 Found 5075 hits with Last Name = 'shi' and Initial = 'w'
Found 5075 hits with Last Name = 'shi' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126829
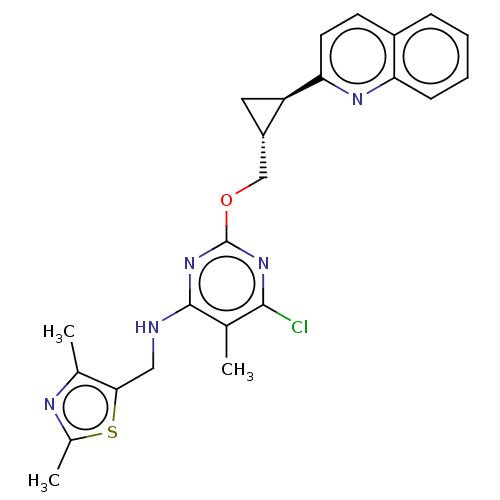
(US8785467, 1-38)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@@H]3C[C@H]3c3ccc4ccccc4n3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126842
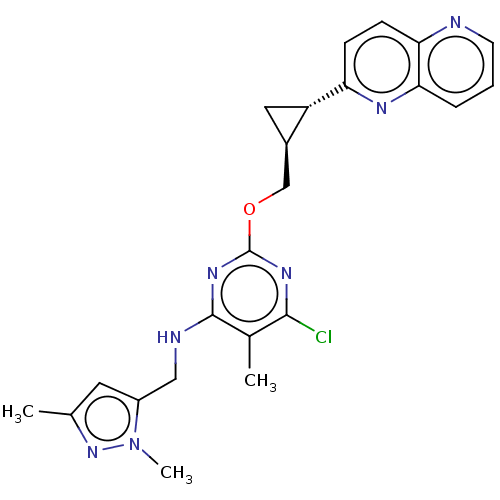
(US8785467, 1-51)Show SMILES Cc1cc(CNc2nc(OC[C@H]3C[C@@H]3c3ccc4ncccc4n3)nc(Cl)c2C)n(C)n1 |r| Show InChI InChI=1S/C23H24ClN7O/c1-13-9-16(31(3)30-13)11-26-22-14(2)21(24)28-23(29-22)32-12-15-10-17(15)18-6-7-19-20(27-18)5-4-8-25-19/h4-9,15,17H,10-12H2,1-3H3,(H,26,28,29)/t15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.00320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
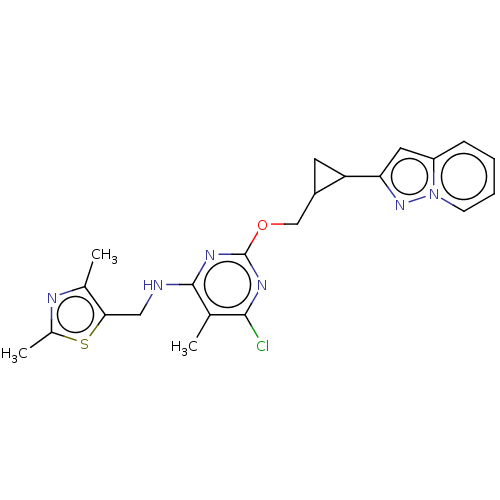
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126826
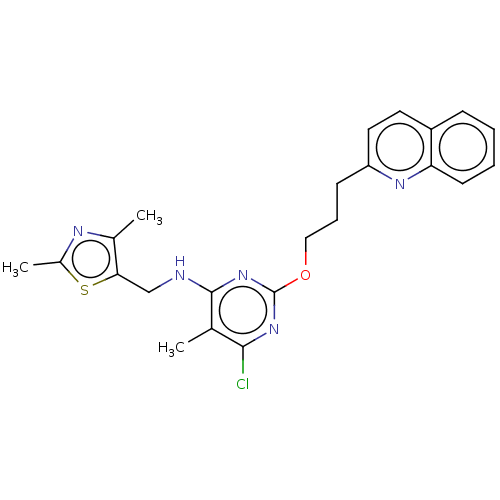
(US8785467, 1-29)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H24ClN5OS/c1-14-21(24)28-23(29-22(14)25-13-20-15(2)26-16(3)31-20)30-12-6-8-18-11-10-17-7-4-5-9-19(17)27-18/h4-5,7,9-11H,6,8,12-13H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126826

(US8785467, 1-29)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H24ClN5OS/c1-14-21(24)28-23(29-22(14)25-13-20-15(2)26-16(3)31-20)30-12-6-8-18-11-10-17-7-4-5-9-19(17)27-18/h4-5,7,9-11H,6,8,12-13H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823
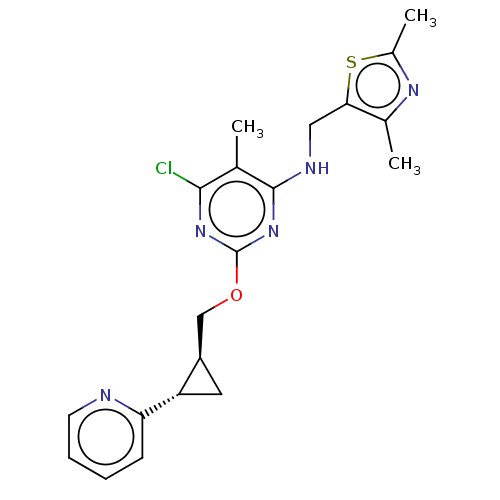
(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126828

(US8785467, 1-37)Show SMILES Cc1c(Cl)nc(OC[C@@H]2C[C@H]2c2ccc3ncccc3n2)nc1NCc1cnn(C)c1 |r| Show InChI InChI=1S/C22H22ClN7O/c1-13-20(23)28-22(29-21(13)25-9-14-10-26-30(2)11-14)31-12-15-8-16(15)17-5-6-18-19(27-17)4-3-7-24-18/h3-7,10-11,15-16H,8-9,12H2,1-2H3,(H,25,28,29)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126825
