 Found 3983 hits with Last Name = 'shafer' and Initial = 'c'
Found 3983 hits with Last Name = 'shafer' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265746
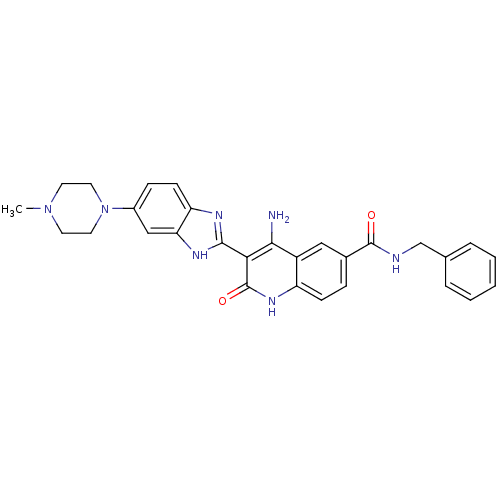
(CHEMBL526507 | {4-Amino-3-[6-(4-methylpiperazinyl)...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2cc(ccc2[nH]c1=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C29H29N7O2/c1-35-11-13-36(14-12-35)20-8-10-23-24(16-20)33-27(32-23)25-26(30)21-15-19(7-9-22(21)34-29(25)38)28(37)31-17-18-5-3-2-4-6-18/h2-10,15-16H,11-14,17H2,1H3,(H,31,37)(H,32,33)(H3,30,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265774
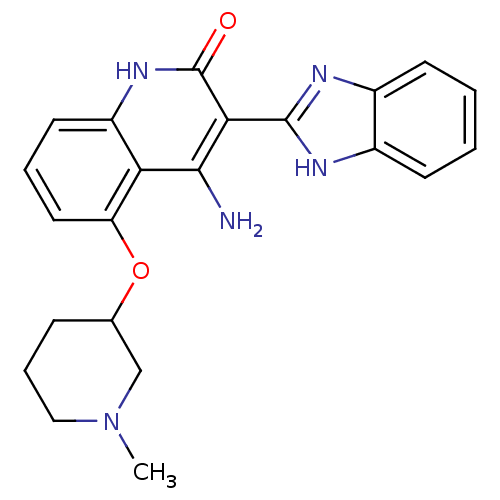
(4-Amino-3-benzimidazol-2-yl-5-(1-methyl(3-piperidy...)Show SMILES CN1CCCC(C1)Oc1cccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N)c12 Show InChI InChI=1S/C22H23N5O2/c1-27-11-5-6-13(12-27)29-17-10-4-9-16-18(17)20(23)19(22(28)26-16)21-24-14-7-2-3-8-15(14)25-21/h2-4,7-10,13H,5-6,11-12H2,1H3,(H,24,25)(H3,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265574
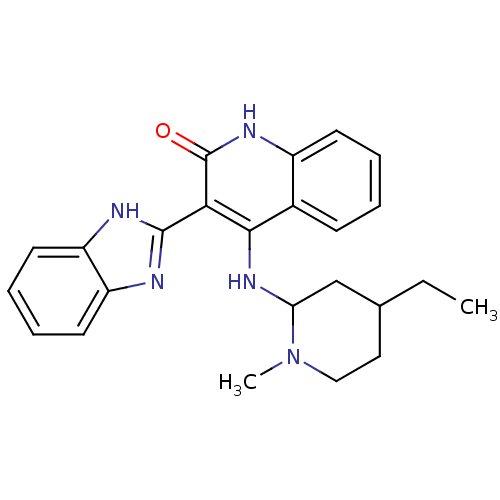
(3-(1H-benzo[d]imidazol-2-yl)-4-(4-ethyl-1-methylpi...)Show SMILES CCC1CCN(C)C(C1)Nc1c(-c2nc3ccccc3[nH]2)c(=O)[nH]c2ccccc12 Show InChI InChI=1S/C24H27N5O/c1-3-15-12-13-29(2)20(14-15)28-22-16-8-4-5-9-17(16)27-24(30)21(22)23-25-18-10-6-7-11-19(18)26-23/h4-11,15,20H,3,12-14H2,1-2H3,(H,25,26)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452149
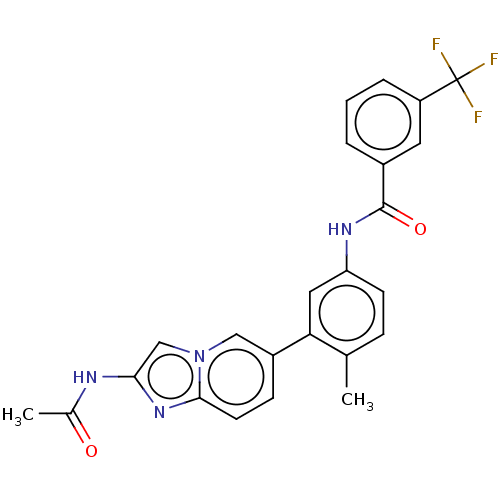
(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50467097

(CHEMBL4292020)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1c(cnc2[nH]ccc12)-c1cc(F)c(O)c(F)c1 Show InChI InChI=1S/C24H22F2N4O/c1-29-8-10-30(11-9-29)17-4-2-15(3-5-17)22-18-6-7-27-24(18)28-14-19(22)16-12-20(25)23(31)21(26)13-16/h2-7,12-14,31H,8-11H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 3197-3201 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.020
BindingDB Entry DOI: 10.7270/Q2F76G78 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452152
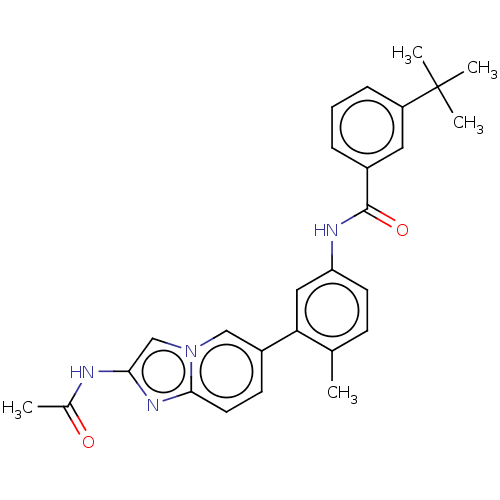
(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265773
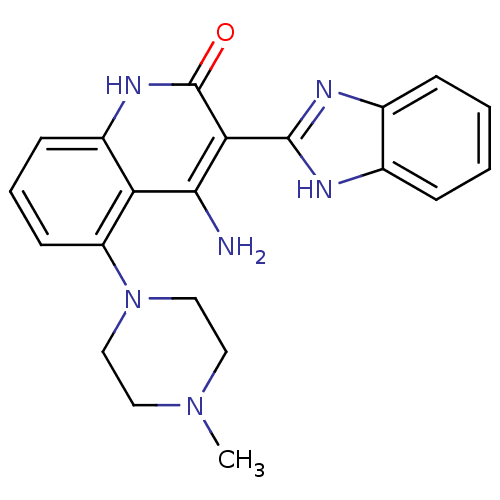
(4-Amino-3-benzimidazol-2-yl-5-(4-methylpiperazinyl...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N)c12 Show InChI InChI=1S/C21H22N6O/c1-26-9-11-27(12-10-26)16-8-4-7-15-17(16)19(22)18(21(28)25-15)20-23-13-5-2-3-6-14(13)24-20/h2-8H,9-12H2,1H3,(H,23,24)(H3,22,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265678
