

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018235 (5-Amino-2-{4-[(2,4-diamino-5-chloro-quinazolin-6-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50002472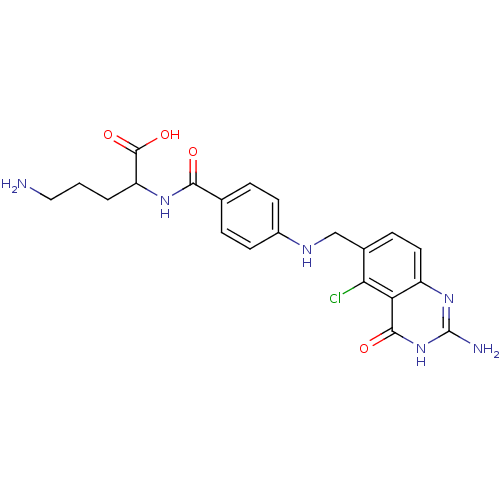 (5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50002472 (5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50003467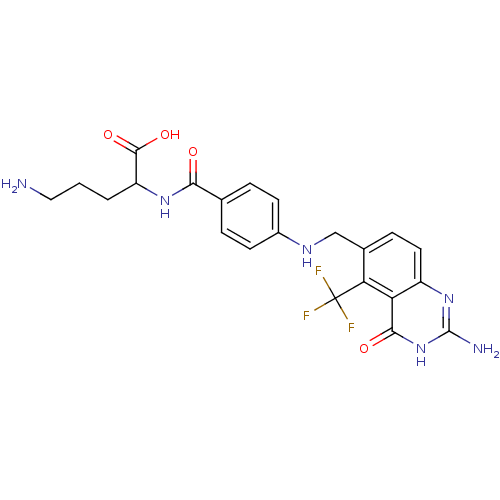 (5-Amino-2-{4-[(2-amino-4-oxo-5-trifluoromethyl-3,4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50003471 (5-Amino-2-{4-[(2-amino-4-oxo-5-trifluoromethyl-3,4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50003470 (5-Amino-2-{4-[(5-fluoro-2-methyl-4-oxo-3,4-dihydro...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018236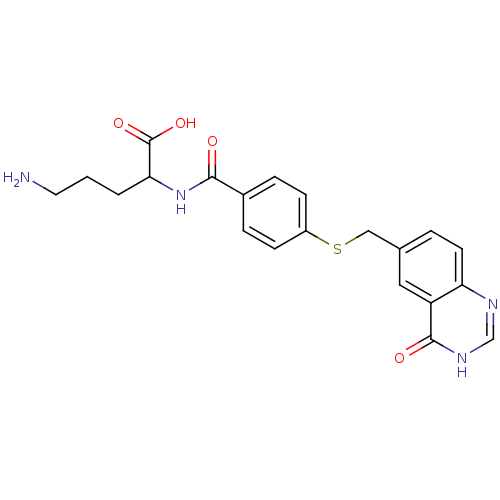 (5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50003468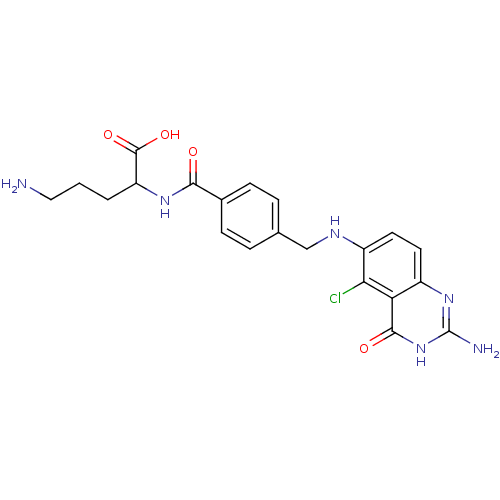 (5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50003469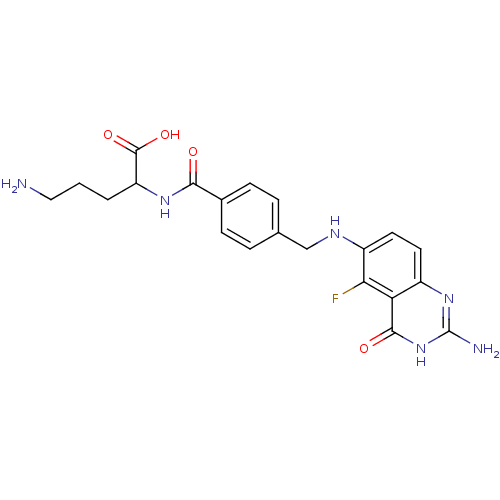 (5-Amino-2-{4-[(2-amino-5-fluoro-4-oxo-3,4-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018232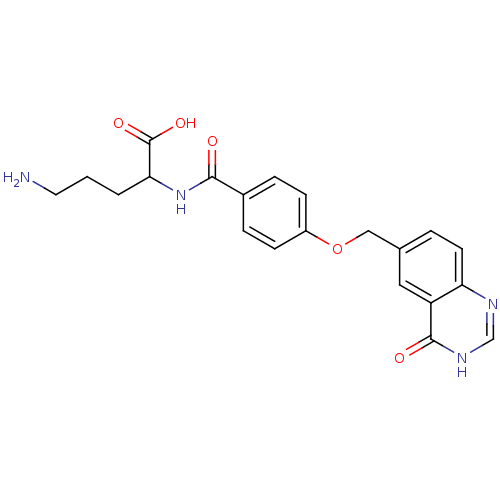 (5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018234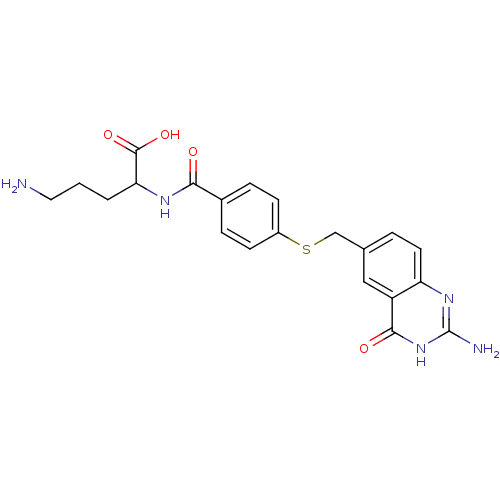 (5-Amino-2-[4-(2-amino-4-oxo-3,4-dihydro-quinazolin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50002473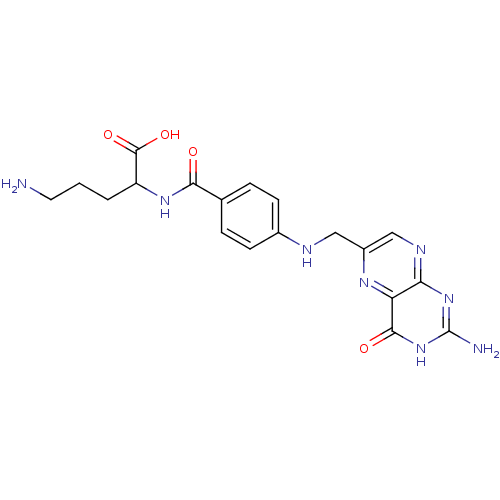 (5-Amino-2-{4-[(2-amino-4-oxo-3,4-dihydro-pteridin-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Binding affinity of the compound for hog liver Folyl-polyglutamate synthase was evaluated | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50002473 (5-Amino-2-{4-[(2-amino-4-oxo-3,4-dihydro-pteridin-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hog liver Folyl polyglutamate synthetase (FPGS) | J Med Chem 35: 4078-85 (1992) BindingDB Entry DOI: 