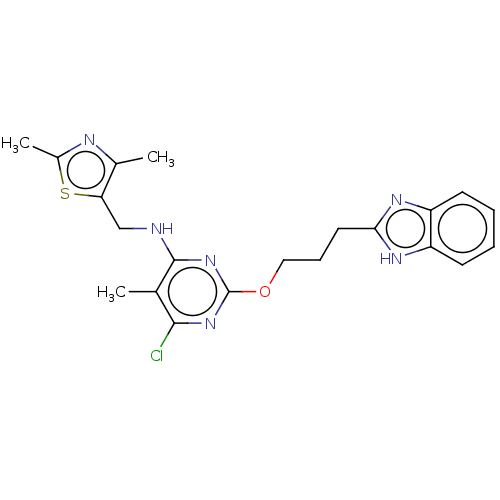
(US8785467, 1-27)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4[nH]3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-12-19(22)27-21(28-20(12)23-11-17-13(2)24-14(3)30-17)29-10-6-9-18-25-15-7-4-5-8-16(15)26-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,25,26)(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
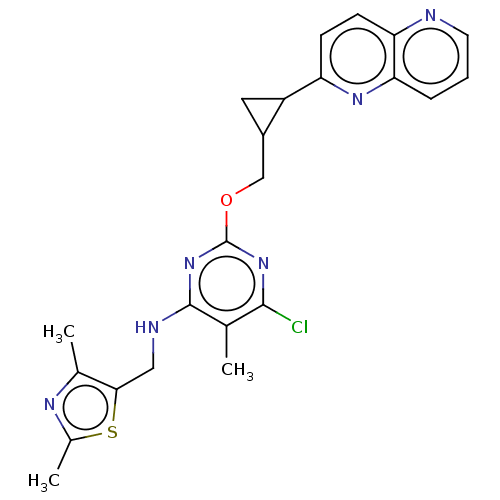
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126825

(US8785467, 1-27)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4[nH]3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-12-19(22)27-21(28-20(12)23-11-17-13(2)24-14(3)30-17)29-10-6-9-18-25-15-7-4-5-8-16(15)26-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,25,26)(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823

(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823

(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126827
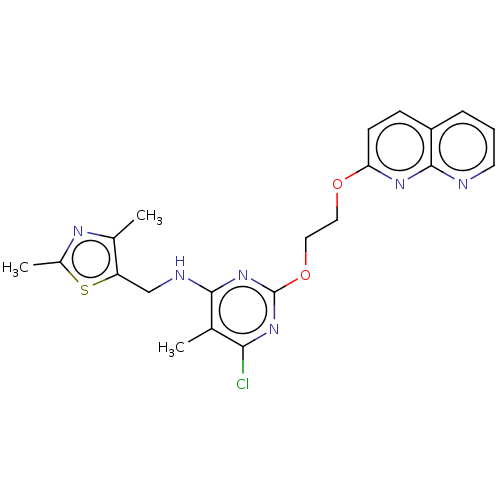
(US8785467, 1-32)Show SMILES Cc1nc(C)c(CNc2nc(OCCOc3ccc4cccnc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H21ClN6O2S/c1-12-18(22)27-21(28-19(12)24-11-16-13(2)25-14(3)31-16)30-10-9-29-17-7-6-15-5-4-8-23-20(15)26-17/h4-8H,9-11H2,1-3H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126827

(US8785467, 1-32)Show SMILES Cc1nc(C)c(CNc2nc(OCCOc3ccc4cccnc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H21ClN6O2S/c1-12-18(22)27-21(28-19(12)24-11-16-13(2)25-14(3)31-16)30-10-9-29-17-7-6-15-5-4-8-23-20(15)26-17/h4-8H,9-11H2,1-3H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124648
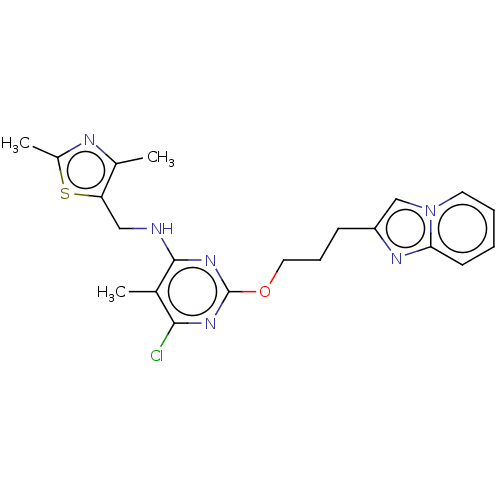
(CHEMBL3622901)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3cn4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-13-19(22)26-21(27-20(13)23-11-17-14(2)24-15(3)30-17)29-10-6-7-16-12-28-9-5-4-8-18(28)25-16/h4-5,8-9,12H,6-7,10-11H2,1-3H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124649