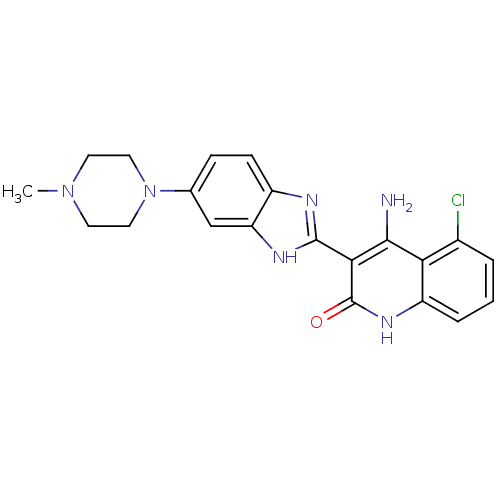
(4-Amino-5-chloro-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(Cl)cccc2[nH]c1=O Show InChI InChI=1S/C21H21ClN6O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452150
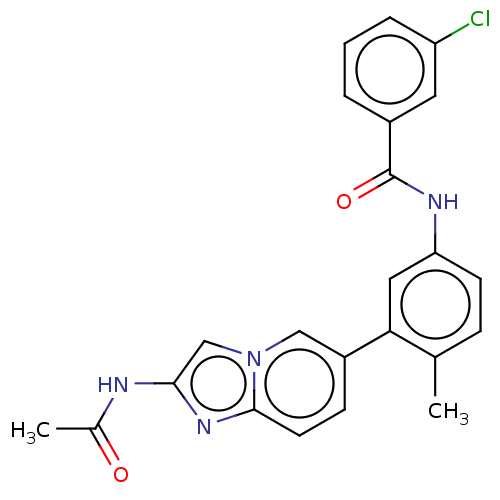
(CHEMBL4216386)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(Cl)c2)ccc1C Show InChI InChI=1S/C23H19ClN4O2/c1-14-6-8-19(26-23(30)16-4-3-5-18(24)10-16)11-20(14)17-7-9-22-27-21(25-15(2)29)13-28(22)12-17/h3-13H,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265750
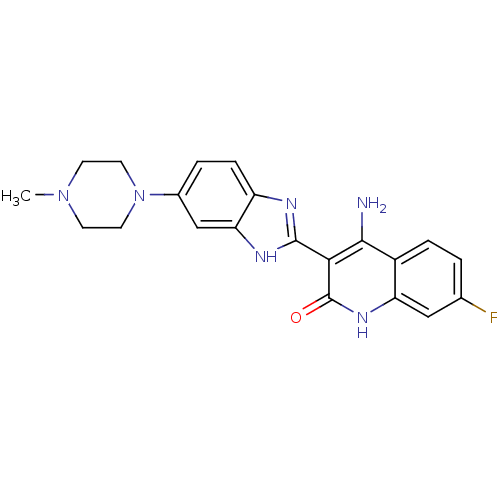
(4-Amino-7-fluoro-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2ccc(F)cc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-6-8-28(9-7-27)13-3-5-15-17(11-13)25-20(24-15)18-19(23)14-4-2-12(22)10-16(14)26-21(18)29/h2-5,10-11H,6-9H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026678
(CHEMBL3335374)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(OC(F)(F)F)c4)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O3/c1-13(31)27-20-12-16(8-9-26-20)32-15-6-7-19-18(11-15)29-21(30(19)2)28-14-4-3-5-17(10-14)33-22(23,24)25/h3-12H,1-2H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026685

(CHEMBL3335373)Show SMILES CC(C)c1cccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)c1 Show InChI InChI=1S/C24H25N5O2/c1-15(2)17-6-5-7-18(12-17)27-24-28-21-13-19(8-9-22(21)29(24)4)31-20-10-11-25-23(14-20)26-16(3)30/h5-15H,1-4H3,(H,27,28)(H,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026695
(CHEMBL3335372)Show SMILES CCc1cccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)c1 Show InChI InChI=1S/C23H23N5O2/c1-4-16-6-5-7-17(12-16)26-23-27-20-13-18(8-9-21(20)28(23)3)30-19-10-11-24-22(14-19)25-15(2)29/h5-14H,4H2,1-3H3,(H,26,27)(H,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088
(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50243389
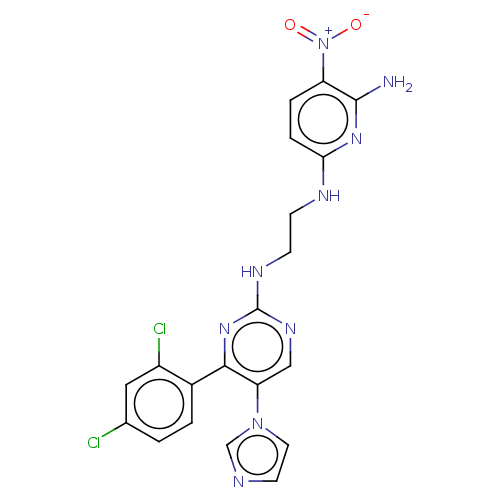
(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313013
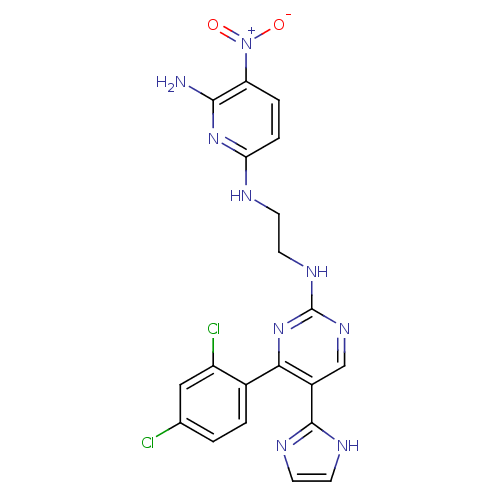
(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265745
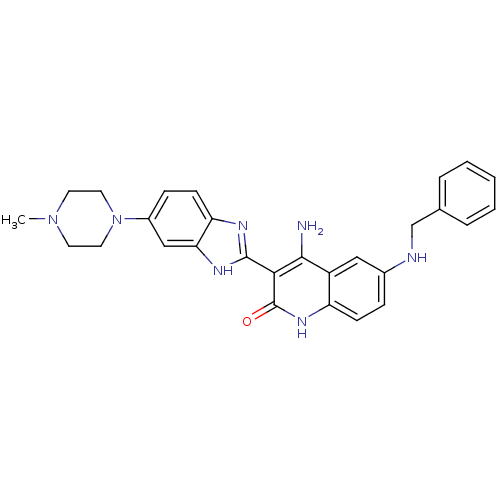
