2-3,5-18,20-21,33,35-38H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.00E+3 | -32.8 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Catholic University of Daegu
| Assay Description
In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... |
Bioorg Chem 72: 273-281 (2017)
BindingDB Entry DOI: 10.7270/Q2ZP450S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50278899
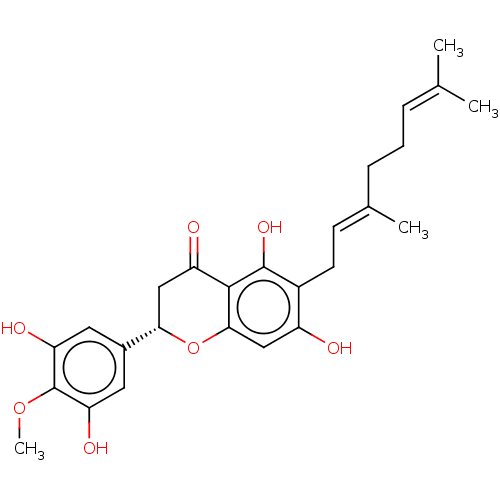
(CHEMBL4174694)Show SMILES [#6]-[#8]-c1c(-[#8])cc(cc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc2-[#8]-1 |r| Show InChI InChI=1S/C26H30O7/c1-14(2)6-5-7-15(3)8-9-17-18(27)12-23-24(25(17)31)19(28)13-22(33-23)16-10-20(29)26(32-4)21(30)11-16/h6,8,10-12,22,27,29-31H,5,7,9,13H2,1-4H3/b15-8+/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... |
J Nat Prod 80: 2659-2665 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00325
BindingDB Entry DOI: 10.7270/Q2RX9FK5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data