(CHEMBL3622902)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4s3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H22ClN5OS2/c1-12-19(22)26-21(27-20(12)23-11-17-13(2)24-14(3)29-17)28-10-6-9-18-25-15-7-4-5-8-16(15)30-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126836
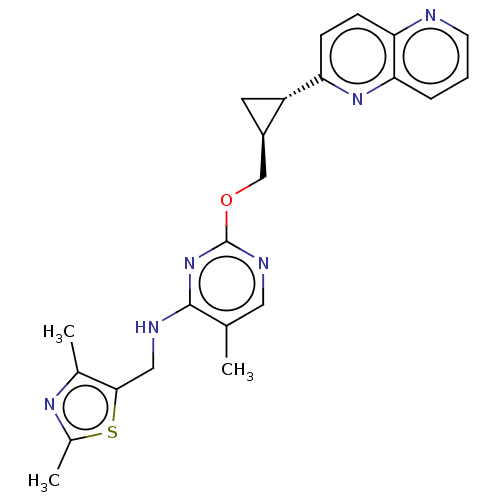
(US8785467, 1-45)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccc4ncccc4n3)ncc2C)s1 |r| Show InChI InChI=1S/C23H24N6OS/c1-13-10-26-23(29-22(13)25-11-21-14(2)27-15(3)31-21)30-12-16-9-17(16)18-6-7-19-20(28-18)5-4-8-24-19/h4-8,10,16-17H,9,11-12H2,1-3H3,(H,25,26,29)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124650
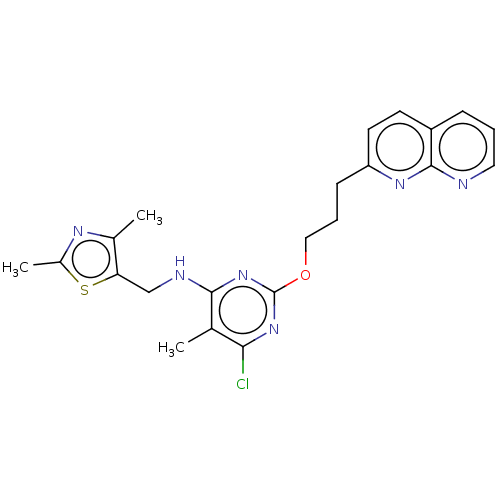
(CHEMBL3622903)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3ccc4cccnc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-13-19(23)28-22(29-20(13)25-12-18-14(2)26-15(3)31-18)30-11-5-7-17-9-8-16-6-4-10-24-21(16)27-17/h4,6,8-10H,5,7,11-12H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126831
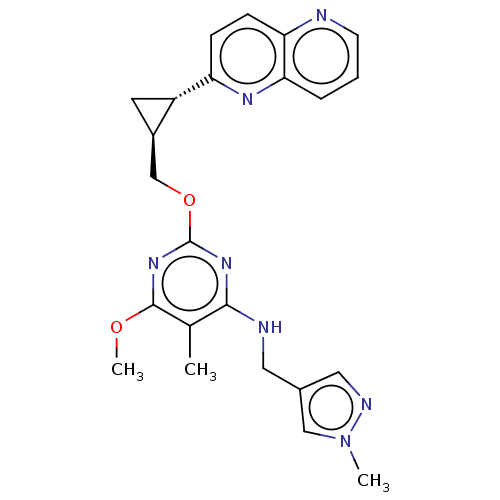
(US8785467, 1-40)Show SMILES COc1nc(OC[C@H]2C[C@@H]2c2ccc3ncccc3n2)nc(NCc2cnn(C)c2)c1C |r| Show InChI InChI=1S/C23H25N7O2/c1-14-21(25-10-15-11-26-30(2)12-15)28-23(29-22(14)31-3)32-13-16-9-17(16)18-6-7-19-20(27-18)5-4-8-24-19/h4-8,11-12,16-17H,9-10,13H2,1-3H3,(H,25,28,29)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126834
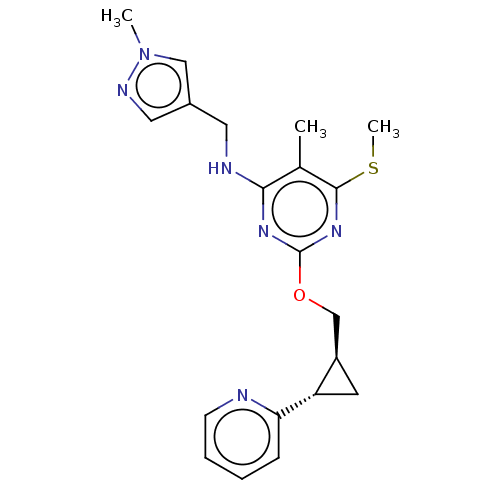
(US8785467, 1-43)Show SMILES CSc1nc(OC[C@H]2C[C@@H]2c2ccccn2)nc(NCc2cnn(C)c2)c1C |r| Show InChI InChI=1S/C20H24N6OS/c1-13-18(22-9-14-10-23-26(2)11-14)24-20(25-19(13)28-3)27-12-15-8-16(15)17-6-4-5-7-21-17/h4-7,10-11,15-16H,8-9,12H2,1-3H3,(H,22,24,25)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM30369
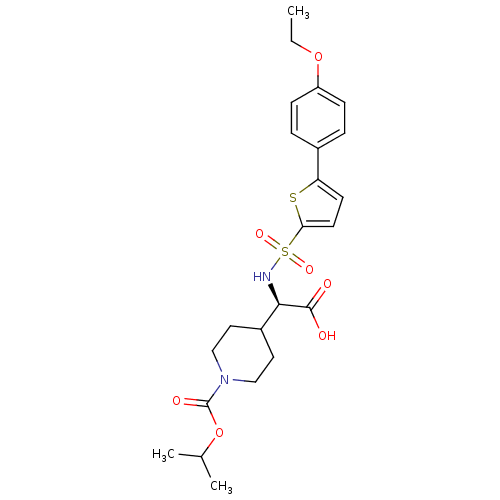