(4-Amino-6-(benzylamino)-3-(6-(4-methylpiperazin-1-...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2cc(NCc3ccccc3)ccc2[nH]c1=O Show InChI InChI=1S/C28H29N7O/c1-34-11-13-35(14-12-34)20-8-10-23-24(16-20)32-27(31-23)25-26(29)21-15-19(7-9-22(21)33-28(25)36)30-17-18-5-3-2-4-6-18/h2-10,15-16,30H,11-14,17H2,1H3,(H,31,32)(H3,29,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265679
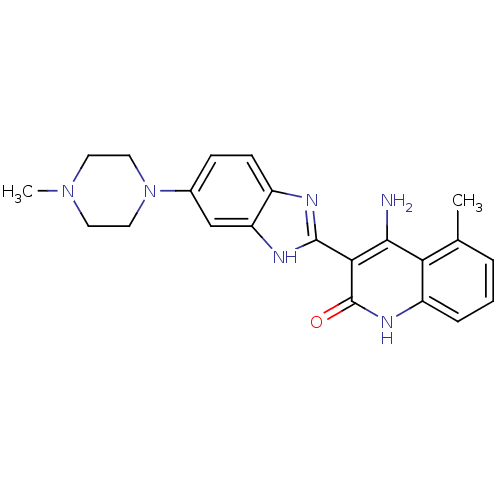
(4-Amino-5-methyl-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(C)cccc2[nH]c1=O Show InChI InChI=1S/C22H24N6O/c1-13-4-3-5-16-18(13)20(23)19(22(29)26-16)21-24-15-7-6-14(12-17(15)25-21)28-10-8-27(2)9-11-28/h3-7,12H,8-11H2,1-2H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500931
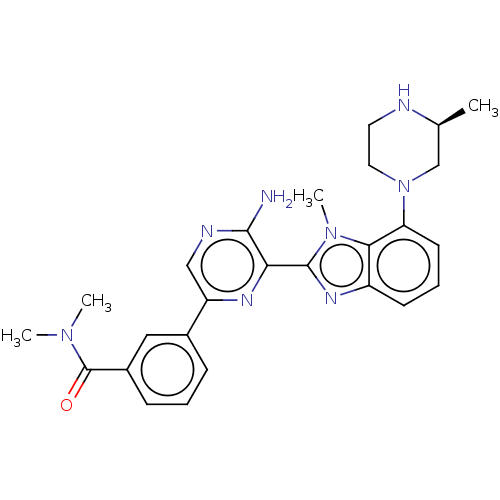
(CHEMBL3797873)Show SMILES C[C@H]1CN(CCN1)c1cccc2nc(-c3nc(cnc3N)-c3cccc(c3)C(=O)N(C)C)n(C)c12 |r| Show InChI InChI=1S/C26H30N8O/c1-16-15-34(12-11-28-16)21-10-6-9-19-23(21)33(4)25(31-19)22-24(27)29-14-20(30-22)17-7-5-8-18(13-17)26(35)32(2)3/h5-10,13-14,16,28H,11-12,15H2,1-4H3,(H2,27,29)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265641
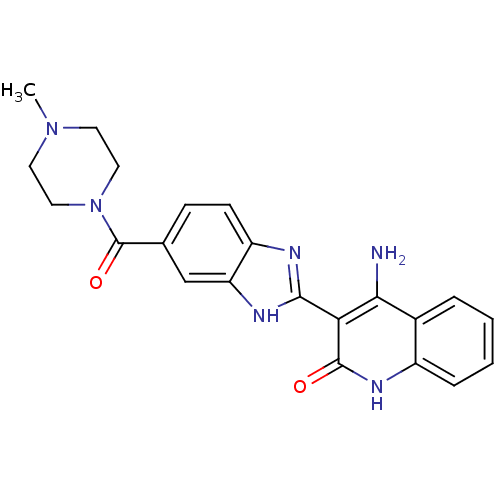
(4-Amino-3-{6-[(4-methylpiperazinyl)carbonyl]benzim...)Show SMILES CN1CCN(CC1)C(=O)c1ccc2nc([nH]c2c1)-c1c(N)c2ccccc2[nH]c1=O Show InChI InChI=1S/C22H22N6O2/c1-27-8-10-28(11-9-27)22(30)13-6-7-16-17(12-13)25-20(24-16)18-19(23)14-4-2-3-5-15(14)26-21(18)29/h2-7,12H,8-11H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452147
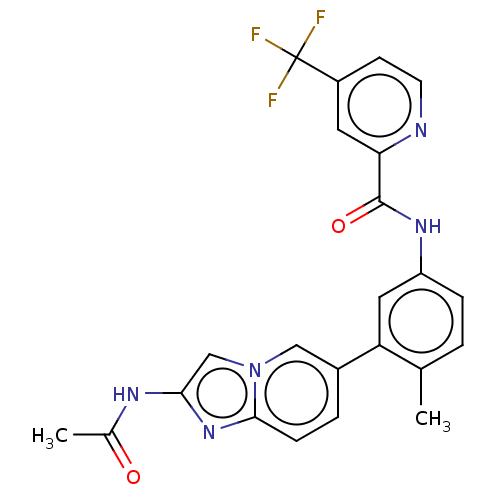
(CHEMBL4204192)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cc(ccn2)C(F)(F)F)ccc1C Show InChI InChI=1S/C23H18F3N5O2/c1-13-3-5-17(29-22(33)19-9-16(7-8-27-19)23(24,25)26)10-18(13)15-4-6-21-30-20(28-14(2)32)12-31(21)11-15/h3-12H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265613
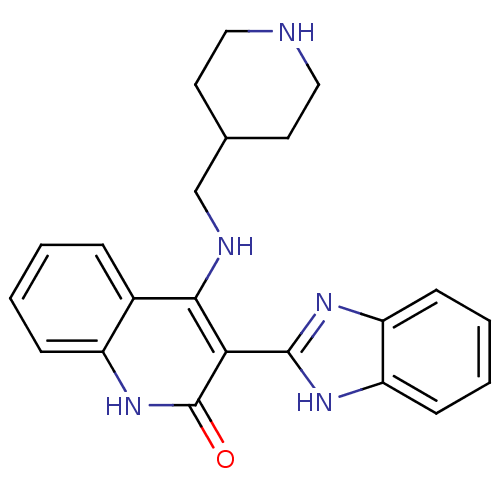
(3-(1H-benzo[d]imidazol-2-yl)-4-(piperidin-4-ylmeth...)Show SMILES O=c1[nH]c2ccccc2c(NCC2CCNCC2)c1-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H23N5O/c28-22-19(21-25-17-7-3-4-8-18(17)26-21)20(15-5-1-2-6-16(15)27-22)24-13-14-9-11-23-12-10-14/h1-8,14,23H,9-13H2,(H,25,26)(H2,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50484715
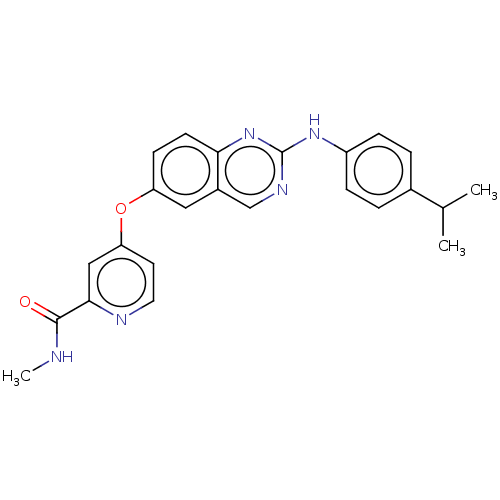
