 Found 1018 hits with Last Name = 'shakespeare' and Initial = 'wc'
Found 1018 hits with Last Name = 'shakespeare' and Initial = 'wc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064027
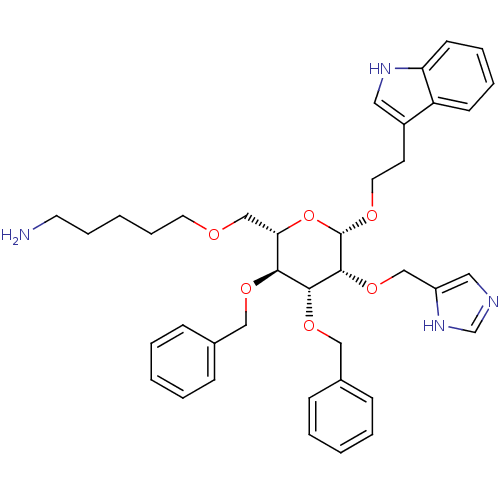
(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064027

(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064025
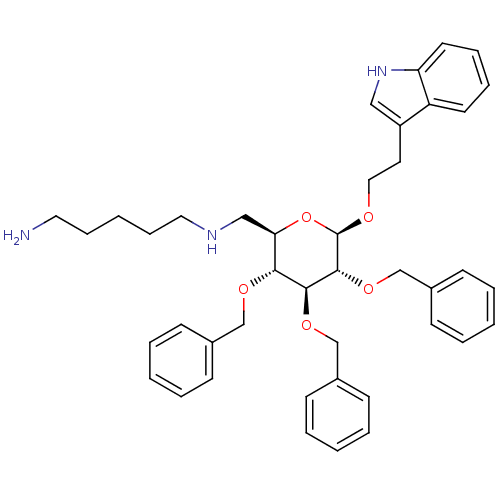
(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064027

(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064027

(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051567
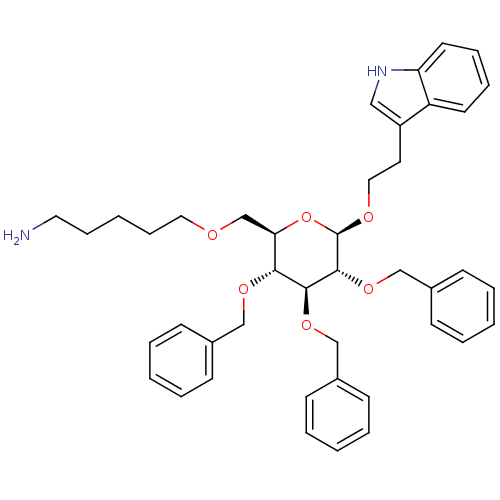
(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051578
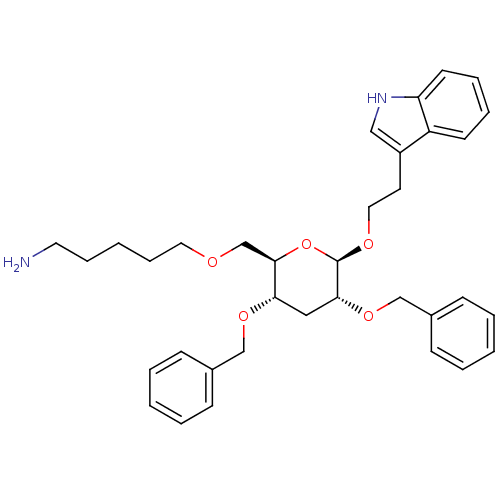
(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064024
(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064026
(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064029
(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
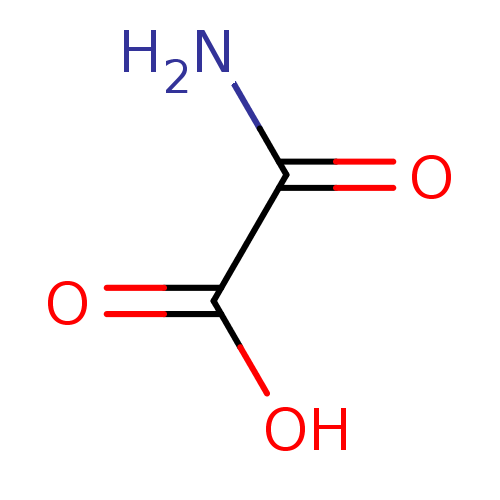
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50062357