(piperidinyl glycine derivative, 24f)Show SMILES CCOc1ccc(cc1)-c1ccc(s1)S(=O)(=O)N[C@H](C1CCN(CC1)C(=O)OC(C)C)C(O)=O |r| Show InChI InChI=1S/C23H30N2O7S2/c1-4-31-18-7-5-16(6-8-18)19-9-10-20(33-19)34(29,30)24-21(22(26)27)17-11-13-25(14-12-17)23(28)32-15(2)3/h5-10,15,17,21,24H,4,11-14H2,1-3H3,(H,26,27)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343977
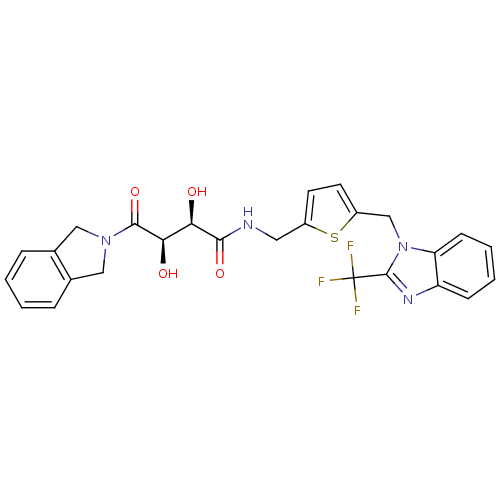
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-4-oxo-N-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1Cc2ccccc2C1)C(=O)NCc1ccc(Cn2c(nc3ccccc23)C(F)(F)F)s1 |r| Show InChI InChI=1S/C26H23F3N4O4S/c27-26(28,29)25-31-19-7-3-4-8-20(19)33(25)14-18-10-9-17(38-18)11-30-23(36)21(34)22(35)24(37)32-12-15-5-1-2-6-16(15)13-32/h1-10,21-22,34-35H,11-14H2,(H,30,36)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147134
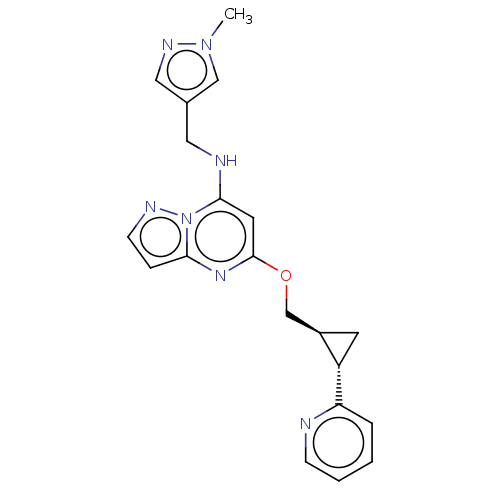
(US8957077, J-5)Show SMILES Cn1cc(CNc2cc(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)cn1 |r| Show InChI InChI=1S/C20H21N7O/c1-26-12-14(11-24-26)10-22-19-9-20(25-18-5-7-23-27(18)19)28-13-15-8-16(15)17-4-2-3-6-21-17/h2-7,9,11-12,15-16,22H,8,10,13H2,1H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.280 | -54.0 | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124651
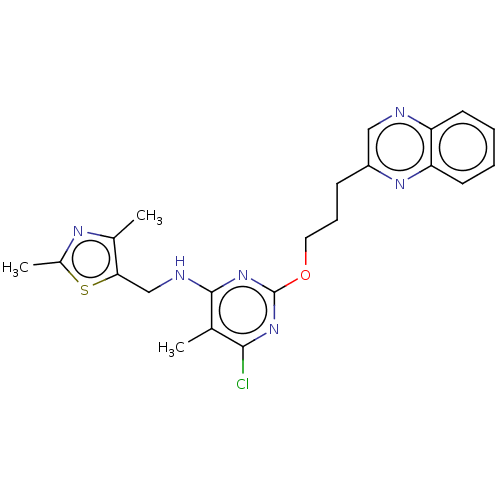
(CHEMBL3622904)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3cnc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-13-20(23)28-22(29-21(13)25-12-19-14(2)26-15(3)31-19)30-10-6-7-16-11-24-17-8-4-5-9-18(17)27-16/h4-5,8-9,11H,6-7,10,12H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500537
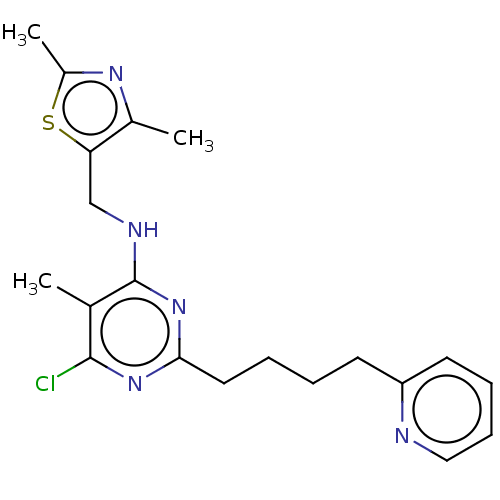
(CHEMBL3746162)Show InChI InChI=1S/C20H24ClN5S/c1-13-19(21)25-18(10-5-4-8-16-9-6-7-11-22-16)26-20(13)23-12-17-14(2)24-15(3)27-17/h6-7,9,11H,4-5,8,10,12H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208973