(CHEMBL1950893)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(cc4)C(C)C)ncc3c2)ccn1 Show InChI InChI=1S/C24H23N5O2/c1-15(2)16-4-6-18(7-5-16)28-24-27-14-17-12-19(8-9-21(17)29-24)31-20-10-11-26-22(13-20)23(30)25-3/h4-15H,1-3H3,(H,25,30)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus His6-tagged Braf V600E mutant expressed in insect Sf9 cells assessed as Mek phosphorylation after 2 hrs by ELISA |
Bioorg Med Chem Lett 22: 1678-81 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.112
BindingDB Entry DOI: 10.7270/Q27H1NF1 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26034
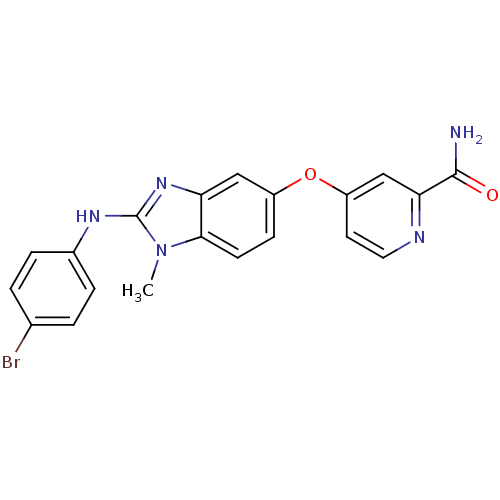
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(N)=O)ccc12 Show InChI InChI=1S/C20H16BrN5O2/c1-26-18-7-6-14(28-15-8-9-23-17(11-15)19(22)27)10-16(18)25-20(26)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50500936
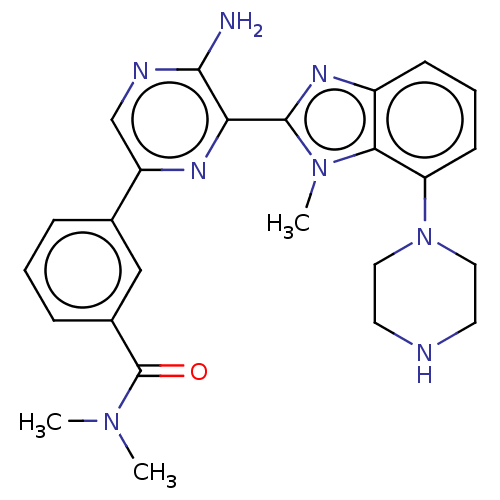
(CHEMBL3798762)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(n1)-c1nc2cccc(N3CCNCC3)c2n1C Show InChI InChI=1S/C25H28N8O/c1-31(2)25(34)17-7-4-6-16(14-17)19-15-28-23(26)21(29-19)24-30-18-8-5-9-20(22(18)32(24)3)33-12-10-27-11-13-33/h4-9,14-15,27H,10-13H2,1-3H3,(H2,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... |
J Med Chem 59: 3034-45 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01657
BindingDB Entry DOI: 10.7270/Q2RB77MH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26023
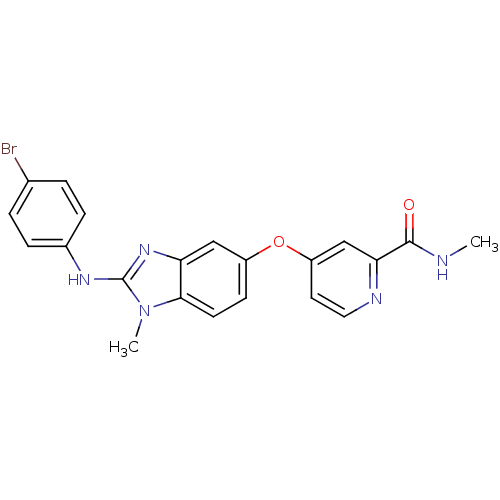
(4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(9-10-24-18)29-15-7-8-19-17(11-15)26-21(27(19)2)25-14-5-3-13(22)4-6-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM247849
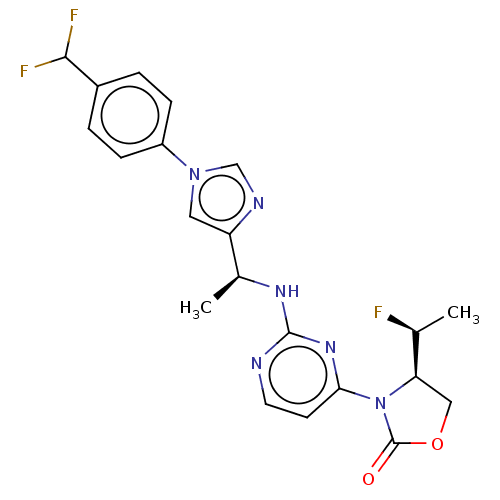
(US10112931, Example 456 | US9434719, 456 | US96886...)Show SMILES C[C@H](F)[C@H]1COC(=O)N1c1ccnc(N[C@@H](C)c2cn(cn2)-c2ccc(cc2)C(F)F)n1 |r| Show InChI InChI=1S/C21H21F3N6O2/c1-12(22)17-10-32-21(31)30(17)18-7-8-25-20(28-18)27-13(2)16-9-29(11-26-16)15-5-3-14(4-6-15)19(23)24/h3-9,11-13,17,19H,10H2,1-2H3,(H,25,27,28)/t12-,13-,17+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG
US Patent
| Assay Description
The IDH1 (R132H) mutant catalyzes the reduced form of NADP+ (NADPH) and α-ketoglutarate (α-KG) to form nicotinamide adenine dinucleotide ph... |
US Patent US10112931 (2018)
BindingDB Entry DOI: 10.7270/Q2PG1TRT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM244193
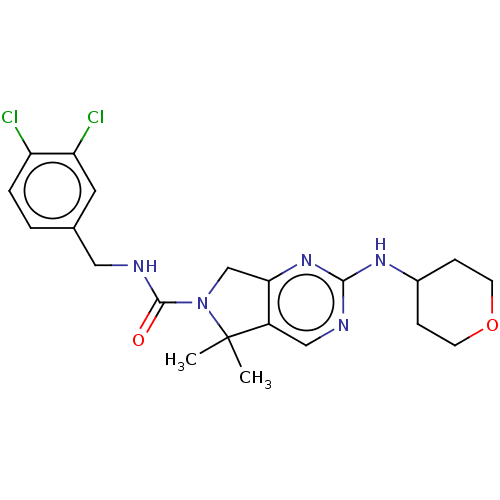