(AP26113 | CHEMBL3397300)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCC(CC1)N(C)C Show InChI InChI=1S/C26H34ClN6O2P/c1-32(2)18-12-14-33(15-13-18)19-10-11-21(23(16-19)35-3)30-26-28-17-20(27)25(31-26)29-22-8-6-7-9-24(22)36(4,5)34/h6-11,16-18H,12-15H2,1-5H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50185287
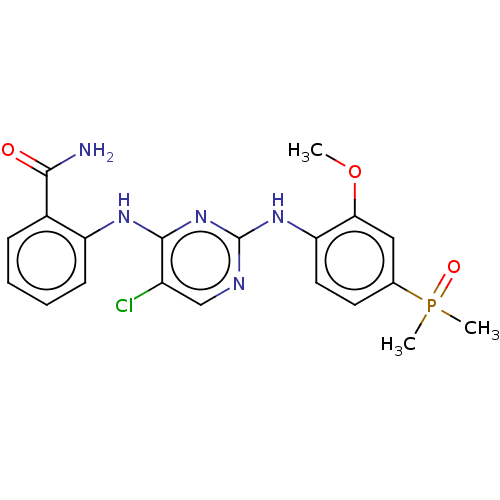
(CHEMBL3823268)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2C(N)=O)n1)P(C)(C)=O Show InChI InChI=1S/C20H21ClN5O3P/c1-29-17-10-12(30(2,3)28)8-9-16(17)25-20-23-11-14(21)19(26-20)24-15-7-5-4-6-13(15)18(22)27/h4-11H,1-3H3,(H2,22,27)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R using KKKSPGEYVNIEFG as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185237

(CHEMBL3824308)Show SMILES CCP(=O)(CC)c1ccccc1Nc1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C31H43ClN7O2P/c1-5-42(40,6-2)29-10-8-7-9-27(29)34-30-25(32)22-33-31(36-30)35-26-12-11-24(21-28(26)41-4)38-15-13-23(14-16-38)39-19-17-37(3)18-20-39/h7-12,21-23H,5-6,13-20H2,1-4H3,(H2,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
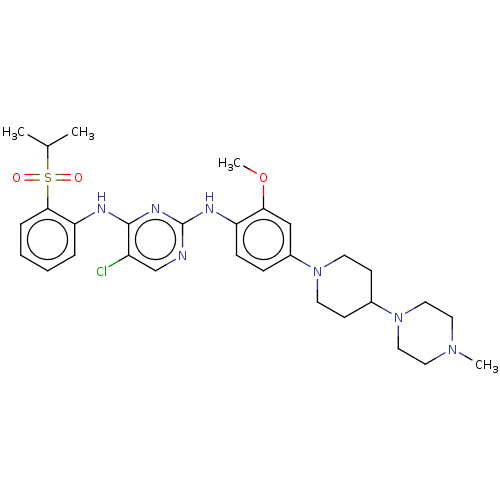
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM482158
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
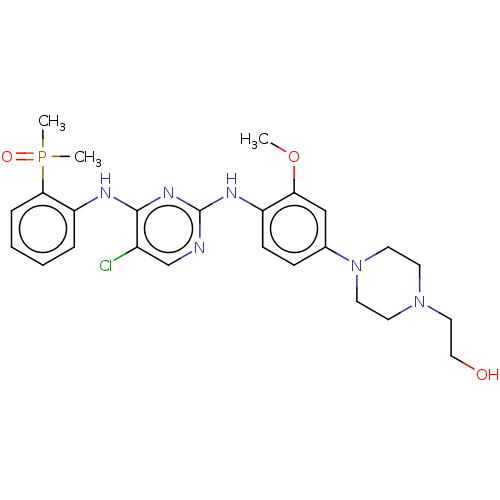
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185275
(CHEMBL3823235)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C25H32ClN6O3P/c1-35-22-16-18(32-12-10-31(11-13-32)14-15-33)8-9-20(22)29-25-27-17-19(26)24(30-25)28-21-6-4-5-7-23(21)36(2,3)34/h4-9,16-17,33H,10-15H2,1-3H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50185280

(CHEMBL3822611)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2P(C)(C)=O)n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H30ClN6O2P/c1-30-11-13-31(14-12-30)17-9-10-19(21(15-17)33-2)28-24-26-16-18(25)23(29-24)27-20-7-5-6-8-22(20)34(3,4)32/h5-10,15-16H,11-14H2,1-4H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) as substrate and [gamma-33P]ATP measured after 1 hr |
J Med Chem 59: 4948-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00306
BindingDB Entry DOI: 10.7270/Q2NK3H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RET using KKKSPGEYVNIEFG by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data