(US9266881, 12-1)Show SMILES Cn1cc(nc1CCn1nc2cccc(N3CCOCC3)n2c1=O)-c1ccccc1 Show InChI InChI=1S/C22H24N6O2/c1-25-16-18(17-6-3-2-4-7-17)23-19(25)10-11-27-22(29)28-20(24-27)8-5-9-21(28)26-12-14-30-15-13-26/h2-9,16H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.410 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126830

(US8785467, 1-39)Show SMILES Cc1c(Cl)nc(OC[C@H]2C[C@@H]2c2ccccn2)nc1NCc1cnn(C)c1 |r| Show InChI InChI=1S/C19H21ClN6O/c1-12-17(20)24-19(25-18(12)22-8-13-9-23-26(2)10-13)27-11-14-7-15(14)16-5-3-4-6-21-16/h3-6,9-10,14-15H,7-8,11H2,1-2H3,(H,22,24,25)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126830

(US8785467, 1-39)Show SMILES Cc1c(Cl)nc(OC[C@H]2C[C@@H]2c2ccccn2)nc1NCc1cnn(C)c1 |r| Show InChI InChI=1S/C19H21ClN6O/c1-12-17(20)24-19(25-18(12)22-8-13-9-23-26(2)10-13)27-11-14-7-15(14)16-5-3-4-6-21-16/h3-6,9-10,14-15H,7-8,11H2,1-2H3,(H,22,24,25)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126839
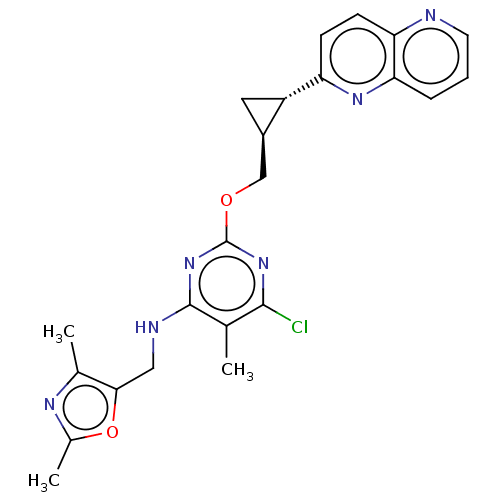
(US8785467, 1-48)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccc4ncccc4n3)nc(Cl)c2C)o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343976
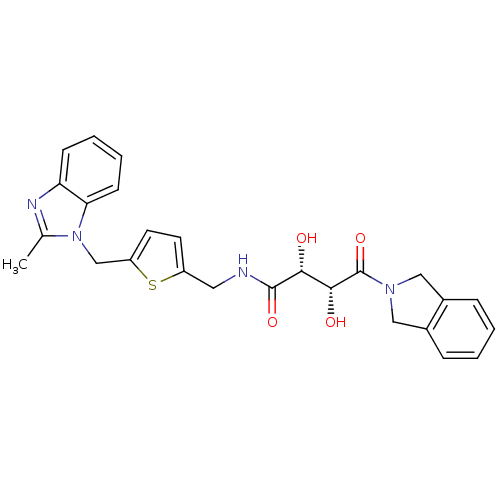
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-N-((5-((...)Show SMILES Cc1nc2ccccc2n1Cc1ccc(CNC(=O)[C@H](O)[C@@H](O)C(=O)N2Cc3ccccc3C2)s1 |r| Show InChI InChI=1S/C26H26N4O4S/c1-16-28-21-8-4-5-9-22(21)30(16)15-20-11-10-19(35-20)12-27-25(33)23(31)24(32)26(34)29-13-17-6-2-3-7-18(17)14-29/h2-11,23-24,31-32H,12-15H2,1H3,(H,27,33)/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM30344
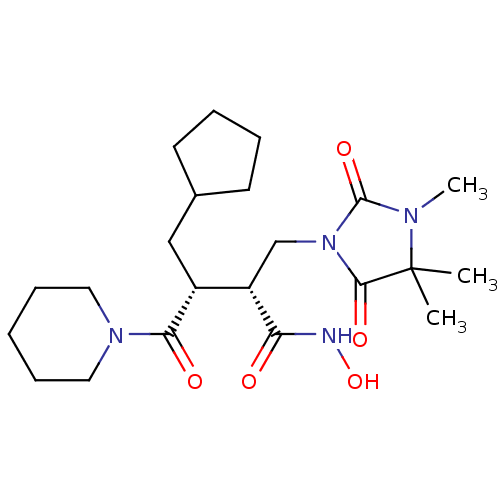
(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | -52.4 | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50375881

(CHEMBL408746)Show SMILES CC(CNCCCCc1ccnnc1)c1c([nH]c2ccc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1 |w:1.0,THB:26:28:30.31:33.34| Show InChI InChI=1S/C37H47N5O/c1-24-18-25(2)20-28(19-24)35-34(26(3)22-38-16-7-6-8-27-15-17-39-40-23-27)32-21-29(9-14-33(32)41-35)37(4,5)36(43)42-30-10-11-31(42)13-12-30/h9,14-15,17-21,23,26,30-31,38,41H,6-8,10-13,16,22H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of GnRH receptor |