(US9546173, cmpd 126 | n-(3,4-dichlorobenzyl)-5,5-d...)Show SMILES CC1(C)N(Cc2nc(NC3CCOCC3)ncc12)C(=O)NCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N5O2/c1-21(2)15-11-24-19(26-14-5-7-30-8-6-14)27-18(15)12-28(21)20(29)25-10-13-3-4-16(22)17(23)9-13/h3-4,9,11,14H,5-8,10,12H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG
US Patent
| Assay Description
Compound potency against activated ERK2 was determined using a kinase assay that measures ERK2-catalyzed phosphorylation of biotinylated ERKtide pept... |
US Patent US9546173 (2017)
BindingDB Entry DOI: 10.7270/Q2GT5Q52 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM247849

(US10112931, Example 456 | US9434719, 456 | US96886...)Show SMILES C[C@H](F)[C@H]1COC(=O)N1c1ccnc(N[C@@H](C)c2cn(cn2)-c2ccc(cc2)C(F)F)n1 |r| Show InChI InChI=1S/C21H21F3N6O2/c1-12(22)17-10-32-21(31)30(17)18-7-8-25-20(28-18)27-13(2)16-9-29(11-26-16)15-5-3-14(4-6-15)19(23)24/h3-9,11-13,17,19H,10H2,1-2H3,(H,25,27,28)/t12-,13-,17+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG
US Patent
| Assay Description
The IDH1 (R132H) mutant catalyzes the reduced form of NADP+(NADPH) and α-ketoglutarate (α-KG) to form nicotinamide adenine dinucleotide pho... |
US Patent US9688672 (2017)
BindingDB Entry DOI: 10.7270/Q2251GBM |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344532
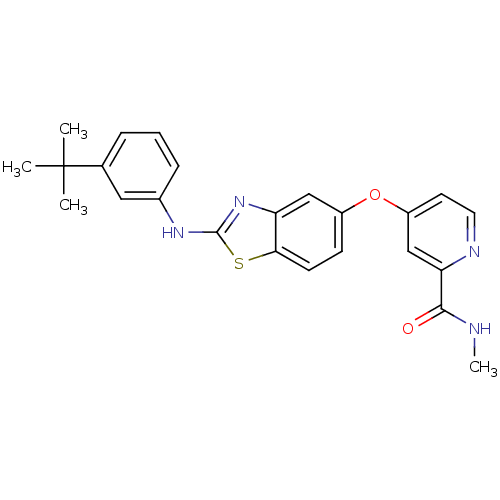
(4-(2-(3-tert-butylphenylamino)benzo[d]thiazol-5-yl...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C24H24N4O2S/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-13-17(8-9-21(19)31-23)30-18-10-11-26-20(14-18)22(29)25-4/h5-14H,1-4H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344540
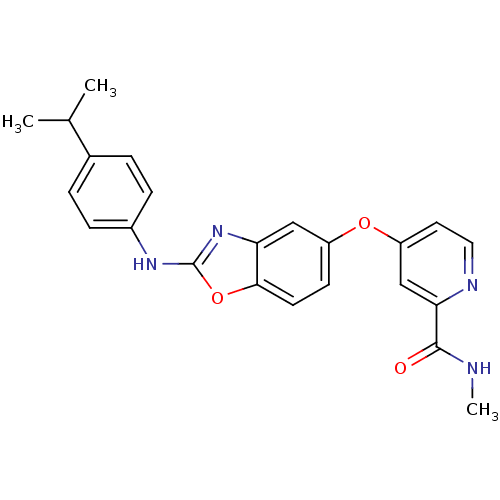
(4-(2-(4-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(cc4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O3/c1-14(2)15-4-6-16(7-5-15)26-23-27-19-12-17(8-9-21(19)30-23)29-18-10-11-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344541

(4-(2-(4-ethylphenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CCc1ccc(Nc2nc3cc(Oc4ccnc(c4)C(=O)NC)ccc3o2)cc1 Show InChI InChI=1S/C22H20N4O3/c1-3-14-4-6-15(7-5-14)25-22-26-18-12-16(8-9-20(18)29-22)28-17-10-11-24-19(13-17)21(27)23-2/h4-13H,3H2,1-2H3,(H,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344548
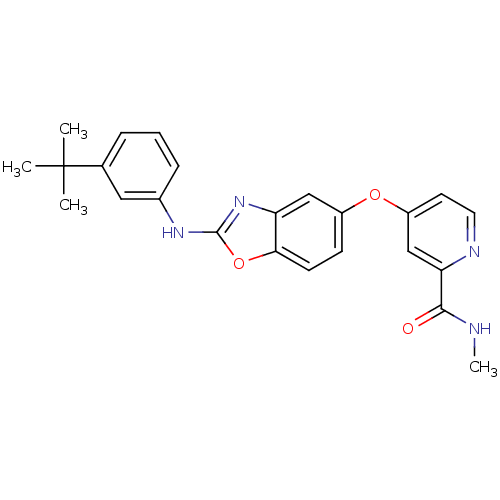