10.7270/Q26M35S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018233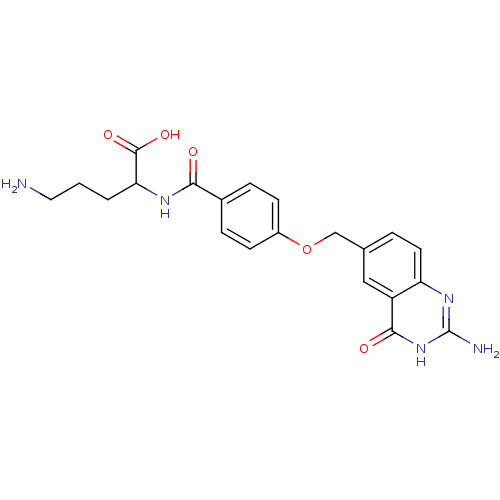 (5-Amino-2-[4-(2-amino-4-oxo-3,4-dihydro-quinazolin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Rattus norvegicus) | BDBM50322368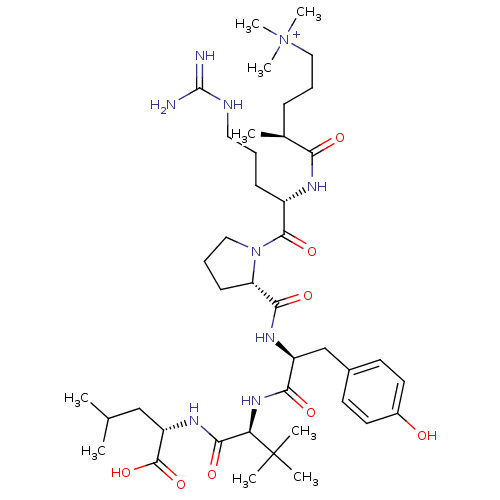 ((S)-5-((S)-1-((S)-2-((S)-1-((S)-1-((S)-1-carboxy-3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Campus Curated by ChEMBL | Assay Description Displacement of [125I]NT from rat NTR2 by gamma counting | J Med Chem 53: 4623-32 (2010) Article DOI: 10.1021/jm100092s BindingDB Entry DOI: 10.7270/Q2DF6RDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50322368 ((S)-5-((S)-1-((S)-2-((S)-1-((S)-1-((S)-1-carboxy-3...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Medical University of South Carolina Campus Curated by ChEMBL | Assay Description Binding affinity to human NTR2 by gamma counting | J Med Chem 53: 4623-32 (2010) Article DOI: 10.1021/jm100092s BindingDB Entry DOI: 10.7270/Q2DF6RDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50322368 ((S)-5-((S)-1-((S)-2-((S)-1-((S)-1-((S)-1-carboxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Medical University of South Carolina Campus Curated by ChEMBL | Assay Description Agonist activity at rat NTR1 expressed in LTK cells assessed as calcium mobilization | J Med Chem 53: 4623-32 (2010) Article DOI: 10.1021/jm100092s BindingDB Entry DOI: 10.7270/Q2DF6RDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50322368 ((S)-5-((S)-1-((S)-2-((S)-1-((S)-1-((S)-1-carboxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Medical University of South Carolina Campus Curated by ChEMBL | Assay Description Binding affinity to human NTR1 by gamma counting | J Med Chem 53: 4623-32 (2010) Article DOI: 10.1021/jm100092s BindingDB Entry DOI: 10.7270/Q2DF6RDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||