Bioorg Med Chem 16: 422-7 (2008)
Article DOI: 10.1016/j.bmc.2007.09.026
BindingDB Entry DOI: 10.7270/Q2Z60PXR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126841
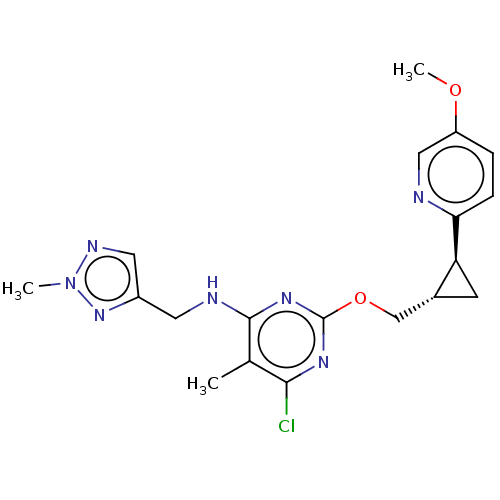
(US8785467, 1-50)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(Cl)c(C)c(NCc2cnn(C)n2)n1 |r| Show InChI InChI=1S/C19H22ClN7O2/c1-11-17(20)24-19(25-18(11)22-7-13-8-23-27(2)26-13)29-10-12-6-15(12)16-5-4-14(28-3)9-21-16/h4-5,8-9,12,15H,6-7,10H2,1-3H3,(H,22,24,25)/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us... |
US Patent US8785467 (2014)
BindingDB Entry DOI: 10.7270/Q2VT1QS1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122657
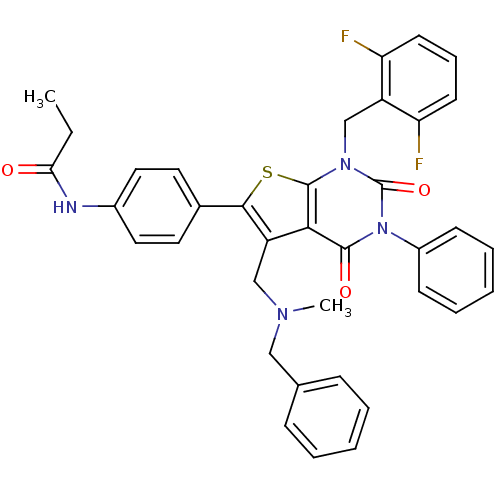
(CHEMBL21126 | N-{4-[5-[(Benzyl-methyl-amino)-methy...)Show SMILES CCC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H32F2N4O3S/c1-3-32(44)40-26-19-17-25(18-20-26)34-29(22-41(2)21-24-11-6-4-7-12-24)33-35(45)43(27-13-8-5-9-14-27)37(46)42(36(33)47-34)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of GnRH receptor |
Bioorg Med Chem 16: 422-7 (2008)
Article DOI: 10.1016/j.bmc.2007.09.026
BindingDB Entry DOI: 10.7270/Q2Z60PXR |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50342963
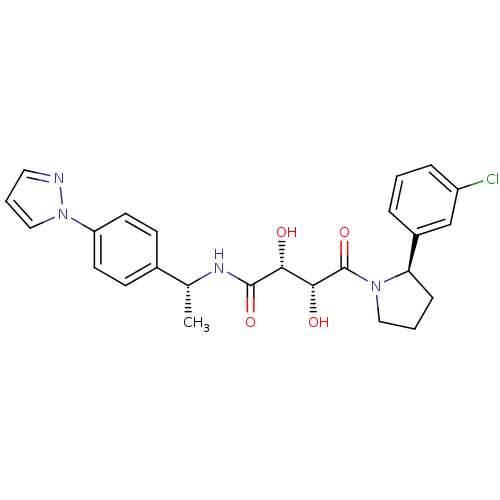
((2R,3R)-N-((R)-1-(4-(1H-pyrazol-1-yl)phenyl)ethyl)...)Show SMILES C[C@@H](NC(=O)[C@H](O)[C@@H](O)C(=O)N1CCC[C@@H]1c1cccc(Cl)c1)c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C25H27ClN4O4/c1-16(17-8-10-20(11-9-17)30-14-4-12-27-30)28-24(33)22(31)23(32)25(34)29-13-3-7-21(29)18-5-2-6-19(26)15-18/h2,4-6,8-12,14-16,21-23,31-32H,3,7,13H2,1H3,(H,28,33)/t16-,21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50375882
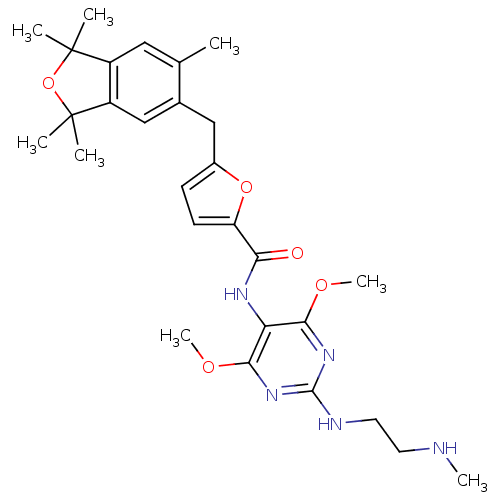