(4-(2-(3-tert-butyl phenylamino)benzo[d]oxazol-5-yl...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C24H24N4O3/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-13-17(8-9-21(19)31-23)30-18-10-11-26-20(14-18)22(29)25-4/h5-14H,1-4H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50380229

(CHEMBL2016996)Show SMILES O=C1NCC2(CCCCC2)c2sc(cc12)-c1ncnc2nc[nH]c12 Show InChI InChI=1S/C17H17N5OS/c23-16-10-6-11(12-13-15(21-8-19-12)22-9-20-13)24-14(10)17(7-18-16)4-2-1-3-5-17/h6,8-9H,1-5,7H2,(H,18,23)(H,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7/DBF4 using MCM-2 as substrate after 1 hr |
ACS Med Chem Lett 2: 720-723 (2011)
Article DOI: 10.1021/ml200029w
BindingDB Entry DOI: 10.7270/Q2SQ91CR |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50380230
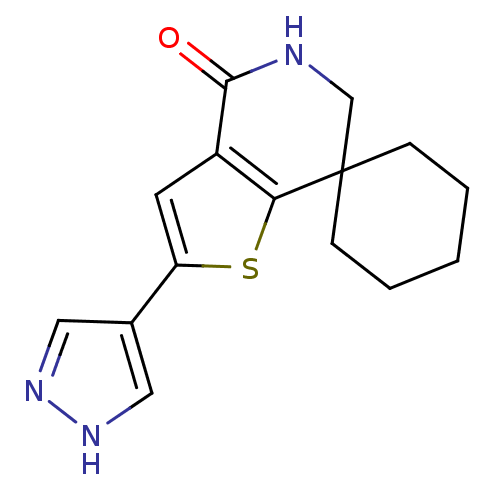
(CHEMBL2016862)Show InChI InChI=1S/C15H17N3OS/c19-14-11-6-12(10-7-17-18-8-10)20-13(11)15(9-16-14)4-2-1-3-5-15/h6-8H,1-5,9H2,(H,16,19)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7/DBF4 using MCM-2 as substrate after 1 hr |
ACS Med Chem Lett 2: 720-723 (2011)
Article DOI: 10.1021/ml200029w
BindingDB Entry DOI: 10.7270/Q2SQ91CR |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase/Protein DBF4 homolog A
(Homo sapiens (Human)) | BDBM50380236
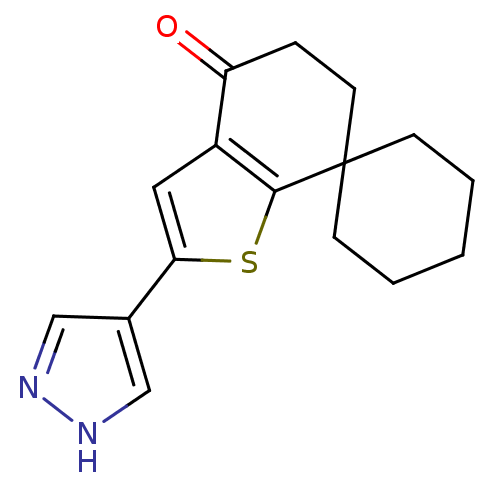
(CHEMBL2016856)Show InChI InChI=1S/C16H18N2OS/c19-13-4-7-16(5-2-1-3-6-16)15-12(13)8-14(20-15)11-9-17-18-10-11/h8-10H,1-7H2,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC7/DBF4 using MCM-2 as substrate after 1 hr |
ACS Med Chem Lett 2: 720-723 (2011)
Article DOI: 10.1021/ml200029w
BindingDB Entry DOI: 10.7270/Q2SQ91CR |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50265773

(4-Amino-3-benzimidazol-2-yl-5-(4-methylpiperazinyl...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N)c12 Show InChI InChI=1S/C21H22N6O/c1-26-9-11-27(12-10-26)16-8-4-7-15-17(16)19(22)18(21(28)25-15)20-23-13-5-2-3-6-14(13)24-20/h2-8H,9-12H2,1H3,(H,23,24)(H3,22,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FGFR1 |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50265774

(4-Amino-3-benzimidazol-2-yl-5-(1-methyl(3-piperidy...)Show SMILES CN1CCCC(C1)Oc1cccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N)c12 Show InChI InChI=1S/C22H23N5O2/c1-27-11-5-6-13(12-27)29-17-10-4-9-16-18(17)20(23)19(22(28)26-16)21-24-14-7-2-3-8-15(14)25-21/h2-4,7-10,13H,5-6,11-12H2,1H3,(H,24,25)(H3,23,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FGFR1 |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344525