(CHEMBL411526)Show SMILES CNCCNc1nc(OC)c(NC(=O)c2ccc(Cc3cc4c(cc3C)C(C)(C)OC4(C)C)o2)c(OC)n1 Show InChI InChI=1S/C28H37N5O5/c1-16-13-19-20(28(4,5)38-27(19,2)3)15-17(16)14-18-9-10-21(37-18)23(34)31-22-24(35-7)32-26(30-12-11-29-6)33-25(22)36-8/h9-10,13,15,29H,11-12,14H2,1-8H3,(H,31,34)(H,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of GnRH receptor |
Bioorg Med Chem 16: 422-7 (2008)
Article DOI: 10.1016/j.bmc.2007.09.026
BindingDB Entry DOI: 10.7270/Q2Z60PXR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500524
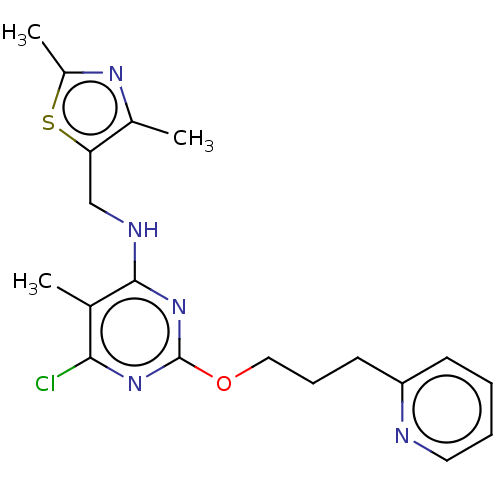
(CHEMBL3746826)Show InChI InChI=1S/C19H22ClN5OS/c1-12-17(20)24-19(26-10-6-8-15-7-4-5-9-21-15)25-18(12)22-11-16-13(2)23-14(3)27-16/h4-5,7,9H,6,8,10-11H2,1-3H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500522
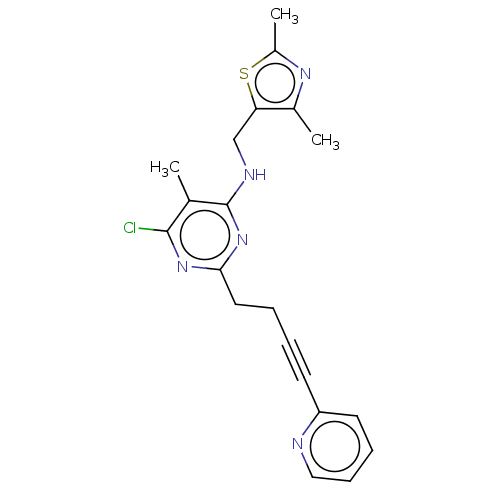
(CHEMBL3747006)Show SMILES Cc1nc(C)c(CNc2nc(CCC#Cc3ccccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C20H20ClN5S/c1-13-19(21)25-18(10-5-4-8-16-9-6-7-11-22-16)26-20(13)23-12-17-14(2)24-15(3)27-17/h6-7,9,11H,5,10,12H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.27 | -50.3 | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis
| Assay Description
Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... |
J Med Chem 52: 3523-38 (2009)
Article DOI: 10.1021/jm801394m
BindingDB Entry DOI: 10.7270/Q2B27SN3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500518
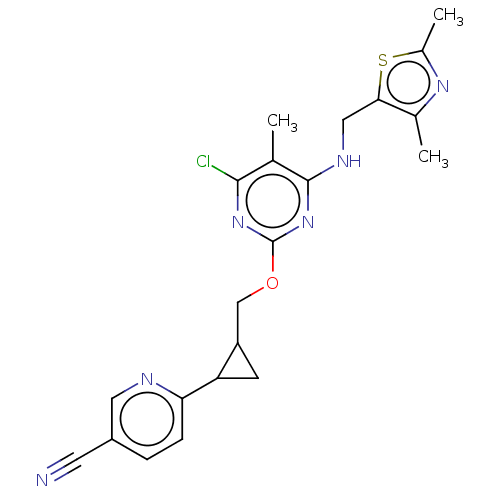
(CHEMBL3747617)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc(cn3)C#N)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H21ClN6OS/c1-11-19(22)27-21(28-20(11)25-9-18-12(2)26-13(3)30-18)29-10-15-6-16(15)17-5-4-14(7-23)8-24-17/h4-5,8,15-16H,6,9-10H2,1-3H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Macaca mulatta (Rhesus macaque)) | BDBM442759