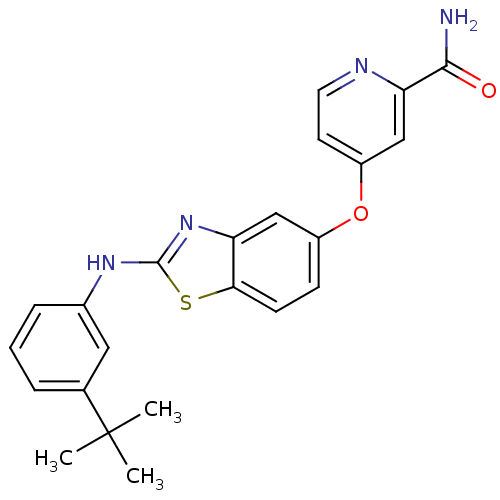
(4-(2-(3-tert-butylphenylamino)benzo[d]thiazol-5-yl...)Show SMILES CC(C)(C)c1cccc(Nc2nc3cc(Oc4ccnc(c4)C(N)=O)ccc3s2)c1 Show InChI InChI=1S/C23H22N4O2S/c1-23(2,3)14-5-4-6-15(11-14)26-22-27-18-12-16(7-8-20(18)30-22)29-17-9-10-25-19(13-17)21(24)28/h4-13H,1-3H3,(H2,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 |
Bioorg Med Chem Lett 16: 3595-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.069
BindingDB Entry DOI: 10.7270/Q2BZ65N7 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265676
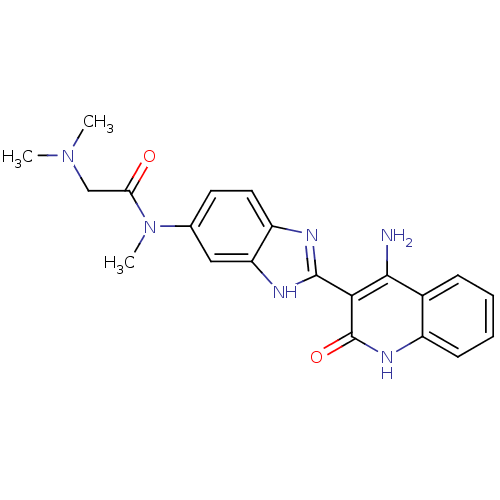
(CHEMBL495962 | N-(2-(4-Amino-2-oxo-1,2-dihydroquin...)Show SMILES CN(C)CC(=O)N(C)c1ccc2nc([nH]c2c1)-c1c(N)c2ccccc2[nH]c1=O Show InChI InChI=1S/C21H22N6O2/c1-26(2)11-17(28)27(3)12-8-9-15-16(10-12)24-20(23-15)18-19(22)13-6-4-5-7-14(13)25-21(18)29/h4-10H,11H2,1-3H3,(H,23,24)(H3,22,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50265738
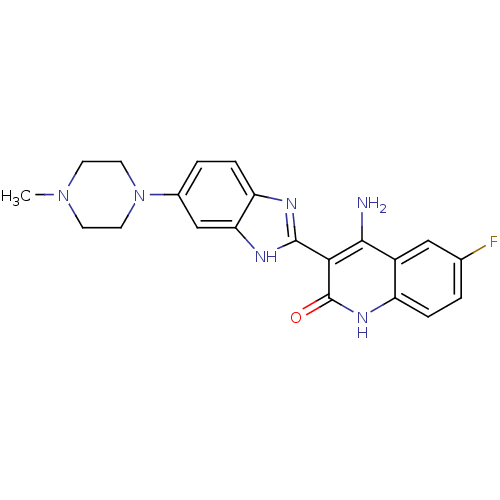
(4-Amino-6-fluoro-3-[6-(4-methylpiperazinyl)benzimi...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2cc(F)ccc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-6-8-28(9-7-27)13-3-5-16-17(11-13)25-20(24-16)18-19(23)14-10-12(22)2-4-15(14)26-21(18)29/h2-5,10-11H,6-9H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 52: 278-92 (2009)
Article DOI: 10.1021/jm800790t
BindingDB Entry DOI: 10.7270/Q2TD9X7X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50181308
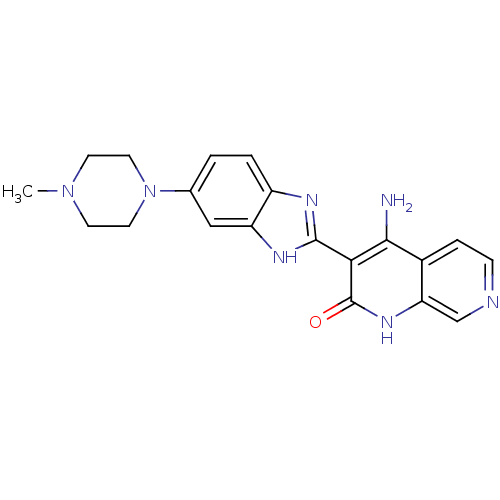
(4-amino-3-(6-(4-methylpiperazin-1-yl)-1H-benzo[d]i...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2ccncc2[nH]c1=O Show InChI InChI=1S/C20H21N7O/c1-26-6-8-27(9-7-26)12-2-3-14-15(10-12)24-19(23-14)17-18(21)13-4-5-22-11-16(13)25-20(17)28/h2-5,10-11H,6-9H2,1H3,(H,23,24)(H3,21,25,28) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
Bioorg Med Chem Lett 16: 2247-51 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.020
BindingDB Entry DOI: 10.7270/Q2NZ8777 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50185178