(6-Chloro-N,1-dimethyl-N-{1-[4-(trifluoromethyl)phe...)Show SMILES CN(c1nc(Cl)nc2n(C)ncc12)C1(CC1)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C17H15ClF3N5/c1-25(13-12-9-22-26(2)14(12)24-15(18)23-13)16(7-8-16)10-3-5-11(6-4-10)17(19,20)21/h3-6,9H,7-8H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... |
US Patent US10647727 (2020)
BindingDB Entry DOI: 10.7270/Q2KS6VK2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500525
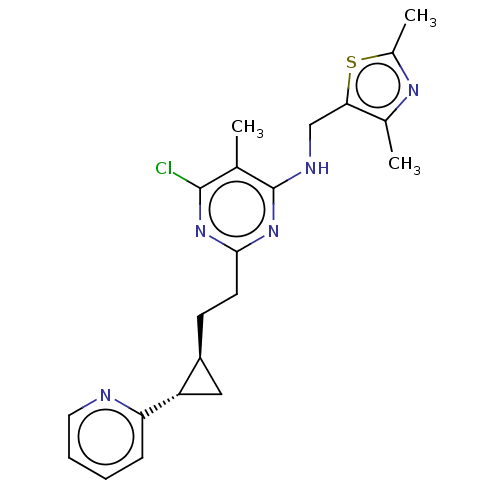
(CHEMBL3747736)Show SMILES Cc1nc(C)c(CNc2nc(CC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C21H24ClN5S/c1-12-20(22)26-19(8-7-15-10-16(15)17-6-4-5-9-23-17)27-21(12)24-11-18-13(2)25-14(3)28-18/h4-6,9,15-16H,7-8,10-11H2,1-3H3,(H,24,26,27)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343967
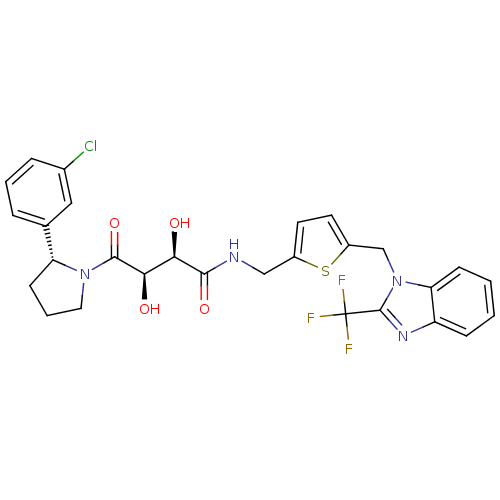
((2R,3R)-4-((R)-2-(3-chlorophenyl)pyrrolidin-1-yl)-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1CCC[C@@H]1c1cccc(Cl)c1)C(=O)NCc1ccc(Cn2c(nc3ccccc23)C(F)(F)F)s1 |r| Show InChI InChI=1S/C28H26ClF3N4O4S/c29-17-6-3-5-16(13-17)21-9-4-12-35(21)26(40)24(38)23(37)25(39)33-14-18-10-11-19(41-18)15-36-22-8-2-1-7-20(22)34-27(36)28(30,31)32/h1-3,5-8,10-11,13,21,23-24,37-38H,4,9,12,14-15H2,(H,33,39)/t21-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124644

(CHEMBL3622894)Show InChI InChI=1S/C18H20ClN5OS/c1-11-16(19)23-18(25-9-7-14-6-4-5-8-20-14)24-17(11)21-10-15-12(2)22-13(3)26-15/h4-6,8H,7,9-10H2,1-3H3,(H,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343984
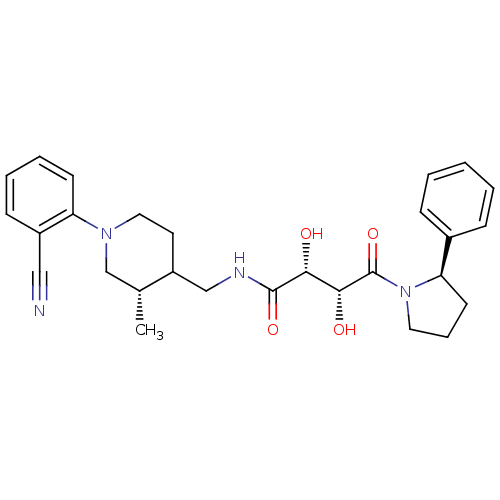
((2R,3R)-N-(((3S)-1-(2-cyanophenyl)-3-methylpiperid...)Show SMILES C[C@@H]1CN(CCC1CNC(=O)[C@H](O)[C@@H](O)C(=O)N1CCC[C@@H]1c1ccccc1)c1ccccc1C#N |r| Show InChI InChI=1S/C28H34N4O4/c1-19-18-31(23-11-6-5-10-21(23)16-29)15-13-22(19)17-30-27(35)25(33)26(34)28(36)32-14-7-12-24(32)20-8-3-2-4-9-20/h2-6,8-11,19,22,24-26,33-34H,7,12-15,17-18H2,1H3,(H,30,35)/t19-,22?,24-,25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data