(1-tert-butyl-3-(3-(5-(4-(piperidin-1-yl)piperidin-...)Show SMILES CC(C)(C)NC(=O)Nc1ccc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCC(CC3)N3CCCCC3)c2c1 Show InChI InChI=1S/C29H38N8O/c1-29(2,3)33-28(38)30-19-7-9-23-22(17-19)26(35-34-23)27-31-24-10-8-21(18-25(24)32-27)37-15-11-20(12-16-37)36-13-5-4-6-14-36/h7-10,17-18,20H,4-6,11-16H2,1-3H3,(H,31,32)(H,34,35)(H2,30,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Bioorg Med Chem Lett 16: 3595-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.069
BindingDB Entry DOI: 10.7270/Q2BZ65N7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026677
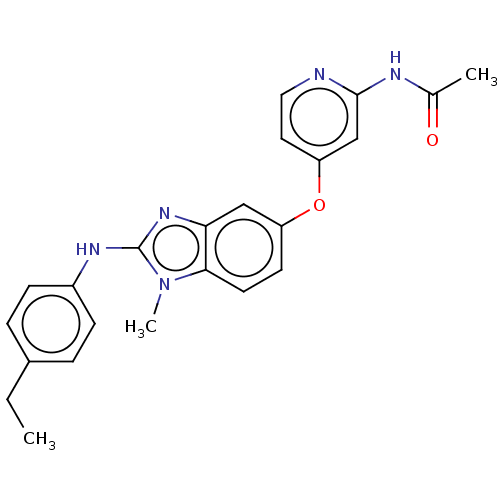
(CHEMBL3335377)Show SMILES CCc1ccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)cc1 Show InChI InChI=1S/C23H23N5O2/c1-4-16-5-7-17(8-6-16)26-23-27-20-13-18(9-10-21(20)28(23)3)30-19-11-12-24-22(14-19)25-15(2)29/h5-14H,4H2,1-3H3,(H,26,27)(H,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50131822
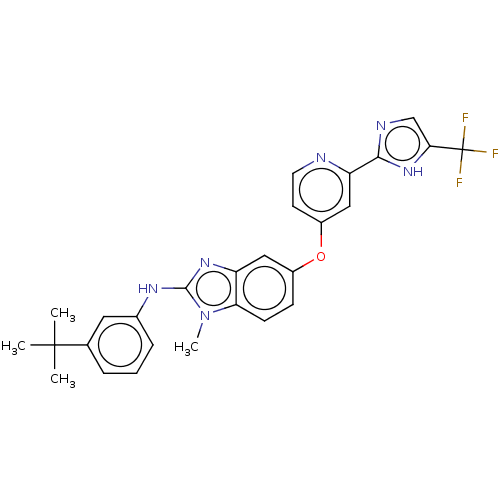
(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50026679
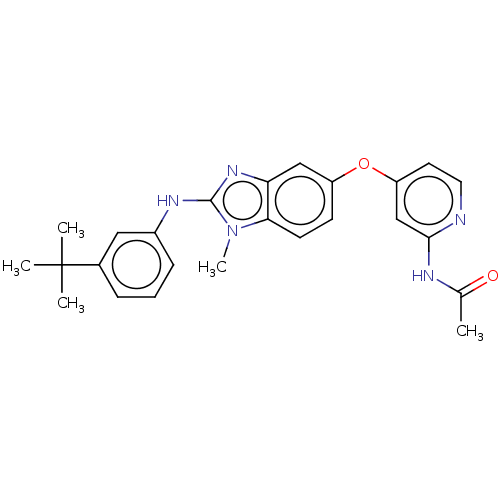
(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026683
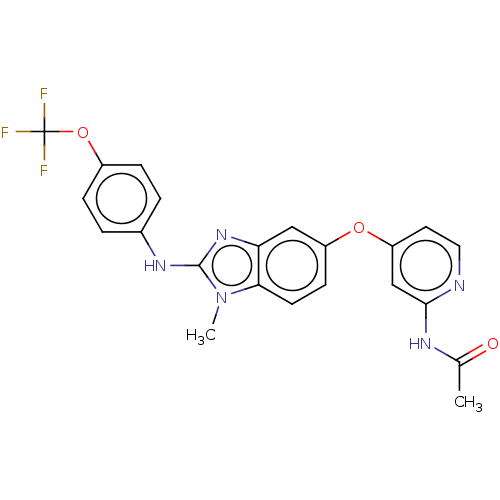
(CHEMBL3335379)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4ccc(OC(F)(F)F)cc4)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O3/c1-13(31)27-20-12-17(9-10-26-20)32-16-7-8-19-18(11-16)29-21(30(19)2)28-14-3-5-15(6-4-14)33-22(23,24)25/h3-12H,1-2H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026692
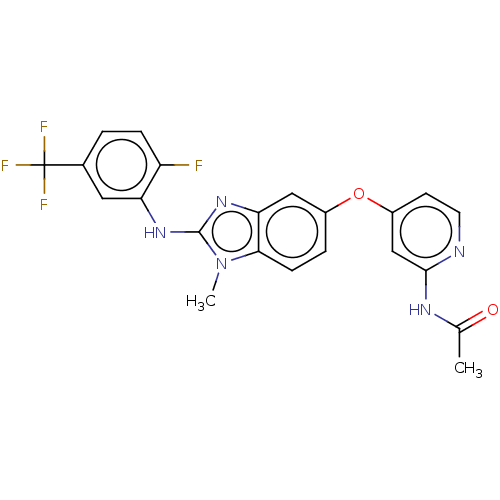
(CHEMBL3335381)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cc(ccc4F)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H17F4N5O2/c1-12(32)28-20-11-15(7-8-27-20)33-14-4-6-19-18(10-14)30-21(31(19)2)29-17-9-13(22(24,25)26)3-5-16(17)23/h3-11H,1-2H3,(H,29,30)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM247849

(US10112931, Example 456 | US9434719, 456 | US96886...)Show SMILES C[C@H](F)[C@H]1COC(=O)N1c1ccnc(N[C@@H](C)c2cn(cn2)-c2ccc(cc2)C(F)F)n1 |r| Show InChI InChI=1S/C21H21F3N6O2/c1-12(22)17-10-32-21(31)30(17)18-7-8-25-20(28-18)27-13(2)16-9-29(11-26-16)15-5-3-14(4-6-15)19(23)24/h3-9,11-13,17,19H,10H2,1-2H3,(H,25,27,28)/t12-,13-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG
US Patent
| Assay Description
The IDH1 (R132H) mutant catalyzes the reduced form of NADP+ (NADPH) and α-ketoglutarate (α-KG) to form nicotinamide adenine dinucleotide ph... |
US Patent US9434719 (2016)
BindingDB Entry DOI: 10.7270/Q2QV3KFR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data